Abstract
This study investigated the effects of age and length on mercury contamination in four fish species; yellow-eye mullet (Aldrichetta forsteri), black bream (Acanthopagrus butcheri), sand flathead (Platycephalus bassensis) and sea-run brown trout (Salmo trutta) from the Derwent Estuary, Tasmania, Australia, and examined the implications of these findings for public health monitoring. Mean mercury levels exceeded the Food Standards Australia and New Zealand maximum permitted level (0.5 mg kg−1) for all species except yellow-eye mullet. Mean levels in black bream were significantly higher (p < 0.05) than other species and consequently are a particular concern for human health. Regional differences (p < 0.05) in mercury levels in sand flathead were not obviously correlated with metal levels in the sediments. However, age and length significantly (p < 0.05) influenced mercury levels in brown trout and sand flathead, with age being more strongly related to intraspecies differences. In addition, movement and distribution within the estuary and trophic status appeared to be important factors in contribution to interspecific variation. Consequently, a sound understanding of fish life history and biology is important in identifying species which may be susceptible to accumulating mercury and hence pose a potential threat to human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fish are increasingly promoted as a healthy and nutritious food source containing minerals, vitamins and essential fatty acids and have been shown to have significant health benefits (Han 1998; Carvalho et al. 2005). However, fish can also contain toxic levels of some metals (Carvalho et al. 2005). In particular, some species of fish have been found to contain elevated mercury concentrations, which may be toxic both for the fish and for humans who consume the fish (Wiener et al. 2003). Mercury poisoning can have devastating effects, one of the first major examples of this was the Minamata Bay disaster in Japan, which has resulted in the deaths of over 1,000 people and left over 2,000 seriously ill from contaminated seafood (Harada 1995). This tragic incident sparked numerous studies into mercury levels in fish and the concomitant health effects on humans (Wang 2002).
The Derwent Estuary adjoins Hobart, the capital city of Tasmania, and as such is an important local industrial, recreational and environmental resource (Green and Coughanowr 2003). The estuary supports a significant recreational fishery, attracting an estimated 11,000 recreational anglers annually (Lyle et al. 2009). The upper estuary is extensively fished for sea-run brown trout (Salmo trutta) and black bream (Acanthopagrus butcheri), whilst the lower reaches are fished for a range of species including sand flathead (Platycephalus bassensis), Australian salmon (Arripis trutta), yellow-eye mullet (Aldrichetta forsteri), school whiting (Sillago bassensis) and greenback flounder (Rhombosolea tapirina; Green and Coughanowr 2003; Lyle 2005; Lyle et al. 2009). The lower reaches of the estuary are also open to commercial fishing, particularly for school whiting and sand flathead (Lyle 2005). Despite its natural values and many recreational uses, the Derwent Estuary is severely contaminated with metals including mercury, the main source being historical industrial effluent from a zinc smelter in the midestuary and a paper mill in the upper estuary (Eustace 1974; Bloom and Ayling 1977; Green and Coughanowr 2003).
Mercury loading in fish may be influenced by many factors; these include both physiological factors (species, size, age, sex, sexual maturity, diet) and broader environmental conditions (water chemistry, salinity, temperature, and levels of contamination; Carvalho et al. 2005). Several studies have shown that mercury accumulation in fish may be strongly influenced by concentrations in the surrounding water and sediments (Blevins and Pancorbo 1986; Calta and Canpolat 2006) whilst interspecific variation may arise as a result of differences in life history strategies, such as feeding habits (Calta and Canpolat 2006) and position in the food chain (Asuquo et al. 2004). Intraspecific variation may also occur in response to location (Asuquo et al. 2004), fish age/size (Calta and Canpolat 2006) and sex (Pourang 1995).
