Abstract
Amphibians, particularly frogs, are increasingly used as bioindicators of contaminant accumulation in pollution studies. Their use for field scientific purposes is limited because most frog species are endangered and protected in most countries; killing of specimens are not allowed. The aim of our study was to work out and assess a method by which the elemental contents of frog bones could be estimated effectively based on the toe bones. For this purpose, Rana esculenta L. individuals were collected from an urban pond in Debrecen city (Hungary). The following large bones were also analysed: skull, spinal, femur, tibia–fibula, tarsal bones, metatarsus, humerus and digits from front and hind limbs. In the bones, P, Ca, Mg, Mn, Na, S and Zn elements were measured. The elemental contents of large bones were significantly correlated with bones weight in the case of each element. The elemental contents of phalanges were also estimated based on the large bones. The measured and the estimated elemental contents of phalanges were not different significantly based on the tibia–fibula, metatarsalis bones, front and hind limb digits. Elemental analysis based on phalanges adds a further way of use of phalanges. Frogs using their phalanges could be useful indicators in the assessment of environmental contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Amphibian populations are declining globally which is caused by several factors such as habitat loss and fragmentation (Icochea et al. 2002; Beebee and Griffiths 2005), ultraviolet radiation and chemical pollution (Blaustein et al. 2003), climate change (Pounds 2001) and epidemic disease, for example chytrig fungus (Pounds et al. 2006). These factors may also cause deformities and abnormalities in development (Blaustein and Johnson 2003). Pond breeding amphibians are especially sensitive to contaminations because of their complex life cycles (Rowe et al. 1996, 1998). Effects of contamination may result in shorter body length, lower body mass and malformations of limbs or other organs (Sparling et al. 2000). The slow development, late metamorphosis and small metamorph size result in increased risk of mortality and exposure to predation (Rowe et al. 2001; Pahkala et al. 2002, 2003). Anurans are increasingly used as bioindicators of accumulation of contaminants in pollution studies (Loumbourdis et al. 2007). Elemental analysis, especially that of heavy metals in amphibians began to attract special attention (Herkovits and Helguero 1998; Stolyar et al. 2008).
The composition of bones is as follows 70% minerals, 20% collagen, 8% water and about 2% non-collageneous components (Klepinger 1984). The concentration of elements in bones may be reported per gram wet weight or dry weight; in the case of bone samples it is given in microgram or milligram per bone sample in analytical studies (Elinder et al. 1994). Calcium and phosphorous are the most important elements in the build of skeleton which are the main components in the formation of hydroxiapatite (Janus et al. 2008).
Toe clipping is a commonly used standard method to identify individuals, particularly frogs and toads (McCarthy and Parris 2004). It is simple, safe and also applicable for genetics (Noonan and Gaucher 2006), histological examinations (Boyle et al. 2004) or amphibian skeletochronological age determination (Castanet and Smirina 1990; Bruce et al. 2002). Toe clipping is an acceptable method which does not cause any serious negative effects (Hartel and Nemes 2006). McCarthy and Parris (2004) found that toe clipping reduces the recapture rate of the clipped individuals by 4–11%, which may be related to possible adverse effect of toe clipping.
Our aim was to show trace elements from bones and develop a method to estimate effectively the elemental concentrations of frog bones based on phalanges. We used hydrogen peroxide to clean the bones. We also tested its effect on the elemental contents of bones to find the shortest period to the cleaning which may not cause element leach out from bone tissue but the bones correctly cleaned from conjunctive tissue. Our hypothesis was that the concentration of trace elements in large bones, for example tibia–fibula, tarsals and metatarsus was similar to the phalanges; i.e. bones have elemental contents commensurable to their weights. Thus, toe phalanges may represent the other parts of the skeletal system. This means that a monitoring procedure based on phalanges is especially useful because it does not imply killing of specimens and it can be used to estimate the environmental contamination.
