Abstract
Macroinvertebrates have been widely used in freshwater ecosystems as surrogates to assess the impacts of waste discharges and water pollution. However, often interpretations have been made on the impact of one pollutant in the presence of others that may provide an unidentified additive effective or otherwise confound the results. There have been few opportunities to study the impact of pollutants without such potentially confounding effects. We studied macroinvertebrates using a replicated kick sampling technique and identified to the family level to assess and compare the effects of zinc-rich coal-mine waste and organic pollution from treated sewage on an otherwise clean upland stream network within a world heritage area. We used multivariate analysis of macroinvertebrate assemblages from polluted and clean sites to measure and compare the effect of each waste impact to community structure. We also calculated three widely used biotic indices (Ephemeroptera, Plecoptera and Trichoptera (EPT) family richness, family richness, and abundance) and found that the EPT index was the only one to respond to both pollution types. Macroinvertebrate abundance was an important attribute of the study, with each source of pollution having a contrasting effect on total abundance. It also helped us to measure the relative response of families to each pollutant. There was an initial significant modification of macroinvertebrate assemblages below the outflow of each of the pollutants, followed by different degrees of recovery downstream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Macroinvertebrates are widely regarded as one of the best indicators of the ecological condition of rivers and streams (Hynes 1960; Hellawell 1986; Rosenberg and Resh 1993; Metzeling et al. 2006). They have been used to assess impacts of different types of water pollution, including sewage wastes (Jolly and Chapman 1966; Pinder and Far 1987; Cosser 1988; Whitehurst and Lindsey 1990; Growns et al. 1995; Wright et al. 1995), mine drainage (Winner et al. 1975; Norris et al. 1982; Mackey 1988; Malmqvist and Hoffsten 1999; Sloane and Norris 2003), urban landuses (Chessman and Williams 1999; Walsh et al. 2001; Gresens et al. 2007) and forestry activities (McCord et al. 2007). However, detailed investigations of freshwater macroinvertebrates have demonstrated the difficulty of isolating the effects of the target impact (e.g. sewage waste, mine drainage pollution) from other disturbances generated by human activities in often highly modified hydrological catchments.
There have been a large number of biotic indices developed to help interpret stream macroinvertebrate results from biological assessment of waterways. Two of the most popular and simply calculated indices are taxa richness and total abundance (see Resh and Jackson 1993). Abundance is often ignored due to the proliferation of qualitative rapid assessment methodologies (e.g. Lenat 1988). The Ephemeroptera, Plecoptera and Trichoptera (EPT) richness index is one of the most widely used biotic indices, based on the taxonomic richness of three common and sensitive macroinvertebrate orders (Lenat 1988; Lenat and Penrose 1996). The EPT index has been widely reported to be a robust and effective index for measuring impairment to stream macroinvertebrates (e.g. Sheehan 1984; Plafkin et al. 1989; Barbour et al. 1992; Hickey and Clements 1998; Camargo et al. 2004; Kitchin 2005; Metzeling et al. 2006). Other biotic indices have been developed, such as the Australian SIGNAL and SIGNAL2 pollution tolerance indices (Chessman 1995, 2003) and the South African Chutter index (Chutter 1972) based on the relative tolerance of macroinvertebrate taxonomic groups to water pollution within a geographical area.
Studies on the response of macroinvertebrates to organic and heavy-metal pollution impacts within a single catchment are very rare. Such situations are ideal for testing the response of the whole community, biotic indices and individual taxonomic groups to different pollution types. One of the only previous examples was the Nent River (Northern England), where macroinvertebrates (Armitage 1980; Armitage and Blackburn 1985) and algae (Say and Whitton 1981) were used to assess the dual impacts of contamination from several centuries of mining and organic pollution wastes within an agricultural catchment. While strong impairment of the target biota was observed, there may have been additional effects on macroinvertebrate communities other than the target pollutants due to background contamination, together with the potential for synergistic and/or overlapping effects (e.g. Connell and Miller 1984) of the pollutants that were the focus of the study. It is desirable, therefore, to conduct studies on macroinvertebrate communities that focus on the contribution of the effects of a single pollutant within a ‘clean’ background.