Mercury levels in Derwent Estuary fish were first examined in the mid-1970s by Ratkowsky et al. (1975) and Dix et al. (1975), who found that levels exceeded maximum permitted levels (0.5 mg kg−1) in several fish species including sand flathead and various sharks. Even though there have been significant improvements in industrial practices, 25 years later, levels in sediments from many areas of the estuary remain high (Jones et al. 2003) and levels in sand flathead from some regions are still markedly higher than the safe maximum permitted level of 0.5 mg kg−1 prescribed by Food Standards Australia and New Zealand (Green and Coughanowr 2003; FSANZ 2004).
This study characterised the potential risk of mercury contamination in seafood from the Derwent Estuary by providing contemporary data on mercury levels in a range of recreationally important fish species to compare with historic records and comparing contamination levels to a range of factors in order to evaluate how environmental conditions and biological factors (including age and/or size) might influence mercury levels in fish. Understanding such relationships is particularly important from a human health perspective as this may influence mercury loading and hence potential health risks of consumption.
2 Materials and Methods
2.1 Study Area
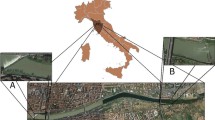
The Derwent Estuary (42° 54′S, 147° 18′E), is approximately 52 km in length and is the largest estuary in southern Tasmania, with depths ranging between 0 to 30 m (Fig. 1). In this study, the estuary was divided into four sampling regions, on the basis of differences in hydrography, sediment metal loading and biological information; these regions are consistent with those used in previous studies (Eustace 1974; Ratkowsky et al. 1975; Green and Coughanowr 2003) (Fig. 1). Region 1 (Tasman Bridge to New Norfolk) has a strong graduation from seawater to freshwater and is subject to significant industrial inputs. Regions 2 (Western shore) and 3 (Eastern shore) are both predominantly marine, although there is a stronger river flow along the eastern shore (Jones et al. 2003). Region 4 (Ralphs Bay) is considered fully marine but as an embayment has several distinct differences from the other regions (shallow water and warmer temperatures) (Jones et al. 2003). Samples were also collected from a control site (Region 5), outside the Derwent Estuary, and relatively unaffected by industrial pollution (Green and Coughanowr 2003) (Fig. 1).
2.2 Sample Collection and Processing
Fish were sampled over 5 months in Winter/Spring 2007 using line fishing and gill netting. Sampling targeted a range of sizes for each species, including individuals below legal minimum size limits. Sand flathead were sampled from all regions, whilst black bream, brown trout and yellow-eye mullet were only sampled from Region 1.
Each fish was weighed (whole ±1 g) and measured (total and fork length ±1 mm), sexed and gonads weighed (±0.1 g). A sample of muscle tissue (approximately 50 g) was removed from each fish from the area posterior of the pectoral fin. The muscle tissue was placed in a labelled plastic zip-lock bag and stored at−18°C until further processing. Sagittal otoliths were extracted, cleaned, dried and stored in plastic sample containers for later age analysis.
2.3 Age Determination
Methods used for fish aging were based on those reported by Ewing et al. (2007), with specific age determination techniques following those previously described for sand flathead (Jordan et al. 1998), black bream (Morison et al. 1998), brown trout (Graynoth 1996) and yellow-eye mullet (Curtis and Shima 2005). Otoliths were examined with no knowledge of fish size, sex or date of capture. Between- and within-reader precision was examined using the index of average percent error (APE; Beamish and Fournier 1981).