2 Materials and Methods
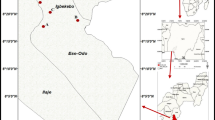
Rana esculenta is a common species in Europe, including Hungary. Frogs and water samples were collected during the summer of 2007. The sampling site was the Frog Pond (Békás tó) in Debrecen City, which is an urban pond with heavy traffic around and other anthropogenic effects (e.g. receiving thermal bath) in the centre of the city (47°33′ N, 21°37′ E). Landscape elements (in percentages) around the pond in a plot of 1 × 1 km size were the following: forest, 39.7%; buildings, 59.9% and pond, 0.4%.
Ten adult frogs were collected by hand net. On arrival at laboratory, frogs were anaesthetized with chloroform. After dissection the following large bones were processed: skull, spinal, femur, tibia–fibula, tarsal bones, metatarsus, humerus and digits from front and hind limbs. Reduced bones were found in the case of one frog specimen where the metatarsal and phalanges from hind limb were missing. In our study, the first phalange of the third digit was also analysed from each hind limb.
Each large bone was placed into a plastic sieve and flushed with 100 ml of double deionised water. After this step, the large bones were placed into 5 ml hydrogen peroxide for 5 days. The cleaning with hydrogen peroxide was important to clean the bones. Femurs were chosen to test the effect of hydrogen peroxide on elemental contents of frogs. Similarly to the other bones, the right femurs were placed into 5 ml 30% (m/m) hydrogen peroxide for 5 days, while the left femurs for 8 days. The hydrogen peroxide contained remarkable concentration of Sn; thus, the elemental analysis the Sn concentration was not considered.
This cleaning procedure was also applied in the case of phalanges. After the flush with double deionised water these smaller toe bones were placed into 0.5 ml hydrogen peroxide for 2 days. After the hydrogen peroxide treatment each sample was flushed with deionised water again and dried overnight at 105°C. The dry weights of bones were measured with analytical balance. Toe bones were measured with a SATORIUS LE 26P micro analytical balance. In the case of each sample, the dry weight material was digested using 2 ml 65% (m/m) nitric acid at 80°C for 4 h. Digested samples were diluted to 100 ml (large bones) and 20 ml (toe bones) using 1% (m/m) nitric acid solution.
Analysis of elements was performed by inductively coupled plasma optical emission spectrometry (ICP-OES) IRIS Intrepid II XSP. We used a seven-point calibration procedure (0.001, 0.005, 0.01, 0.05, 0.1, 0.5 and 1.0 mg L−1) with multi-element calibration solution (Merk ICP multi-element standard solution IV). The elemental content of bone samples was expressed in milligram or microgram per bone in our study.
Classical analytical method was used to determine the trace elemental concentration of the water sample. The water sample (1,000 ml) was filtered on 0.45 µm membrane. Concentrations of metals were analysed by ICP-OES. Other compounds of water were measured by ionchromatography and titration.
Calculations were performed by the SPSS/PC+ software package. Linear regression models were used to evaluate the relationships between the element contents and the weight of bones. In this analysis, the average of element contents per specimens was used. The measured and estimated elemental contents of phalanges were tested with paired samples t test (Zar 1996).
3 Results and Discussion
There was no significant difference between the 5 and 8 days long hydrogen peroxide cleaning for the studied elemental concentrations except the manganese. In the case of manganese content we found weak significant decrease in the concentration (Table 1). Thus, we recommend using the 5 days cleaning for the large bones. In the cases of phalanges 2 days was always sufficient for the hydrogen peroxide cleaning.