We used quantitative surveys to compare the effects of contamination from two separate discharges of heavy-metal contamination and treated sewage on stream macroinvertebrates within a small otherwise clean stream network to investigate the impact of each of these pollution types on resident macroinvertebrate communities. The use of a small catchment for the study increased the likelihood of waterways sharing similar fauna (Corkum 1989) and minimised biogeographic variation of animals across sampling sites (Cranston 1995). Although our preference would have been to conduct a before versus after, control versus impact (BACI) design (see Underwood 1991), both waste discharges in the Grose River catchment were constructed many decades previously (Wright 2006). To compensate, we sought to compare macroinvertebrate and water quality results from waste affected sites with results from multiple reference sites (Fairweather 1990) across the catchment, away from the influence of any known disturbance or waste discharges to represent the spectrum of undisturbed catchment physio-chemical and biological conditions.
The questions we addressed in this study are (1) do macroinvertebrates respond differently to different types of pollutants, (2) what is the relative effectiveness of commonly used biotic indices, and (3) is measurement of macroinvertebrate abundance important for pollution assessment?
2 Materials and Methods
2.1 Study Area and Sampling Sites
Field work was carried out on waterways (Table 1) in the upper Grose River catchment in the Blue Mountains (33°35′ S, 150°15′ E), which forms part of the Great Dividing Range in southeastern Australia (Fig. 1). Most of the study area is protected as part of Blue Mountains National Park estate, nested within the Greater Blue Mountains World Heritage Area (NPWS 2001; BMCC 2002). Whilst most of the study area is undisturbed wilderness, roads run along the outer rim of the catchment to service urban centres, including Mount Victoria and Blackheath (NPWS 1999). Only a very small proportion of the two townships lie within the hydrological catchment of the study area, and urban lands cover less than 2% of the study area. Further details of the study area are given in Wright (2006).
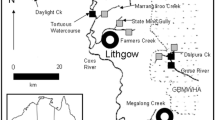
Map of survey sites (square symbols), waterways and waste discharge points in the upper Grose River. Site DAL was sampled only for water chemistry. Approximate catchment boundary is dashed line. Inset shows location of study area in southeastern Australia. Details on sampling sites given in Table 1
Two waste sources discharge into tributaries of the upper Grose River. One is coal-mine drainage from a disused underground coal mine ‘Canyon Colliery’ which operated, under various owners, from the 1920s (Macqueen 2007) until 1997 (EPA 2001). A horizontal mine drainage shaft (Macqueen 2007) discharges from the abandoned mine into Dalpura Creek, which shortly thereafter flows into the Grose River (Fig. 1). The second point source is the Blackheath sewage treatment plant (STP). It was constructed in the 1930s and, at the time of sampling, discharged approximately 0.92 ML/day of secondary treated effluent to Hat Hill Creek (Sydney Water 2004). Previous monitoring results reported ammonia levels in the STP effluent discharged to Hat Hill Creek at mean levels of 4 mg/L (Sydney Water 2004)
Ten sampling sites were selected in the study area (Table 1; Fig. 1). Four sites were clean reference sites, unaffected by waste discharges, to represent natural conditions (GEN, GDK, VIC, HHU). The remaining six sites were downstream from waste discharges. Three received mine-drainage (DAL, GDD, GBK) and two sewage effluent (HHD, HHG). DAL was not sampled for macroinvertebrates but was sampled only for water quality, as it was considered to be a point-source impact of mine drainage into the Grose River. The site GHU was the furthest downstream in the study and was subject to a mixture of the two waste sources (Fig. 1).
2.2 Macroinvertebrate Sampling
Macroinvertebrates were collected from nine sites in the upper Grose River catchment on three occasions (Fig. 1; Table 1) between April and June 2003. On each sampling occasion and at each of the nine macroinvertebrate sites, five quantitative benthic samples were collected from cobble riffle zones (cf. Resh and Jackson 1993; Wright et al. 1995). The location of each replicate was randomly selected within a 15-m stream reach.