2.4 Mercury Analysis
The determination of total mercury in fish muscle tissue followed National Association of Testing Authorities (NATA) approved methods. A measurement of total Hg can be used an approximate of MeHg in fish muscle due to the fact that MeHg generally accounts for 80% to 100% of the total Hg content in fish (Wiener et al. 2003). A 1-g portion of skinless and boneless muscle tissue was digested in mercury digestion acid (HNO3 67% vol/vol plus H2SO4 33% vol/vol), with the addition of 5 mL of K2S2O8 solution and KMnO4 as required. Mercury analysis was undertaken using cold vapour atomic fluorescence spectrometry (CV-AFS) on an atomic fluorescence analyser (Melenium Merlin, UK). All data are given as milligrams per kilogram (mg kg−1) wet weight of sample, with a reporting limit of 0.02 mg kg−1. Quality control measures included the running of a blank, blank matrix spike, duplicate sample, standard reference material (SRM) and a matrix spike for every 20 samples analysed and indicated that overall detection levels were reliable. For all sample batches, the blank matrix spikes were within 25% of the theoretical value of 1.00 µg L−1, duplicates agreed within 20% of the mean, and preparation blanks were below the minimum reporting level (MRL) of 0.02 mg kg−1.
2.5 Data Analysis
Analysis of variance was used to test for the effects of species and region (sand flathead only) upon mercury concentrations. In all cases, the type III sums of squares was used to test the null hypothesis because the experimental design was unbalanced. Assumptions of homogeneity and normality of data were assessed through examination of residual plots, and data were appropriately transformed (natural log) where assumptions of homogeneity were not met. For all analysis of variances, significance value was set at p < 0.05. Where effects were significant, Tukey’s pair-wise post hoc tests were used to further examine these differences.
The relationships between mercury levels and age and length were evaluated using regression analysis; analysis of covariance (ANCOVA) was employed to allow for comparisons between regions where significant relationships were observed between factors. All statistical analyses were carried out using SPSS version 15.0 (SPSS, Chicago, IL).
3 Results
3.1 Interspecific Comparisons
Mean mercury levels in black bream, brown trout and sand flathead all exceeded the Food Standards Australia and New Zealand (FSANZ) maximum permitted level (ML) of 0.5 mg kg−1, with maximum mercury levels recorded for individual sand flathead, black bream and brown trout being well in excess of this guideline (Table 1). Brown trout and sand flathead had significantly higher mean levels than yellow-eye mullet (df 3, 108, F = 92.79, p < 0.01), and black bream had significantly higher mean levels than all of the other species (Table 1).
Of the legal sized fish sampled in this study, all of the black bream, 60% of the brown trout and 46% of the sand flathead had mercury levels that exceeded the ML (Table 2). In contrast, none of the legal-sized yellow-eye mullet had concentrations that exceeded the ML (Table 2).
Sand flathead and brown trout displayed significant, positive relationships between mercury concentration and age and length, with length accounting for slightly more of the observed variability than age in both species (Fig. 2). However, it should be noted that there was a high degree of autocorrelation between age and length in brown trout.
3.2 Regional Comparisons—Sand Flathead
Comparison of mercury levels in sand flathead between the five regions suggests that there were significant differences between locations (df 4, 148, F = 34.77, p < 0.001; Fig. 3). Mean mercury levels in sand flathead from regions 1, 3 and 4 (0.62, 0.57 and 0.64 mg kg−1, respectively) were significantly higher than that from the other regions, whilst levels in fish sampled from the control region were significantly lower (region 5; 0.22 mg kg−1) than other areas, including region 2 (0.33 mg kg−1; Fig. 3). The length–age relationships for sand flathead within each region suggest that age explained more of the variation in mercury levels (Table 3).
The size and age structure of sand flathead were not consistent between regions, with samples from regions 1 and 5 having a much larger range of age/sizes than other regions (Table 4). Given that mercury levels were found to vary with age and length, the data need to be standardised for age in order to accurately evaluate regional differences. The adjusted (least-squares means) mercury concentrations shown in Table 4 reflect the values that might be expected had fish from each region been of similar age. Examination of these adjusted means suggests that regional differences were more pronounced; importantly, the adjusted mean levels for regions 3 and 4 exceeded that for region 1, with levels in region 4 being the highest (Table 4). In the ANCOVA, age was found to have a significant effect on mercury levels in sand flathead from all regions (Table 5).
