Abstract
For more than a decade, Lakeland, FL, has invested in restoring its urban Lake Hollingsworth from a hypereutrophic state to its natural eutrophic state. The lake bottom was dredged of nearly 2 million m3 of accumulated organic sediments, and treatment wetlands, storm water curb inlet strainers, and a storm water baffle box were installed within the lake’s catchment area to reduce the loading of dirt, leaves, and trash to the lake. After dredging ceased, the lake was dosed one time with alum to improve water clarity and reduce phosphorus recycling from its sediments. Water quality surrogates for algal biomass— Secchi disk transparency and water column total nitrogen, total phosphorus, and chlorophyll-α concentrations— were reviewed to assess Lakeland’s progress towards its goal. In the years since dredging has stopped, algal biomass concentration in Lake Hollingsworth has significantly declined. Even with these improvements, however, the lake still remains hypereutrophic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
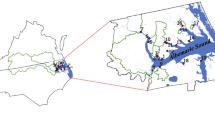
Lake Hollingsworth is a 144-ha lake in Lakeland, Polk County, FL, USA (Fig. 1) with a mean postdredging depth of 2.7 m (Harper and Medley 2004) and a reference stage of 40 m above mean sea level. For more than 100 years, this lake has been popular with residents and tourists for a range of activities including water sports and bird watching. During the twentieth century as the lake’s surrounding 668-ha catchment developed, storm water collected from streets and parking lots was discharged through pipes directly to Lake Hollingsworth. Organic-rich sediments accumulated on the lake’s floor and recurring nutrient inputs and resuspension caused frequent algal blooms and loss of water clarity (Harper and Medley 2004).
Lake Hollingsworth, Lakeland, FL (latitude 28°01′26″, longitude −081°56′42″). The US Census (2009) reported that in 2007, the estimated population of Lakeland was 92,796 persons
Lake Hollingsworth is classified as hypereutrophic based on average Secchi disk (SD) transparency and on concentrations of total nitrogen (TN), total phosphorus (TP), and chlorophyll-α (CHLA; Forsberg and Ryding 1980). Since 1995, the City of Lakeland has implemented control measures intended to reduce nutrient inputs to the lake and suppress nutrient recycling within the lake (Table 1). We examined the interim impact of these lake management efforts on lake trophic state.
Lake Hollingsworth is located on Lakeland Ridge, an ancient sand dune that once protruded from a sea but today divides the Hillsborough River basin to the west from the Peace River basin to the east (Martin 1998; Sacks et al. 1998). To the west and south of Lakeland Ridge extends Bone Valley, a fossil-rich formation of ∼518,000 ha that lies ∼10 m below the surface, which is extensively mined for its phosphate (Hoppe 2005). Lake Hollingsworth is typical of a ridge lake in mantled karst topography, and as the nearly round perimeter of the lake suggests, it was created from a sinkhole (Sacks et al. 1998). The lake is underlain by unconsolidated phosphate-rich sands and clay.
Lake Hollingsworth receives inflow from two upstream lakes: Lake Horney and, during heavy rainfall events, Lake Morton (Sacks et al. 1998). Water is discharged from Lake Hollingsworth via Banana Creek and Banana Lake to Lake Hancock and from Lake Hancock via Lower Saddle Creek to the Peace River. The 1,800-ha Lake Hancock and its 34,000-ha watershed forms the headwaters of the Peace River, which flows to 190 km to the southwest and empties into the Charlotte Harbor estuary at the Gulf of Mexico (see FL Department of Environmental Protection (FDEP) 2007 for maps). Lakes within the Lake Hancock watershed, including Lake Hollingsworth, Banana Lake, and Lake Hancock, are classified by FDEP for designated uses that include “recreation, propagation, and maintenance of healthy well-balanced population of fish and wildlife in fresh water” but are listed as “verified impaired” for nutrients (FDEP 2005). Reductions of water column nutrient concentrations in Lake Hollingsworth were part of a broader management strategy for restoring the ecosystem of the Peace River basin and ultimately the health and productivity of the Charlotte Harbor estuary (FDEP 2007).