Samples were collected by ‘kick sampling’. A ‘kick’ net with a frame of 30 × 30 cm and 250 μm mesh was used (Rosenberg and Resh 1993; Wright 1994). Sampling was achieved by disturbing the stream bottom for a period of 1 min over a 900-cm2 area, immediately upstream of the net. The net contents, including stream detritus and macroinvertebrates, were immediately placed into a labelled storage container and preserved in 70% ethanol.
In the laboratory, the sediment below 250 μm was washed from the sample. The remaining material was then sorted under a dissecting microscope (×40) to extract the macroinvertebrates from stream detritus (e.g. leaves, sticks, rocks, gravel). Macroinvertebrate identification was determined using the identification keys recommended by Hawking (1994). All insect groups were identified to family as these data have been demonstrated to provide adequate taxonomic resolution for impact assessment (Wright 1994; Wright et al. 1995). Some non-insect groups (Oligochaeta, Temnocephalidae, Hydracarina, non-Ancylidae Gastropoda) were not identified to the family level due to identification difficulties.
2.3 Water Quality Sampling
Water quality data were collected from ten sites on three occasions, including samples from Dalpura Creek downstream of the mine drainage outflow (Fig. 1). They were collected immediately prior to the macroinvertebrates to minimise disturbance due to kick-sampling. At each site, on each occasion, water quality was monitored in situ at the centre of the waterway using a portable field chemistry meter (WTW Multiline P4; Universal Meter, Weilheim, Germany) to measure stream electrical conductivity, pH and water temperature. Water samples were also collected in 200 mL plastic bottles for later laboratory analysis. Water samples were cooled and analysis was conducted within 72 hours of collection. Replicated measurement of water quality samples was conducted with multiple field meter readings taken on each sampling occasion and duplicate bottles collected for later laboratory analysis on three different sampling occasions.
These samples were analysed using standard chemical analysis methods (APHA 1998). Chemical analysis comprised total zinc (TZn), hardness, alkalinity, total nitrogen (TN) and total phosphorus (TP). On the first sampling occasion, samples were also assessed for the metals aluminium, arsenic, boron, barium, cadmium, chromium, cobalt, copper, iron, lead, manganese, mercury, molybdenum, nickel, selenium, silver, tin, uranium and zinc. When only zinc was found to exceed ANZECC (2000) guidelines for ecosystem protection, subsequent metal analyses were restricted to zinc, and it is the only metal data presented.
2.4 Data Analysis
Multivariate analyses of macroinvertebrate community studies have been demonstrated to be a sound technique to evaluate the ecology of macroinvertebrates (Corkum 1989) of freshwater (Norris et al. 1982; Marchant et al. 1994; Wright et al. 1995) and marine pollution (Clarke 1993; Warwick 1993). Non-metric multidimensional scaling (NMDS) was performed on the similarity matrix, computed with square-root transformed macroinvertebrate taxon abundance data, using the Bray-Curtis dissimilarity measure (Clarke 1993; Warwick 1993). Two-dimensional ordination plots represented the dissimilarity among samples. All reference sites were grouped to test differences by two-way analysis of similarity (ANOSIM: Clarke 1993) between reference sites and sites downstream of the waste discharges. In the ordinations, the influence of particular taxa on dissimilarities between communities was quantified using the similarity percentage procedure (SIMPER). These multivariate analyses were achieved using the software package PRIMER version 5 (Clarke 1993).
Macroinvertebrate and chemical data were also analysed using a mixed model analysis of variance (SPSS V14) with ‘sites’ treated as a fixed factor and sampling ‘time’ as a random factor. Data were checked for normality using PP plots and for homogeneity of variance using Levene’s test. Linear contrasts were used to test for differences between clean reference sites and those polluted with either waste discharge.