4 Discussion
Variability in contamination levels in fish is influenced by two main factors: (1) exposure, the overall level of pollutant in the environment (Carvalho et al. 2005), and (2) the capacity of the contaminant to accumulate in an organism and the organism’s ability to excrete the contaminant (Sorensen 1991). Benthic environmental conditions, in particular sediments, will influence mercury loading (Carvalho et al. 2005), particularly where fish rely either directly or indirectly on the sediment for food. Fish that dwell and feed primarily in the sediments are thus likely to uptake more mercury since most benthic invertebrates (which are common prey items) obtain nutrition through the digestion of sediment material (Campbell 1994).
Sediment mercury levels are elevated in much of the Derwent estuary; region 1 is the most contaminated area, and there is a tendency for levels to decline towards the mouth of the estuary (Green and Coughanowr 2003; Table 6). As a result, benthic feeders, such as sand flathead (Jordan 2001), that feed on prey in areas exposed to higher metal loads are expected to accumulate higher levels of mercury (Blevins and Pancorbo 1986). Furthermore, sand flatheads are generally regarded as sedentary, moving within relatively restricted ranges at least in the short term (Dix et al. 1975; Jordan 2001) and thus might be expected to reflect local regional differences in mercury levels. While mercury levels in sand flathead were moderately elevated compared with the other species examined and also demonstrated significant regional variability, this variability did not match the spatial variability in sediment load. For instance, fish from region 1 had moderate levels of mercury, whereas those from region 4 (Ralphs Bay) had the highest levels, despite sediment levels in that region being an order of magnitude lower than in region 1. Previous studies from the Derwent have revealed similar spatial patterns of mercury contamination in sand flathead and shellfish, oysters (Crassostrea gigas) and mussels (Mytilus edulis planulatus) (Ratkowsky et al. 1975; Green and Coughanowr 2003), implying that mercury contamination is not directly linked to sediment levels and that other mechanisms influence bioaccumulation.
The other species in this study differed in the degree to which they utilise the estuary, and this is expected to influence their potential exposure to contaminants. Brown trout and yellow-eye mullet are mobile species, which move in and out of estuaries (Graynoth 1996; Curtis and Shima 2005) and thus may have a lower overall exposure to contaminants in the Derwent estuary. Black bream, on the other hand, is an estuarine resident species that, while actively moving within a given estuarine system, especially the mid and upper regions of the estuary (Hindell et al. 2008), show little evidence of movement between systems. Since these mid and upper regions also represent the most polluted areas of the Derwent Estuary, black breams are likely to have greater overall exposure to contaminants than either brown trout or yellow-eye mullet. This may in part account for the higher mercury levels observed in black bream. Although there is no information on mercury levels for black bream from “uncontaminated” estuaries in Tasmania, black bream from a relatively unpolluted estuary in Victoria had considerably lower levels (0.22 mg kg−1; Fabris et al. 1999) than those observed in the current study (1.57 mg kg−1).
The trophic status of a species also influences metal loadings, and many studies that have shown that species from higher trophic levels tend to accumulate higher concentrations of mercury than species of lower trophic status (Ratkowsky et al. 1975; Mason et al. 1995). Yellow-eye mullet, a herbivore (Curtis and Shima 2005) and therefore at the lowest trophic level of the four species sampled, correspondingly had the lowest levels of mercury in its flesh. Brown trout and sand flathead, which are opportunistic carnivores (Graynoth 1996; Jordan 2001), both showed relatively high mercury levels. By contrast, black breams are generally regarded as omnivores (Sarre et al. 2000) yet had substantially higher levels of mean mercury than did the other species. A possible explanation for this is that specific components of the diet may be particularly susceptible to mercury accumulation. For instance, previous studies have indicated that bivalves, crabs and macrophytes represent the main components of the black bream diet (Sarre et al. 2000), and in the Derwent, bivalves (eg. blue mussels) have been shown to contain high concentrations of mercury, with levels of up to 1.3 mg kg−1 reported (Green and Coughanowr 2003). As such, this prey group may represent an important contributor to the elevated levels of mercury observed. Thus, while trophic status may provide a general characterisation of mercury levels, bioaccumulation may also be influenced by specific dietary components.