Tables 2 and 3 summarize water quality indicators for Lake Hollingsworth. Alkalinity, conductivity, and pH were substantially higher for lake water than in water from the surficial aquifer for samples taken from wells near the lake (Sacks et al. 1998). Concentrations of bioavailable forms of nitrogen, e.g., ammonia, nitrite, and nitrate nitrogen were low, but organic nitrogen was high. Concentrations of organic nitrogen were on the average 99% of the total nitrogen. Lake water conditions were consistent with phytoplankton-dominated photosynthesis that consumes available inorganic nitrogen and carbon, increases pH, and thus stimulates phosphorus release from sediments (Reynolds 1998; Hullebusch et al. 2003; Xie et al. 2003).
Warm, shallow, poorly buffered, and phosphate-rich lake waters, low nitrogen to phosphorus ratios, and the presence of organic-rich sediments had favored growth of cyanobacteria in Lake Hollingsworth. Smith (1983) suggested that cyanobacteria out-compete other algal species when water column TN/TP <29.
In freshwater, the type and distribution of algae, intensity of algal blooms, and production of toxins depend either directly or indirectly on nutrient and light levels, temperature, and possibly metal concentrations (de Figueiredo et al. 2004; Camargo and Alonso 2006). Species of cyanobacteria, e.g., Microcystis and Anabaena, are known to release toxins that pose a health threat to both humans and animals (de Figueiredo et al. 2004; Camargo and Alonso 2006), although the risk posed by these toxins is not well characterized. Bigham (2008) reported that a small percentage of water samples drawn from hypereutrophic FL lakes contained the toxin microcystin above a concentration of 20 µg l−1, which is a World Health Organization guideline concentration based on a moderate adverse health risk during recreational contact.
Brenner et al. (1999) presented evidence from stable isotope ratios, radioactive isotope dating, and diatom composition in lake sediment cores that prior to the twentieth century, Lake Hollingsworth was eutrophic and possibly macrophyte-dominated. Algal pigments in lake sediments cores revealed that blooms of cyanobacteria likely occurred prior to the 1900s; however, peak concentrations appeared between 1960 and 1990 (Riedinger-Whitmore et al. 2005). Further nutrient enrichment of Lake Hollingsworth evolved after 1930 as land use shifted from citrus groves to residential and urban development, an inference based on sustained increases in upper sediment core layers of total chlorophyll concentration, diatom-marked trophic state, and δ13C of organic matter (Riedinger-Whitmore et al. 2005).
Building on the work of Brenner et al. (1999), Paul and Gerritsen (2002) developed nutrient criteria for FL lakes based not only on paleolimnological surveys but also on statistical distributions of water column concentrations, trophic lake condition indices, and multiple linear regression analyses of trophic state parameters including total nitrogen, total phosphorus, chlorophyll-α, and Secchi disk transparency. Nutrient criteria were based on one of five lake classifications, and for an alkaline-colored lake such as Lake Hollingsworth (Tables 2 and 3), total nitrogen and total phosphorus criteria, respectively, were 1,100 and 40 µg l−1, which corresponded to eutrophic conditions as defined by Forsberg and Ryding (1980; Table 4).
2 Methods and Materials
Observations of trophic state parameters including Secchi disk transparency, chlorophyll-α, total nitrogen, and total phosphorus were obtained from the Polk County Water Atlas (USF 2009). For these parameters, the study dataset combined the data records (i.e., sampling dates) from 1984 to 2009 that were reported by three different entities: LAKEWATCH (2001–2008, N = 32), Polk County (1984–2009, N = 73), and City of Lakeland (1984–2009, N = 205). A few records had missing data, and no data qualifiers were present in the dataset.
LAKEWATCH, Polk County, and City of Lakeland sampling and analyses differed on some protocols. LAKEWATCH methods for sampling and analyses are described by Canfield et al. (2002). Sampling frequencies varied within and between agencies, but one or more agencies sampled water from Lake Hollingsworth at least once per quarter from 1988 to present. In general, all three agencies collected water samples by boat during daylight hours at multiple (e.g., three to five) midlake middepth locations. For each sampling date and parameter, the reported value was the average value of measurements from samples taken at the several stations. Secchi disk measurements were made in accordance with a FDEP protocol (FDEP 2004).