3 Results
3.1 Macroinvertebrates
A total of 48,069 (54 taxa) macroinvertebrates were collected with a majority being insects (Table 2). Family (F 8,108 = 18.95, p < 0.001) and EPT family richness (F 8,108 = 27.46, p < 0.001) differed significantly among sites. Linear contrasts showed that family and EPT family richness were significantly lower immediately downstream of both waste sources compared to reference sites (Table 3; Fig. 2). Total abundance also differed significantly between sites (F 8,108 = 5.25, p = 0.002) and was significantly higher immediately downstream of the STP organic outflow compared to the reference sites and was significantly lower downstream of the zinc-rich coal-mine effluent compared to the reference sites (Table 3; Fig. 2). When biotic indices were compared between the most downstream site sampled (i.e. where both waste sources were mixed; GHU) and the reference sites using linear contrasts, only total abundance was significantly different (Table 3; Fig. 2).
Based on community structure, multivariate analysis showed that the site immediately downstream of the STP (HHD) and the two sites downstream of the mine outflow (GDD, GBK) were well separated from all other sites which tended to cluster (i.e. samples were grouped in the NMDS ordination; Fig. 3). Stress values (range = 0.20–0.17) indicated that, in two dimensions, the MDS was a fair representation of the original data (cf. Clarke 1993). The ANOSIM results (Table 4) showed that the differences between sampling sites were more influential than time (Global R 0.772 vs. 0.126). Pairwise comparison of sites (Table 4) confirmed that there were differences in community structure in the presence of the waste discharges (GDD, HHD) compared to reference sites (R-statistic values, 0.930 and 0.927). Comparison of assemblages at the two sites downstream of the zinc pollution point source (GBK, GDD) and the organic pollution outflow (HHG, HHD) showed that the coal-mine waste sites were more similar (R-statistic 0.297) than the sewage discharge sites (R-statistic 0.980). Different degrees of recovery were detected below each waste source. Community structure at the lower site downstream of the zinc pollution (GBK) was less similar to reference sites (R-statistic 0.770) than at the lower sewage site (HHG) compared to the reference sites (R-statistic 0.323).
NMDS ordination of macroinvertebrate data. Stress = 0.2. Each symbol represents a centroid of five macroinvertebrate samples from the Grose River and its tributaries, from each of three sampling occasions (four from GBK on second occasion). Reference sites are squares: black VIC, light grey GEN, white GDK, dark grey HHU. Sites immediately below waste discharges are diamonds: black GDD (coal-mine drainage), white HHD (sewage). Sites further downstream of waste discharges are triangles: white HHG, dark grey GBK, black GHU (site abbreviations given in Table 1)
Using SIMPER, data from the reference sites were compared with the sites immediately downstream of the STP (HHD) and mine drainage (GDD) site (Table 5). Of the ten taxa that contributed most to the separation between the mine drainage and reference sites, all except Hydropsychidae had lower abundance at the mine drainage site than at reference sites. In contrast, of the ten taxa that contributed most to the separation between the site immediately downstream of the sewage inflow (HHD) and the reference sites, six (Ancylidae, Nemertea, non-Ancylidae gastropods, Simuliidae, Hydroptilidae, Corbiculidae) had higher abundance in the presence of sewage than at the reference sites, while the other four common taxa (Leptophlebiidae, Baetidae, Elmidae, Scirtidae) were in higher abundance at the reference sites than in the presence of the highest influence of organic pollution (Table 5).
The affinity of each common taxon to each pollution type was compared, based on the SIMPER results (Table 6). Leptophlebiidae was the only family that had a highly negative response to both types of pollution. Elmidae (larvae), Baetidae and Psephenidae had highly negative responses to zinc pollution and moderately negative responses to organic pollution (Table 6). In contrast, the response of Ancylidae was highly positive to organic pollution and highly negative in the presence of zinc pollution. Other taxa that demonstrated a negative response to zinc pollution and a positive response to organic pollution were the Chironomidae and Simuliidae. Hydropsychidae was the only common taxon that showed a negative response to organic pollution and a positive response to zinc pollution (Table 6).