Mercury is a persistent contaminant, and excretion from fish is slow (Sorensen 1991). Consequently, mercury tends to accumulate over time, reaching high levels in long lived fish (Wiener et al. 2003). Several studies have shown that mercury levels in muscle tissue increase with size and age in fish, for example, black marlin (Mackay et al. 1975), deep water sharks (Hornung et al. 1993), largemouth bass (Park and Curtis 1997) and pacific cod (Burger 2007). Consistent with these findings, mercury levels in sand flathead and brown trout increased with age and length. The relationship between age and mercury concentration was more pronounced in brown trout than sand flathead, although this may reflect the larger size range of brown trout examined. Contrary to expectations, mercury levels did not increase significantly with age in black bream. A possible explanation for this is that the sampled black breams were relatively old fish (13-28 years), with 71% of individuals falling into just two age classes (18 and 19 years). A relationship may have been observed had a greater range of sizes and ages been examined. It is also worth noting that the black breams were significantly older than the other species sampled, a factor that may have further contributed to the observed high levels of mercury in this species. As has been noted, mercury bioaccumulation is a function of both exposure opportunity and duration of exposure; black breams in the Derwent Estuary were in the highest category for both of these factors. These results highlight that age and size, in conjunction with other factors, need to be considered when assessing the environmental impact or health effects of mercury or similar metal pollutants in estuaries.
A key component of this study was to provide an assessment of the risks to public health associated with mercury in commonly caught fish species. The findings indicate that mercury levels in several key estuarine fish species exceeded the safe maximum permitted level (FSANZ 2004). Despite major improvements in industrial practices, Derwent sediment levels have remained elevated in many areas (Green and Coughanowr 2003) and mercury levels in sand flathead have remained largely unchanged since the 1970s (Ratkowsky et al. 1975; Dix et al. 1975). As a consequence, there are potential public health issues associated with the consumption of fish caught in the Derwent Estuary. This study has also shown that mercury levels in brown trout and black bream exceeded the maximum permissible residue level, with black bream concentrations being three times higher than levels in sand flathead. Black breams are a highly sought-after sport fish and are regularly consumed by recreational fishers (Lyle 2005), and therefore, such concentrations represent an important public health concern. In recognition of this, a health advisory was issued in 2007 recommending that consumption of sand flathead should be limited to no more than three meals a week and that pregnant women and children should limit their consumption to one meal per week. Furthermore, it was recommended that black bream not be used for human consumption at all.
In addition, data from the current study suggest that there may be considerable regional variability in mercury levels for sand flathead. This has implications for human health, as fish from areas such as region 4 appear to pose a greater risk than those from elsewhere. Furthermore, depending on differences in fishing and fish usage practices, the risk to human health may vary between ethnic or socioeconomic groups. Consequently, understanding the biology, ecology and behaviour of key fish species in contaminated estuaries is not only important in establishing contamination pathways but also in the identification of high-risk groups and the subsequent response to the management of potential health issues.
References
Asuquo, F. E., Ewa-Oboho, I., Asuquo, E. F., & Udo, P. J. (2004). Fish species used as biomarker for heavy metal and hydrocarbon contamination for Cross River, Nigeria. The Environmentalist, 24, 29–37.
Beamish, R. J., & Fournier, D. A. (1981). A method for comparing the precision of a set of age determinations. Canadian Journal of Fisheries and Aquatic Sciences, 38, 982–983.
Blevins, R. D., & Pancorbo, O. C. (1986). Metal concentrations in muscle of fish from aquatic systems in east Tennessee, USA. Water, Air, and Soil Pollution, 29, 361–371.
Bloom, H., & Ayling, G. M. (1977). Heavy metals in the Derwent Estuary. Environmental Geology, 2, 3–22.