For total phosphorus and total Kjeldahl nitrogen (TKN) analyses, Polk County and Lakeland collected water samples in high density polyethylene (HDPE) 500-ml bottles rinsed with lake water. Samples were preserved with sulfuric acid and returned to the laboratory on ice. Analyses of total phosphorus and total Kjeldahl nitrogen were by US Environmental Protection Agency (US EPA) methods 365.4 and 351.2, respectively (US EPA 1974, 1993b). Polk County and Lakeland collected water for ammonia nitrogen, nitrite/nitrate nitrogen, and chlorophyll-α analyses in a 2-l HDPE bottle and a 1-l amber Nalgene bottle, respectively, without preservation. Bottles were rinsed with lake water before sampling. Samples were returned to the laboratory on ice. Measurement of ammonia nitrogen and nitrite/nitrate nitrogen in lake water samples were by US EPA methods 350.1 and 353.2, respectively (US EPA 1993a, c). Total nitrogen was calculated as the sum of total Kjeldahl nitrogen and nitrate/nitrite-nitrogen. All agencies determined chlorophyll-α in lake water samples by standard method 10200 H (APHA 1992). Polk County corrected chlorophyll-α concentrations for pheophytin-α concentrations while LAKEWATCH and Lakeland did not.
Table 3 provides summary statistics for each of the trophic state parameters and histograms of parameter data are shown in Fig. 2. A one-sample Kolmogorov–Smirnov test at the 95% confidence level confirmed that parameter observations were not normally distributed. Log-transformed observations were, however, normally distributed (Fig. 3). Outlier analyses and statistical testing were accomplished with log-transformed distributions.
Nonlinear relationships between SD transparency and CHLA, TP, and TN concentrations are shown in Figs. 4, 5, and 6. Pearson product moment correlation coefficients for log-transformed data were r = −0.87, −0.54, and −0.85 between Secchi disk transparency and CHLA, TP, and TN concentrations, respectively. Trophic state parameters agreed reasonably well between sampling entities Lakeland, LAKEWATCH, and Polk County, with two notable exceptions: Lakeland’s total phosphorus concentrations between 1997 and 2005 and LAKEWATCH’s chlorophyll-α concentrations between 2002 and 2004 diverged from general trends, as can be seen in Fig. 7.
To identify possible outliers, “studentized residuals” were calculated in SYSTAT® for a least squares fit of a linear model to log-transformed data of paired parameters (e.g., SECCHI versus CHLA, SECCHI versus TP, SECCHI versus TN, CHLA versus SECCHI, CHLA versus TP, CHLA versus TN, etc.). Observations with a studentized residual >|3| were removed as outliers and the process was repeated until no observations were flagged as outliers by this method. A total of 12 outliers were removed by this method: two outliers of Secchi disk transparency, three of chlorophyll-α, one of total phosphorus, and six of total nitrogen concentrations.
For hypothesis testing, our study dataset was divided into two periods: 1984–2001 and 2002–2009, as all of the dredging occurred before 2002 and much of the storm water capture and treatment equipment was installed between 2001 and 2002 (Table 1). Our study’s null hypothesis was that no change in the mean algal biomass concentration occurred between the two periods. The alternate hypothesis was that mean algal mass biomass changed between the two periods. We used a student’s two-sided t test assuming unequal variances on log-transformed observations to test the null hypothesis at the 95% confidence level. Removal of outliers did not change either the direction or significance of the hypothesis testing results.
Carlson (1977) developed a trophic state index (TSI) on the premise that a doubling of algal biomass defined a change in trophic state. His TSI for Secchi disk transparency (TSIS; Eq. 1) was based on the range over which transparency was measured and scaled such that a 10-unit change would reflect a change in trophic state. He then expanded this concept to two other surrogates for algal biomass, chlorophyll-α and total phosphorus concentrations, by replacing log-transformed Secchi disk transparency in Eq. 1 with an equation derived from a least squares fit of a linear model to log-transformed data, e.g., chlorophyll-α versus Secchi disk transparency and total phosphorus versus Secchi disk transparency, as shown in Table 5. Kratzer and Brezonik (1981) expanded this series to include total nitrogen.