3.2 Physical and Chemical Indicators
The effects of the two waste discharges on the water chemistry of local streams (Table 7; Fig. 4) were clearly apparent, although distinctly different. TP (F 9,50 = 8.05, p < 0.001; Table 8) and TN (F 9,50 = 9.10, p < 0.001; Table 8) both varied highly significantly among sites. Linear contrasts revealed that both were higher immediately below the STP (HHD) (TP, 506.8 μg/L and TN, 14,316.7 μg/L) compared to the reference sites (TP, 3.8–5.0 μg/L and TN, 55.0–101.7 μg/L). Five kilometres below the STP (HHG), there was some reduction (TP, 189.2 μg/L, TN, 7,533.3 μg/L; Fig. 4)
Mean total phosphorus (grey bar), mean total zinc (black bar) and mean total nitrogen (white bar), in μg/L, collected from duplicate samples, at each site, on three sampling occasions April to June 2003 (plus one standard error). Grose River sites are grouped to the left and tributary sites to the right. Arrows and text indicate the location that mine drainage and Hat Hill Ck enters the Grose River and where STP effluent flows into Hat Hill Creek
Mean total zinc levels also varied significantly among sites (F 9,44 = 74.72, p < 0.001; Table 8), and linear contrasts revealed that total zinc was significantly higher (594.7 μg/L) in Dalpura Ck, the tributary containing the coal-mine outflow, compared to reference sites (4.2–6.2 μg/L). With further distance downstream of Dalpura Ck, the level gradually dropped, although at the most downstream site sampled (GHU), levels remained elevated (70.7 μg/L; Fig. 4).
Given that water hardness was classified as ‘soft’ (ANZECC 2000), the recommended ‘trigger level’ for protecting aquatic ecosystems for New South Wales upland streams for total zinc levels (5 μg/L) were violated at all sites sampled downstream of the coal mine (see Tables 7 and 8).
4 Discussion
Treated sewage from Blackheath STP and mine drainage, from the disused Canyon Colliery, resulted in different and distinct pollution-related changes to macroinvertebrate communities and water chemistry of surface waters in the upper reaches of the Grose River system. Comparison of the water quality and ecological effects of the two pollution gradients in this study was enhanced by the lack of other human impacts, apart from the waste discharges, in an otherwise predominantly naturally vegetated (c. 95%) upland catchment within a largely protected National Park reserve (NPWS 2001).
We found that biological indices (macroinvertebrate abundance, family richness and EPT family richness) responded differently to the two waste discharges in this study. Family richness declined to a greater degree downstream from the coal-mine drainage compared to only a modest reduction below the STP (Fig. 2). A similar difference in taxonomic richness to dual disturbance gradients was observed by Metzeling et al. (2006) who reported that family richness performed poorly against a salinity gradient but better against a habitat simplification gradient. EPT richness was the only one of the indices that responded with a reduction of similar magnitude below both the mine and STP discharge. In comparison, macroinvertebrate abundance declined below the mine but increased immediately below the STP. Measuring abundance is often not included in many pollution studies, perhaps partly due to the popularity of rapid assessment methodologies that use non-quantitative techniques (e.g. Chessman 1995). Our findings illustrate how abundance data can be a very important ecological attribute in pollution studies. Abundance of individual families in this study helped reveal differences in community structure at polluted and unpolluted sites, further details of which are discussed further below.
A group of six taxa were particularly abundant and strongly influenced the organically polluted macroinvertebrate community, below Blackheath STP: Ancylidae, non-Ancylidae gastropods, Nemertea, Simuliidae, Hydroptilidae and Corbiculidae. This group of biota collectively increased their abundance, in the presence of sewage effluent, more than three times that found at unpolluted reference sites. However, in contrast, the macroinvertebrate community below the coal mine was depauperate, with only one influential taxa, Hydropsychidae, being more abundant here than at unpolluted sites. Hydropsychidae was much less abundant below the STP.
Our finding that Hydropsychidae was tolerant of mine drainage contrasts with findings from some Australian metal pollution studies. For example, Norris (1986) reported that Hydropsychidae responded negatively to metal-pollution in the Molongolo River and Mackey (1988) also made the same observation in the River Dee. Metal-pollution tolerance of Hydropsychidae was also observed in Daylight Creek (NSW) where they were the second most abundant taxa at a highly copper- and zinc-polluted site (Napier 1992), and in the South Esk River (Tasmania), they were abundant at all but one metal-polluted site (Norris et al. 1982). Tolerance of Hydropsychidae to mine pollution has been documented in other parts of the world; for example, they were recorded in New Zealand metal-polluted waterways (Hickey and Clements 1998), an English zinc contaminated river (Armitage 1980) and in acid mine drainage (AMD)-affected waters in Kentucky (Short et al. 1990).