In the case of large bones we found strong correlation between the elemental contents and dry weights: P (r 2 = 0.96, p < 0.001, df = 9) and Ca (r 2 = 0.95, p < 0.001, df = 9). Similar relationships were found in the cases of other elements: Mg (r 2 = 0.97, p < 0.001, df = 9), S (r 2 = 0.91, p < 0.001, df = 9), Na (r 2 = 0.89, p < 0.001, df = 9), Mn (r 2 = 0.73, p < 0.01, df = 9), Ba (r 2 = 0.63, p < 0.01, df = 9) and Zn (r 2 = 0.57, p < 0.05, df = 9). The elemental concentrations of bones were significantly correlated with bones weight in the case of each element (p < 0.05). The significant relationships between the weight of bones and elemental contents mean that the elemental contents of bones were commensurable to their weights. Our result showed that strong correlation was between the main components of bone (P, Ca, Mg, S and Na) and weights. In the case of Ba and Zn the relatively lower correlation may have resulted from their smaller concentration in bones.
There was no significant difference between the elemental contents of phalanges from right and left hind limbs (p > 0.05). In the case of phalanges, the following elemental contents were found: Ca, 0.4 ± 0.1 mg bone−1; P, 0.3 ± 0.1 mg bone−1; Mg, 4.0 ± 1.0 µg bones−1 and Zn, 0.4 ± 0.01 µg bones−1. Because the weights of phalanges were very small the following elements were not measurable with this technique: Ba, Mn, Na and S; i.e. the concentration of Ba (<0.006 mg L−1), Mn (<0.001 mg L−1), Na (<0.002 mg L−1) and S (<0.01 mg L−1) was below the detection limit.
The aim of our study was to work out a method by which the elemental contents of frog bones could be estimated effectively based on phalanges. We found that the elemental contents of large bones significantly correlated with bones weight. Thus, we can estimate the elemental contents of phalanges based on this correlation. Measured elemental contents of phalanges were compared to the estimated elemental contents based on large bones to test the usefulness of the method. The measured and the estimated elemental contents of phalanges were not different significantly based on the tibia–fibula, metatarsalis bones, front and hind limb digits (Table 2). We found significant differences for the skull, spinal, femur and humerus between the measured and estimated elemental contents.
In the case of other large bones the weakly significant differences were caused by the high concentration of elements in the large bones. Our results indicated that the elemental contents of phalanges represented reliably the elemental contents of tibia–fibula, metatarsalis bones and digits. Thus, elemental contents of these bones were represented by the smaller phalanges. Frogs by phalange elemental analysis may be useful indicator organism for environmental pollution assessment.
Our results show that the main elements were calcium, phosphorous and magnesium in the bones, similar to the study of Oudadesse et al. (2004). The concentration of sodium and barium were also present in the large bones similarly to other findings (Klepinger 1984). The concentration of strontium was below detection limits in our study which is similar to Alexander et al. (1955). Similar result was reported by other studies in the case of zinc contents in the large bones (Flyaks and Borkin 2004). Pavel and Kucera (1986) studied the elemental contents in the body of R. esculenta by atomic absorption spectrophotometry. In contrast with their work, the concentrations of Cu, Cr and Pb were below detection limits in our study. They found that a few elemental contents (Fe and Mn) in frogs were significantly affected by the ecological characteristics and the pollution of the localities. Puky and Oertel (1997) reported that R. esculenta can accumulate metals in higher concentration than Bombina bombina.
With ionchromatography the following water compounds were analysed: F−, 0.4 mg L−1; Cl−, 4.0 mg L−1; NO −2 , <0.01 mg L−1; NO −3 , 0.7 mg L−1; PO 3−4 , <0.01 mg L−1 and SO 2−4 , 0.5 mg L−1. The results of titration of water were the following: HCO −3 , 119.8 mg L−1 and CO 2−3 , 117.8 mg L−1. The following trace elements were analysed in the water: Ca, 32.8 mg L−1; K, 1.5 mg L−1; Mg, 18.7 mg L−1; Na, 63.2 mg L−1; Fe and Mn, <0.05 mg L−1; Zn, <0.001 mg L−1; Ba, 0.3 mg L−1 and Sr, 0.1 mg L−1. The Cd and Pb concentration in the water were below the detection limit (<0.001 mg L−1) similarly to earlier studies (Stolyar et al. 2008). The pond was receiving thermal water, which resulted in the high Ba and Sr concentration (Wang et al. 2001). All the above water chemistry analyses indicated that the Frog Pond was rich in carbonates and hydrogen carbonates.