Carlson (1977) recognized that none of the algal biomass surrogates were a perfect measure of algal biomass. For example, along with algal biomass concentration, a lake’s water color or suspended nonalgal particle burden could attenuate light, in which case the Secchi disk transparency (TSIS) would overestimate the trophic state. In this study, Carlson’s steps in TSI development are repeated with trophic state parameter data from Lake Hollingsworth (Figs. 4, 5, and 6; Table 5) to improve inferences regarding limiting nutrients.
3 Results and Discussion
Secchi disk transparency increased, and chlorophyll-α, total nitrogen, and total phosphorus average concentrations decreased over the 25-year study period (Table 6; Fig. 7). Moreover, for chlorophyll-α, total nitrogen, and total phosphorus concentrations, the amplitude of annual cycling also declined (Table 6; Fig. 7). For each of these surrogate measures of algal biomass, changes in mean transparency or concentration between the two periods were statistically significant (Fig. 3). Trophic state parameters, however, were still in the range of hypereutrophic as defined by Forsberg and Ryding (1980; Table 5). Nutrient criteria of 1,100 and 40 µg l−1 for total nitrogen and total phosphorus appropriate for an alkaline and colored lake (Paul and Gerritsen 2002), respectively, had yet to be sustained in Lake Hollingsworth.
Pre- and post-dredging, annual cycling of algal biomass is evident from time series plots (Fig. 7). For Secchi disk transparency, transparency minima and maxima occurred in March through May and July through October, respectively, and for chlorophyll-α and total nitrogen concentrations, minima and maxima correspondingly occurred in July through October and March through May, respectively. Such cycling is consistent with higher springtime temperatures and dilution effects or higher flushing rates from rainfall during summer and early fall. Annual cycling of total phosphorus concentrations was not as strongly evident, possibly obscured by wind-induced resuspension of lake sediment (Bachmann et al. 2000). Brown et al. (1998) did not find a compelling seasonal cycle for trophic state indicators across FL’s hypereutrophic lakes.
From 2000 to 2001, an extended drought caused water levels in Lake Hollingsworth to fall by 2 m and dredging that had begun in 1997 was stopped. Storm water controls were put into place in 2001 (Table 1) and by the end of 2002 when lake levels returned to normal, trophic state parameters responded to a recontoured lake and reduced nutrient loading (Fig. 7). The effect of a one-time alum addition in December 2003 was evident in the correspondingly high Secchi disk transparency and low chlorophyll-α concentration and, to a lesser extent, low total nitrogen and total phosphorus concentrations observed in January 2004 (Fig. 7). This suggested that alum was effective over the short term in removing suspended algal biomass but in the highly productive environment not at permanently binding phosphorus in the sediments (Hullebusch et al. 2003).
Figure 8 shows the change in TSIs pre- and post-dredging. According to Carlson, a lake with a TSI of 50 is mesotrophic and 60 eutrophic; presumably, a lake with a TSI of 70 is hypereutrophic. Note that when Carlson’s (1977) TSI for chlorophyll-α (TSIC) and TSI for phosphorus (TSIP) and Kratzer and Brezonik’s (1981) TSI for nitrogen (TSIN) were used, the results show a 10-unit change or by Carlson’s definition a change in trophic state. Such a large change was not seen when TSIs produced from regressions of Lake Hollingsworth data were used (Figs. 4, 5, and 6). The latter results were more consistent with transparency and concentration data (Table 6).