The mayfly family Leptophlebiidae emerged as the most sensitive family in this study with equal and absolute intolerance of both the mine drainage and sewage. No individual specimen was collected at either the mine-polluted site or the STP-polluted site. Our results reinforce the reputation of Leptophlebiidae as one of the most pollution-sensitive macroinvertebrate families worldwide. They have been reported as being completely missing from other heavily acid mine drainage-affected reaches of rivers and streams such as the River Dee in Queensland (Mackey 1988), Bob’s Creek in Kentucky (Short et al. 1990) and the River Vascão in Portugal (Gerhadt et al. 2004). Some pollution tolerance has been reported with AMD-affected streams in New Zealand (Winterbourn 1998) containing Leptophlebiidae tolerant of highly acidic waters (pH 3.5). Leptophlebiidae are also frequently reported to be very sensitive to organic pollution with several researchers reporting their complete absence at the most affected sites (Cosser 1988; Whitehurst and Lindsey 1990; Wright et al. 1995).
Four animals that strongly contributed to community structure at the polluted sites exhibited opposite affinities towards each of the two waste-types. Hydropsychidae was discussed above. The other three taxa were highly abundant in the organic pollution below the STP (Ancylidae, Chironomidae and Simuliidae) and were absent or at very low abundances, below the mine drainage. The gastropod Ancylidae had the most strongly diverging relationship to the waste sources. It was more than 1,000% more abundant in the presence of sewage effluent than at the unpolluted reference sites, yet it displayed intolerance of mine pollution. This differential tolerance is supported by the metal (SIGNAL-MET 8/10) and organic pollution (SIGNAL-SEW 2/10) grades in Chessman and McEvoy (1998). Ancylidae have also been found to be intolerant of mine drainage in Spain (Marqués et al. 2003) and were reported as being tolerant of sewage pollution in NSW (Wright 1994; Wright et al. 1995) and nutrient enrichment in the Eresma River in Central Spain (Camargo et al. 2004), although they were absent from the most sewage-polluted sites on the River Adur (Whitehurst and Lindsey 1990), possibly due to other human influences in the disturbed Adur catchment.
The biological and chemical changes resulting from pollution has been illustrated by the classic model developed by Hynes (1960) with a steady increase of ‘pollution fauna’ below the waste discharge then a gradual and progressive reduction with further distance below the point-source and a corresponding inverse relationship with pollution sensitive animals. We found some evidence of recovery below each waste source compared to sites located in the zone of highest contamination. Considerable recovery was evident further downstream below the STP discharge in Hat Hill Creek, yet a lower degree of recovery was observed in the Grose River below the coal mine, until the sewage-enriched waters combined with the Grose River.
This study constitutes some of the first Australian evidence that coal mining can result in freshwater ecosystem damage due to heavy-metal contamination. Such cases may appear to be unusual in Australia, but this is not the case internationally, where coal mining has been more frequently associated with AMD and elevated heavy metal levels in the USA (e.g. Herlihy et al. 1990), Europe (e.g. Armitage 1980; Malmqvist and Hoffsten 1999; Johnson 2003) and New Zealand (Winterbourn 1998).
This study builds upon previous northern hemisphere studies that also used macroinvertebrates to measure sewage and mine drainage impacts. They were carried out on the Nent River system in England where they contended with contaminated runoff from urban and agricultural landuses as well as mine and sewage impacts (Armitage 1980; Armitage and Blackburn 1985). Although strong changes in macroinvertebrate community structure were detected in the Nent River, there were difficulties clearly differentiating the specific sewage and mining impacts due to overlapping contamination from multiple sources of mine pollution. Our current study was able to limit the confounding effects of multiple overlapping sources of pollution and disturbance due to it being situated in a small catchment that was predominantly naturally vegetated.