In this study we demonstrated that the elemental contents of bones can be estimated using phalanges; thus, there is no need to kill specimens for extracting large bones (e.g. tibia–fibula, metatarsalis bones and digits) for environmental quality assessment studies. The elemental analysis of phalanges had some advantages. The method may apply with live frog, the analytical analysis has small chemical reagent demand and a high number of samples can be analysed by this method. In spite of the small size of the phalanges, the elemental concentrations can be measured reliably. Our result showed that the elemental analysis based on phalanges adds a further way of use of these bones for environmental quality assessment. Thus, frogs by their phalanges may be useful biological indicators of contamination in the pollution studies. With further technical development or using highly sensitive analytical technique (ICP-MS) the microelements (Ba and Mn) may also be measured in the phalanges.
References
Alexander, G. V., Nusbaum, R. E., & MacDonald, N. S. (1955). The relative retention of strontium and calcium in bone tissue. Journal of Biological Chemistry, 218, 911–919.
Beebee, T. C. J., & Griffiths, R. A. (2005). The amphibian decline crisis: A watershed for conservation biology? Biological Conservation, 125, 271–285.
Blaustein, A. R., & Johnson, P. T. J. (2003). The complexity of deformed of amphibians. Frontiers in Ecology and the Environment, 1, 87–94.
Blaustein, A. R., Romansic, J. M., Kiesecker, J. M., & Hatch, A. C. (2003). Ultraviolet radiation, toxic chemicals, and amphibian population declines. Diversity and Distribution, 9, 123–140.
Boyle, D. G., Boyle, D. B., Olsen, V., Morgan, J. A. T., & Hyatt, A. D. (2004). Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organism, 60, 141–148.
Bruce, R. C., Castanet, J., & Francillon-Vieillot, H. (2002). Skeletochronological analysis of variation in age structure, body size, and life history in three species of Desmognathine salamanders. Herpetologica, 58, 181–193.
Castanet, J., & Smirina, E. (1990). Introduction to the skeletochronological method in amphibians and reptiles. Annales des Sciences Naturelles, Zoologie, 11, 191–196.
Elinder, C. G., Friberg, L., Kjellström, T., Nordberg, G., & Oberdoerster, G. (1994). Biological monitoring of metals. Chemical safety monographs. Geneva: World Health Organization.
Flyaks, N. L., & Borkin, L. J. (2004). Morphological abnormalities and heavy metal concentrations in anurans of contaminated areas, Eastern Ukraine. Applied Herpetology, 1, 229–264.
Hartel, T., & Nemes, Sz. (2006). Assessing the effect of toe clipping on Yellow Bellied Toads. Acta Zoologica Academiae Scientiarum Hungaricae, 52, 359–366.
Herkovits, J., & Helguero, L. A. (1998). Copper toxicity and copper–zinc interactions in amphibian embryos. Science of the Total Environment, 221, 1–10.
Icochea, J., Quispitupac, E., Portilla, A., & Ponce, E. (2002). Framework for assessment and monitoring of amphibians and reptiles in the lower Urubamba Region, Peru. Environmental Monitoring and Assessment, 76, 55–67.
Janus, A. M., Faryna, M., Haberko, K., Rakowska, A., & Panz, T. (2008). Chemical and microstructural characterization of natural hydroxyapatite derived from pig bones. Microchimica Acta, 161, 349–353.
Klepinger, L. L. (1984). Nutritional assessment from bone. Annual Review of Anthropology, 13, 75–96.
Loumbourdis, N. S., Kostaropoulos, I., Theodoropoulou, B., & Kalmanti, D. (2007). Heavy metal accumulation and methallothionein concentration in the frog Rana ridibunda after exposure to chromium or a mixture of chromium and cadmium. Environmental Pollution, 145, 787–792.