Change in trophic state index (TSI) pre- and postdredging of Lake Hollingsworth, Lakeland, FL. In both graphics a and b, TSIS is calculated according to Carlson (1977). In graphic a, TSIC and TSIP are calculated according to Carlson (1977) and TSIN according to Kratzer and Brezonik (1981); in graphic b, TSIC, TSIP, and TSIN were determined by a least squares fit of a linear model to log-transformed paired data from Lake Hollingsworth
Both nitrogen and phosphorus appeared to limit primary production in Lake Hollingsworth, a preliminary conclusion that is consistent with previous assessments for shallow hypereutrophic FL lakes (Canfield 1983; Havens 1995). Water column chlorophyll-α in Lake Hollingsworth was compared with average and maximum concentrations seen for FL lakes (Fig. 9). According to Brown et al. (2000), average and maximum chlorophyll concentrations can be predicted from total phosphorus concentrations by Eqs. 2 and 3. Chlorophyll (e.g., biomass) production is not limited by phosphorus alone if observations plot below the predicted maximum chlorophyll line, as shown in Fig. 9, although factors other than nitrogen or phosphorus—temperature, light intensity, carbon (e.g., dissolved carbon dioxide), flushing or mixing rate, and predation by zooplankton, as examples—can limit algal growth (Reynolds 1998).
Chlorophyll-α versus total phosphorus concentration in Lake Hollingsworth. Predicted average and maximum chlorophyll-α concentrations were based on an analysis of FL lakes by Brown et al. (2000)
Temporal plots of TSIN–TSIP and TSIC–TSIP (Fig. 10) revealed time-dependent changes in limiting nutrients, as was discussed by Havens (1995) for a subtropical nitrogen-limited lake and validated for that lake with nutrient bioassay experiments. The temporal trend of TSIN–TSIP suggested that phosphorus limitation occurred through 1997 with a shift toward nitrogen limitation after 1997, the trend of TSIC–TSIS that a light limitation abated after 1997. This apparent shift in limiting factors is a likely consequence of the removal by dredging of ∼1 m of organic layer of sediment from the lake’s bottom. The deepened lake is now less susceptible to wind-induced sediment resuspension (Bachmann et al. 2000).
Table 7 shows average concentrations of chlorophyll-α for combinations total nitrogen and total phosphorus concentrations above and below their median concentrations. The highest average chlorophyll-α concentration occurred when both total nitrogen and total phosphorus concentrations were high.
A plot of log-transformed total nitrogen versus log-transformed total phosphorus is presented in Fig. 11, along with TN/TP ratios identified by Forsberg and Ryding (1980) and Sakamoto (1966, cited therein) as thresholds for nitrogen (TN/TP <10) and phosphorus limiting (TN/TP >17) algal growth. TN/TP ratios observed in Lake Hollingsworth ranged from 4.32 to 50.7, with an average of 17.1, and spanned both phosphorus- and nitrogen-limiting conditions. Use of such ratios, however, oversimplifies the nutrient–chlorophyll relationship in a lake system (Smith 1983; Reynolds 1998; Lewis and Wurtzbaugh 2008). Phytoplankton thrive at optimal nitrogen to phosphorus ratios as low as 4.1 for Microcystis species and as high as 38 for Scenedesmus species (Smith 1982), and the distribution of phytoplankton species within a lake may depend not only on the immediate shortage of one or more growth factors but on historical dominance (Reynolds 1998). Moreover, measures of total nitrogen and total phosphorus include both bioavailable and refractory compounds and offer no information regarding the time-dependent cycling between soluble and insoluble, organic and inorganic, fractions (Lewis and Wurtzbaugh 2008).
Can Lake Hollingsworth switch from a turbid state to a clear state? It is difficult to predict if and under what conditions such a switch could occur (Beisner et al. 2003). Observations and modeling reinforce the theory that shallow lakes may have two stable states: a turbid phytoplankton-dominated state and a clear macroplankton-dominated state (Moss 1990; Blindow et al. 1993; Scheffer et al. 1993). Data from subtropical FL lakes analyzed by Bachmann et al. (2002) generally support the theory that shallow lakes have both a turbid and a stable state, but stop short of defining a critical turbidity threshold. Altering the number and type of biota in a lake—biomanipulation—has been proffered as a way to catalyze the change from one stable state to another (Moss 1990; Hilt et al. 2006; Jeppesen et al. 2007). Reducing nutrient loads to a lake can reduce turbidity, but reestablishing submerged aquatic vegetation may be necessary for a shallow lake to reach and maintain its clear state (Moss 1990; Scheffer et al. 1993; Bachmann et al. 2002). Blindow et al. (1993) postulated that lakes may have a third, labile or transition, state that is dominated by angiosperms (such as hydrilla). Submerged aquatic macrophytes appear to play a key role in maintaining water clarity, as examples, they serve as a nutrient reservoir, reduce sediment resuspension, and provide protection for algae-eating zooplankton against predators (Blindow et al. 1993; Jeppesen et al. 2007; Takamura et al. 2003).