5 Conclusions
Comparison of ecological effects of two different types of pollution (organic and inorganic) in a small catchment with otherwise unpolluted waterways flowing upstream of the two waste discharges provided an unusual opportunity to observe the relationship of macroinvertebrates to the different waste sources. The ecological effects of each of the two waste sources (STP and mine drainage) were clearly apparent from observed changes to the taxonomic assemblages of stream macroinvertebrates. We found that multivariate analysis of quantitative family level data allowed detailed assessment of the pollution impacts. Three biotic indices family richness, total abundance and EPT richness were also valuable for comparing effects of the two wastes. EPT richness was particularly sensitive at detecting biological impairment from both pollution sources.
References
ANZECC (Australian and New Zealand Environment and Conservation Council). (2000). Australian and New Zealand guidelines for fresh and marine waters. Canberra: Australian and New Zealand Environment and Conservation Council.
APHA (American Public Health Association). (1998). Standard Methods for the Examination of Water and Wastewater (20th ed.). Washington, DC: American Public Health Association.
Armitage, P. D. (1980). The effects of mine drainage and organic enrichment on benthos in the River Nent system, Northern Pennines. Hydrobiologia, 74, 119–128. doi:10.1007/BF00014563.
Armitage, P. D., & Blackburn, J. H. (1985). Chironomidae in a Pennine stream system receiving mine drainage and organic enrichment. Hydrobiologia, 121, 165–172. doi:10.1007/BF00008720.
Barbour, M. T., Plafkin, J. L., Bradley, B. P., Graves, C. G., & Wisseman, R. W. (1992). Evaluation of EPA’s rapid bioassessment benthic metrics: Metric redundancy and variability among reference stream sites. Environmental Toxicology and Chemistry, 11, 437–449. doi:10.1897/1552-8618(1992)11[437:EOERBB]2.0.CO;2.
BMCC (Blue Mountains City Council). (2002). State of the Environment Report 2001/2002. Katoomba, NSW: Blue Mountains City Council.
Camargo, J. A., Alonso, A., & De La Puente, M. (2004). Multimetric assessment of nutrient enrichment in impounded rivers based on benthic macroinvertebrates. Environmental Monitoring and Assessment, 96, 233–249. doi:10.1023/B:EMAS.0000031730.78630.75.
Chessman, B. C. (1995). Rapid assessment of rivers using macroinvertebrates: A procedure based on habitat-specific sampling, family level identification and a biotic index. Australian Journal of Ecology, 20, 122–129. doi:10.1111/j.1442-9993.1995.tb00526.x.
Chessman, B. C. (2003). New sensitivity grades for Australian river macroinvertebrates. Marine & Freshwater Research, 54, 95–103. doi:10.1071/MF02114.
Chessman, B. C., & McEvoy, P. K. (1998). Towards diagnostic biotic indices for river macroinvertebrates. Hydrobiologia, 364, 169–182. doi:10.1023/A:1003142819625.
Chessman, B. C., & Williams, S. A. (1999). Biodiversity and conservation of river macroinvertebrates. Pacific Conservation Biology, 5, 36–55.
Chutter, F. M. (1972). An empirical biotic index of the quality of water in South African streams and rivers. Water Research, 6, 19–30. doi:10.1016/0043-1354(72)90170-4.
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology, 18, 117–143. doi:10.1111/j.1442-9993.1993.tb00438.x.
Connell, D. W., & Miller, G. J. (1984). Chemistry and Ecotoxicology of Pollution. New York, Brisbane: John Wiley & Sons.
Corkum, L. D. (1989). Patterns of benthic invertebrate assemblages in rivers of northwestern North America. Freshwater Biology, 21, 191–205. doi:10.1111/j.1365-2427.1989.tb01358.x.
Cosser, P. R. (1988). Macroinvertebrate community structure and chemistry of an organically polluted creek in south-east Queensland. Australian Journal of Marine and Freshwater Research, 39, 671–683. doi:10.1071/MF9880671.
Cranston, P. S. (1995). Biogeography. In P. Armitage, et al. (Ed.), The Chironomidae: The biology and ecology of non-biting midges (pp. 180–197). Melbourne: Chapman & Hall.