Burger, J. (2007). Heavy metals in pacific cod (Gadus macrocephalus) from the aleutians: location, age, size, and risk. Journal of Toxicology and Environmental Health. Part A, 70, 1897–1911.
Calta, M., & Canpolat, O. (2006). The comparison of three cyprinid species in terms of heavy metals accumulation in some tissues. Water Environment Research, 78, 548–551.
Campbell, K. R. (1994). Concentrations of heavy metals associated with urban runoff in fish living in stormwater treatment ponds. Archives of Environmental Contamination and Toxicology, 27, 352–356.
Carvalho, M. L., Santiago, S., & Nunes, M. L. (2005). Assessment of the essential element and heavy metal content of edible fish muscle. Analytical and Bioanalytical Chemistry, 382, 426–432.
Curtis, T. D., & Shima, J. S. (2005). Geographic and sex-specific variation in growth of yellow-eyed mullet, Aldrichetta forsteri, from estuaries around New Zealand. New Zealand Journal of Marine and Freshwater Research, 39, 1277–1285.
Dix, T. G., Martin, A., Ayling, G. M., Wilson, K. C., & Ratkowsky, D. A. (1975). Sand flathead (Platycephalus bassensis) an indicator species for mercury pollution in Tasmanian waters. Marine Pollution Bulletin, 6, 142–143.
Eustace, I. J. (1974). Zinc, cadmium, copper and manganese in species of finfish and shellfish caught in the Derwent Estuary, Tasmania. Australian Journal of Marine and Freshwater Research, 25, 209–220.
Ewing, G. P., Lyle, J. M., Murphy, R. J., Kalish, J. M., & Ziegler, P. E. (2007). Validation of age and growth in a long-lived temperate reef fish using otolith structure, oxytetracycline and bomb radiocarbon methods. Marine and Freshwater Research, 58, 1–12.
Fabris, G. J., Theodoropoulos, T., Sheehan, A., & Abbott, B. (1999). Mercury levels and organochlorines in black bream, Acanthopagrus butcheri, from the Gippsland Lakes, Victoria, Australia: evidence for temporal increases in mercury levels. Marine Pollution Bulletin, 38, 970–976.
FSANZ (Food Standards Australia and New Zealand). (2004). Regulations for mercury in fish. <http://www.foodstandards.gov.au/newsroom/factsheets/factsheets2004/mercuryinfishfurther2394.cfm>. [accessed 2008 Feb 2]
Graynoth, E. (1996). Determination of the age of Brown and Rainbow trout in a range of New Zealand lakes. Marine and Freshwater Research, 47, 749–756.
Green, G., Coughanowr, C. (2003). State of the Derwent Estuary 2003: a review of pollution sources, loads and environmental quality data from 1997–2003. Derwent Estuary Program, DPIWE, Tasmania.
Han, B. C. (1998). Estimation of target hazard quotients and potential health risks for metals by consumption of seafood in Taiwan. Archives of Environmental Contamination and Toxicology, 35, 711–720.
Harada, M. (1995). Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Critical Reviews in Toxicology, 25, 1–24.
Hindell, J. S., Jenkins, G. P., & Womersley, B. (2008). Habitat utilization and movement of black bream Acanthopagrus butcheri (Sparidae) in an Australian estuary. Marine Ecology Progress Series, 336, 219–229.
Hornung, H., Krom, M. D., Cohen, Y., & Bernhard, M. (1993). Trace metal content in deep-water sharks from the eastern Mediterranean Sea. Marine Biology, 115, 331–338.
Jones, B. G., Chenhall, B. E., Debretsion, F., & Hutton, A. C. (2003). Geochemical comparisons between estuaries with non-industrialised and industrialised catchments: the Huon and Derwent River estuaries, Tasmania. Australian Journal of Earth Sciences, 50, 653–667.