4 Conclusions
Strategies employed to improve the water clarity of Lake Hollingsworth included substantial reductions in external nutrient loading by capturing influent detritus, suppressing internal cycling of nutrients by removing organic sediments, replanting submerged and emergent aquatic vegetation, and settling suspended particles including algal cells through alum addition (Table 1). These strategies straddled dramatically lower lake water levels during a 2-year drought.
The trophic state of Lake Hollingsworth in Lakeland, FL, has significantly improved in response to reductions in both external and internal loading of phosphorus and nitrogen. Lake water quality data indicated that algal growth is limited by both phosphorus and nitrogen but further reductions in external nutrient loading may be important for the lake to return to its natural state.
References
APHA. (1992). Standard methods for the examination of water and wastewater (18th ed.). Washington, D.C.: American Public Health Association. 1100 pp.
Bachmann, R. W., Hoyer, M. V., & Canfield, D. E., Jr. (2000). The potential for wave disturbance in shallow Florida lakes. Lake and Reservoir Management, 16, 281–291.
Bachmann, R. W., Horsburgh, C. A., Hoyer, M. V., Mataraza, L. K., & Canfield, D. E., Jr. (2002). Relations between trophic state indicators and plant biomass in Florida lakes. Hydrobiologia, 470, 219–234.
Beisner, B. E., Haydon, D. T., & Cuddington, K. (2003). Alternative stable states in ecology. Frontiers in Ecology and the Environment, 1, 376–382.
Bigham, D. (2008). Toxic algae: should Floridians be worried? Florida LAKEWATCH, 42, 1–3.
Blindow, I., Andersson, G., Hargeby, A., & Johansson, S. (1993). Long-term pattern of alternative stable states in two shallow eutrophic lakes. Freshwater Biology, 30, 159–167.
Brenner, M., Whitmore, T., Curtis, J., Hodell, D., & Schelske, C. (1999). Stable isotope (δ13C and δ15N) signatures of sedimented organic matter indicators of historic lake trophic state. Journal of Paleolimnology, 22, 205–221.
Brown, C. D., Canfield, D. E., Jr., Bachmann, R. W., & Hoyer, M. V. (1998). Seasonal patterns of chlorophyll, nutrient concentrations, and Secchi disk transparency in Florida lakes. Journal of Lake and Reservoir Management, 14, 60–76.
Brown, C. D., Hoyer, M. V., Bachmann, R. W., & Canfield, D. E., Jr. (2000). Nutrient–chlorophyll relationships: an evaluation of empirical nutrient–chlorophyll models using Florida and north-temperate lake data. Canadian Journal of Fisheries and Aquatic Science, 87, 1574–1583.
Camargo, J., & Alonso, A. (2006). Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environment International, 32, 831–849.
Canfield, D. E. (1983). Prediction of chlorophyll a concentrations in Florida lakes: the importance of phosphorus and nitrogen. Water Resources Bulletin, 19, 255–262.
Canfield, D., Brown, C., Bachmann, R., & Hoyer, M. (2002). Volunteer lake monitoring: testing the reliability of data collected by the Florida LAKEWATCH program. Lake and Reservoir Management, 18, 1–9.
Carlson, R. E. (1977). A trophic state index for lakes. Limnology and Oceanography, 22, 361–369.
De Figueiredo, D., Azeiteiro, U., Esteves, S., Gonçalves, F., & Pereira, M. (2004). Microcystin-producing blooms—a serious global public health issue. Ecotoxicology and Environmental Safety, 59, 151–163.