McCarthy, M. A., & Parris, K. M. (2004). Clarifying the effect of toe clipping on frogs with Bayesian statistics. Journal of Applied Ecology, 41, 780–786.
Noonan, B. P., & Gaucher, P. (2006). Refugial isolation and secondary contact in the dyeing poison frog Dendrobates tinctorius. Molecular Ecology, 15, 4425–4435.
Oudadesse, H., Martin, S., Derrien, A. C., Lucas-Girot, A., Cathelineau, G., & Blondiau, G. (2004). Determination of Ca, P, Sr and Mg in the synthetic biomaterial aragonite by NAA. Journal of Radioanalytical and Nuclear Chemistry, 262, 479–483.
Pahkala, M., Rasanen, K., Laurila, A., Johanson, U., Björn, L. O., & Merila, J. (2002). Lethal and sublethal effects of UV-B/pH synergism on common frog embryos. Conservation Biology, 16, 1063–1073.
Pahkala, M., Laurila, A., & Merila, J. (2003). Effects of ultraviolet-B radiation on behaviour and growth of three species of amphibian larvae. Chemosphere, 51, 197–204.
Pavel, J., & Kucera, M. (1986). Cumulation of heavy metals in frog. Ekológia, 5, 431–440.
Pounds, J. A. (2001). Climate and amphibian declines. Nature, 410, 639–640.
Pounds, J. A., Bustamante, M. R., Coloma, L. C., Consuegra, J. A., Michael, P. L., Fogden, M. P. L., et al. (2006). Widespread amphibian extinctions from epidemic disease driven by global warming. Nature, 439, 161–167.
Puky, M., & Oertel, N. (1997). On the protective role of maternal organism in amphibians. Opusculla Zoologica, 29–30, 125–132.
Rowe, C. L., Kinney, O. M., Fiori, A. P., & Congdon, J. D. (1996). Oral deformities in tadpoles (Rana catesbeiana) associated with coal ash deposition: Effects on grazing ability and growth. Freshwater Biology, 36, 723–730.
Rowe, C. L., Kinney, O. M., & Congdon, J. D. (1998). Oral deformities in tadpoles of the bullfrog (Rana catesbeiana) caused by conditions in a polluted habitat. Copeia, 1998, 244–246.
Rowe, C. L., Hopkins, W. A., & Coffman, V. R. (2001). Failed recruitment of southern toads (Bufo terrestris) in a trace element-contaminated breeding habitat: Direct and indirect effects that may lead to a local population sink. Archives of Environmental Contamination & Toxicology, 40, 399–405.
Sparling, D. W., Linder, G., Bishop, C. A. (Eds.) (2000). Ecotoxicology of amphibians and reptiles. Pensacola, FL: Society of Environmental Toxicology and Chemistry (SETAC). 904 p.
Stolyar, O. B., Loumbourdis, N. S., Falfushinska, H. I., & Romanchuk, L. D. (2008). Comparison of metal bioavailability in frogs from urban and rural sites of Western Ukraine. Archives of Environmental Contamination and Toxicology, 54, 107–113.
Wang, Y., Shen, Z., & Moisevich, S. G. (2001). Strontium hydrogeochemistry of thermal groundwaters from Baikal and Xinzhou. Science in China (Series E), 44, 138–143.
Zar, J. H. (1996). Biostatistical analysis. NJ: Prentice Hall.
Acknowledgement
We are grateful to an anonymous reviewer for the proposals in improving the manuscript. The Hertelendi Laboratory of Environmental Studies (Hungarian Academic of Science, ATOMKI, Debrecen) and ANALAB Ltd. provided the instrumental background to the studies presented in this contribution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simon, E., Braun, M. & Tóthmérész, B. Non-destructive Method of Frog (Rana esculenta L.) Skeleton Elemental Analysis Used During Environmental Assessment. Water Air Soil Pollut 209, 467–471 (2010). https://doi.org/10.1007/s11270-009-0214-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0214-6