Jordan, A. R. (2001). Reproductive biology, early life-history and settlement distribution of sand flathead (Platycephalus bassensis) in Tasmania. Marine and Freshwater Research, 52, 589–601.
Jordan, A. R., Mills, D. M., Ewing, G., & Lyle, J. M. (1998). Assessment of inshore habitats around Tasmania for life-history stages of commercial finfish species. FRDC Final Report. University of Tasmania, Tasmanian Aquaculture and Fisheries Institute.
Lyle, J. M. (2005). 2000/01 Survey of recreational fishing in Tasmania. Tasmanian Aquaculture and Fisheries Institute. Technical Report.
Lyle, J. M., Tracey, S. R., Stark, K. E., & Wotherspoon, S. (2009) 2007–08 survey of recreational fishing in Tasmania. Tasmanian Aquaculture and Fisheries Institute Report.
MacKay, N. J., Kazacos, M. N., Williams, R. J., & Leedow, M. I. (1975). Selenium and heavy metals in black marlin. Marine Pollution Bulletin, 6, 57–61.
Mason, R. P., Reinfelder, J. R., & Morel, F. M. M. (1995). Bioaccumulation of mercury and methylmercury. Water, Air, and Soil Pollution, 80, 915–921.
Morison, A. K., Coutin, P. C., & Robertson, S. G. (1998). Age determination of black bream, Acanthopagrus butcheri (Sparidae), from the Gippsland Lakes of south-eastern Australia indicates slow growth and episodic recruitment. Marine and Freshwater Research, 49, 491–498.
Park, J. G., & Curtis, L. R. (1997). Mercury distribution in sediments and bioaccumulation by fish in two Oregon reservoirs: point-source and nonpoint-source impacted systems. Archives of Environmental Contamination and Toxicology, 33, 423–429.
Pourang, N. (1995). Heavy metal bioaccumulation in different tissues of two fish species with regards to their feeding habits and trophic levels. Environmental Monitoring and Assessment, 35, 207–219.
Ratkowsky, D. A., Dix, T. G., & Wilson, K. C. (1975). Mercury in fish in the Derwent Estuary, Tasmania, and its relation to the position of the fish in the food chain. Australian Journal of Marine and Freshwater Research, 26, 223–231.
Sarre, G. A., Platell, M. E., & Potter, I. C. (2000). Do the dietary compositions of Acanthopagrus butcheri in four estuaries and a coastal lake vary with body size and season and within and amongst these water bodies? Journal of Fish Biology, 56, 103–122.
Sorensen, E. M. B. (1991). Metal poisoning in fish. Boston: CRC Press.
Wang, W. X. (2002). Interactions of trace metals and different marine food chains. Marine Ecology Progress Series, 243, 295–309.
Wiener, J. G., Krabbenhoft, D. P., Heinz, G. H., & Scheuhammer, A. M. (2003). Ecotoxicology of mercury. In D. J. Hoffman, B. A. Rattner, G. A. Burton, & J. Cairns (Eds.), Handbook of ecotoxicology (2nd ed., pp. 409–443). New York: Lewis Publishers.
Acknowledgement
This work was funded by Nystar Pty Ltd. and a Fishwise Community Grant. We would like to thank all those who assisted us in the field, notably Mr. L. Mackenzie, Mr. M. Stalker and Mr. A. Hunt. We are grateful to Dr. A. Featherstone, Mr. S. Black and Dr. D. Norman from Analytical Services Tasmania for their assistance with the metal analyses. Thanks must also go to Mr. G. Ewing for his practical and theoretical advice on fish processing and aging and to Mr. D. Padula for his guidance with respect to metal residues monitoring and analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verdouw, J.J., Macleod, C.K., Nowak, B.F. et al. Implications of Age, Size and Region on Mercury Contamination in Estuarine Fish Species. Water Air Soil Pollut 214, 297–306 (2011). https://doi.org/10.1007/s11270-010-0424-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0424-y