FDEP. (2004). Field measurement of light penetration (Secchi Depth and Transparency). DEP-SOP-001/01 FT 1700, Florida Department of Environmental Protection, ftp://ftp.dep.state.fl.us/pub/labs/assessment/sopdoc/2004sops/ft1700.pdf, 9 pp.
FDEP. (2005). Total maximum daily loads, adopted verified lists of impaired waters for the group 3 basins, Sarasota–Peace–Myakka Rivers group 3 basins, verified list. Florida Department of Environmental Protection, http://www.dep.state.fl.us/water/tmdl/docs/303d/group3/adopted/Sara_VerifiedFinal.pdf, 12 pp.
FDEP. (2007). Peace river basin resource management plan. Florida Department of Environmental Protection, ftp://ftp.dep.state.fl.us/pub/minerec/peaceriver/FinalPeaceRiverManagementPlan/, 102 pp.
Forsberg, C., & Ryding, S.-O. (1980). Eutrophication parameters and trophic state indices in 30 Swedish waste-receiving lakes. Archiv für Hydrobiologie, 89, 189–207.
Harper, H., & Medley, G. (2004). Lake Hollingsworth alum treatment. Proceedings of the 15th Annual Conference of the Florida Lake Management Society, June 7–10, 2004, Saddlebrook Resort, Tampa, Florida, 5A 11–12.
Havens, K. E. (1995). Secondary nitrogen limitation in a subtrophical lake impacted by non-point source agricultural pollution. Environmental Pollution, 89, 241–246.
Hilt, S., Gross, E., Hupfer, M., Morscheid, H., Mählmann, J., Melzer, A., et al. (2006). Restoration of submerged vegetation in shallow eutrophic lakes—a guideline and state of the art in Germany. Limnologica, 36, 155–171.
Hoppe, M. K. (2005). Phosphate at the crossroads: a special report. Bay Soundings, 4, 2005, available at http://www.baysoundings.com/sum05/index.html.
Hullebusch, E., Auvray, F., Deluchat, V., Chazal, P., & Baudu, M. (2003). Phosphorus fractionation and short-term mobility in the surface sediment of a polymictic shallow lake treated with a low dose of alum (Courtille Lake, France). Water, Air, and Soil Pollution, 146, 75–91.
Jeppesen, E., Søndergaard, M., Meerhoff, M., Lauridsen, T., & Jensen, J. (2007). Shallow lake restoration by nutrient loading reductions—some recent findings and challenges ahead. Hydrobiologia, 584, 239–252.
Kratzer, C., & Brezonik, P. (1981). A Carlson-type trophic state index for nitrogen in Florida lakes. Water Resources Bulletin, 17, 713–715.
Lakeland. (2005). City of Lakeland stormwater utility overview and status report. Public Works Department, Lakes & Stormwater Division, available at http://www.lakelandgov.net/publicworks/lakes/reports.html, April 2005.
Lakeland. (2009). City of Lakeland, public works—lakes and stormwater division. Current Capital Project Updates, Lake Hollingsworth Westside Regional Stormwater Treatment Project, available at http://www.lakelandgov.net/publicworks/lakes/reports.html, March 2009.
Lewis, W. M., Jr., & Wurtsbaugh, W. A. (2008). Control of lacustrine phytoplankton by nutrients: erosion of the phosphorus paradigm. International Review of Hydrobiology, 93, 446–465.
Martin, T. (1998). Florida's ancient islands. The Lake Wales Ridge Ecosystem Working Group, Archbold Biological Station, available at http://www.archbold-station.org/fai/index.html.
Moss, B. (1990). Engineering and biological approaches to the restoration from eutrophication of shallow lakes in which aquatic plant communities are important components. Hydrobiologia, 200(201), 367–377.
Paul, M., & Gerritsen, J. (2002). Nutrient criteria for Florida lakes: A comparison of approaches. Tetra Tech, Inc., Owings Mill, Maryland, prepared for Florida Department of Environmental Protection, Tallahassee, Florida, December 2009, 78 pp.
Reynolds, C. S. (1998). What factors influence the species composition of phytoplankton in lakes of different trophic status? Hydrobiologia, 369(370), 11–26.
Riedinger-Whitmore, M., Whitmore, T., Smoak, J., Brenner, M., Moore, A., Curtis, J., et al. (2005). Cyanobacterial proliferation is a recent response to eutrophication in many Florida lakes: A paleolimnological assessment. Lake and Reservoir Management, 21, 423–435.
Sacks, L., Swancar, A., & Lee, T. (1998). Estimating ground-water exchange with lakes using water-budget and chemical mass-balance approaches for ten lakes in ridge areas of Polk and Highlands Counties, Florida. U.S. Geological Survey, Water Resources Investigations Report 98-4133. Tallahassee, Florida, 52 pp.
Sakamoto, M. (1966). Primary production by phytoplankton communities in some Japanese lakes and its dependence on lake depth. Archiv für Hydrobiologie, 62, 1–28.
Scheffer, M., Hosper, S., Meijer, M.-L., Moss, B., & Jeppesen, E. (1993). Alternative equilibria in shallow lakes. Trends in Ecology and Evolution, 8, 275–279.
Smith, V. H. (1982). The nitrogen and phosphorus dependence of algal biomass in lakes: an empirical and theoretical analysis. Limnology and Oceanography, 27, 1101–1112.
Smith, V. H. (1983). Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science, 221, 669–671.
Takamura, N., Kadono, Y., Fukushima, M., Nakagawa, M., & Kim, B.-H. (2003). Effects of aquatic macrophytes on water quality and phytoplankton communities in shallow lakes. Ecological Research, 18, 381–395.
US Census. (2009). US Census Bureau, Population Division, Population Estimates, Cities and Towns, 2000–2007, http://www.census.gov/popest/cities/SUB-EST2007-4.html, accessed June 10, 2009.
US EPA. (1974). Phosphorous, total (Colorimetric, Automated, Block Digester AA II). http://www.epa.gov/waterscience/methods/method/files/365_4.pdf, 5 pp.
US EPA. (1993a). Method 350.1, Rev. 2, Determination of ammonia nitrogen by semi-automated colorimetry. In J. sO’Dell (Ed.), Inorganic Chemistry Branch, Chemistry Research Division, http://www.epa.gov/waterscience/methods/method/files/350_1.pdf, 15 pp.
US EPA. (1993b). Method 351.2, Rev. 2, Determination of total Kjeldahl nitrogen by semi-automated colorimetry. In J. O’Dell (Ed.), Inorganic Chemistry Branch, Chemistry Research Division, http://www.epa.gov/waterscience/methods/method/files/351_2.pdf, 15 pp.
US EPA. (1993c). Method 353.2, Rev. 2, Determination of nitrate–nitrite nitrogen by automated colorimetry. In J. O’Dell (Ed.), Inorganic Chemistry Branch, Chemistry Research Division, http://www.epa.gov/waterscience/methods/method/files/353_2.pdf, 14 pp.
USF. (2009). Polk County Water Atlas. http://www.polk.wateratlas.usf.edu/, accessed June 2, 2009.
Xie, L., Xie, P., & Tang, H. (2003). Enhancement of dissolved phosphorus release from sediment to lake water by Microcystis blooms—an enclosure experiment in a hyper-eutrophic, subtropical Chinese lake. Environmental Pollution, 122, 391–399.
Acknowledgments
We appreciate the help provided by the City of Lakeland Lakes and Storm Water Division, the City of Lakeland Glendale Wastewater Treatment Plant Laboratory, the University of Florida Institute of Food and Agricultural Science, Polk County Extension, and the Polk County Board of County Commissioners Environmental Management Department. This research was funded in part by Chastain-Skillman, Inc. of Lakeland, FL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poor, N.D. Effect of Lake Management Efforts on the Trophic State of a Subtropical Shallow Lake in Lakeland, Florida, USA. Water Air Soil Pollut 207, 333–347 (2010). https://doi.org/10.1007/s11270-009-0140-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0140-7