EPA (NSW Environment Protection Authority) (2001). Canyon Colliery, Environment Protection Licence. Licence 558. http://www.epa.nsw.gov.au/prpoeo/licences/L558.pdf Accessed 19 February 2009.
Fairweather, P. G. (1990). Sewage and the biota on seashores: Assessment of impact in relation to natural variability. Environmental Monitoring and Assessment, 14, 197–210. doi:10.1007/BF00677916.
Gerhadt, A., Janssens de Bisthoven, L., & Soares, A. M. V. M. (2004). Macroinvertebrate response to acid mine drainage: Community metrics and on-line behavioural toxicity bioassay. Environmental Pollution, 130, 263–274. doi:10.1016/j.envpol.2003.11.016.
Gresens, S. E., Belt, K. T., Tang, J. A., Gwinn, D. C., & Banks, P. A. (2007). Temporal and spatial responses of Chironomidae (Diptera) and other benthic invertebrates to urban stormwater runoff. Hydrobiologia, 575, 173–190. doi:10.1007/s10750-006-0366-y.
Growns, J. E., Chessman, B. C., McEvoy, P. K., & Wright, I. A. (1995). Rapid assessment of rivers using macroinvertebrates: Case studies in the Nepean River and Blue Mountains, NSW. Australian Journal of Ecology, 20, 130–141. doi:10.1111/j.1442-9993.1995.tb00527.x.
Hawking, J. H. (1994). A preliminary guide to keys and zoological information to identify invertebrates from Australian freshwaters. Cooperative Research Centre for Freshwater Ecology Identification Guide No. 2.Albury, NSW: Cooperative Research Centre for Freshwater Ecology.
Hellawell, J. M. (1986). Biological Indicators of Freshwater Pollution and Environmental Management. London: Elesvier.
Herlihy, A. T., Kaufman, P. R., Mitch, M. E., & Brown, D. D. (1990). Regional estimates of acid mine drainage impact on streams in the mid-Atlantic and southeastern United States. Water, Air, and Soil Pollution, 50, 91–107. doi:10.1007/BF00284786.
Hickey, C. W., & Clements, W. H. (1998). Effects of heavy metals on benthic macroinvertebrate communities in New Zealand streams. Environmental Toxicology and Chemistry, 17, 2338–2346. doi:10.1897/1551-5028(1998)017<2338:EOHMOB>2.3.CO;2.
Hynes, H. B. N. (1960). The Biology of Polluted Waters. Liverpool: Liverpool University Press.
Johnson, D. B. (2003). Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines. Water, Air, and Soil Pollution, 3, 47–66.
Jolly, V. H., & Chapman, M. A. (1966). A preliminary biological study of the effects of pollution on Farmers’s Creek and Cox’s River, New South Wales. Hydrobiologia, 27, 160–192. doi:10.1007/BF00161494.
Keith, D. A., & Benson, D. H. (1988). Natural vegetation of the Katoomba area. Cunninghamia, 2, 107–144.
Kitchin, P. L. (2005). Measuring the amount of statistical information in the EPT index. Environmetrics, 16, 51–59. doi:10.1002/env.670.
Lenat, D. R. (1988). Water quality assessment of streams using a qualitative collection method for benthic macroinvertebrates. Bulletin of the North American Benthological Society, 7, 222–233. doi:10.2307/1467422.
Lenat, D. R., & Penrose, D. L. (1996). History of the EPT taxa richness metric. Bulletin of the North American Benthological Society, 13, 305–307.
Mackey, A. P. (1988). The biota of the River Dee (central Queensland, Australia) in relation to the effects of acid mine drainage. Proceedings of the Royal Society of Queensland, 99, 9–19.
Macqueen, A. (2007). Back from the Brink. Blue Gum Forest and the Grose Wilderness. Second Edition. 8 Angel St, Wentworth Falls, NSW; self-published.
Malmqvist, B., & Hoffsten, P. (1999). Influence of drainage from old mine deposits on benthic macroinvertebrate communities in central Swedish streams. Water Research, 33, 2415–2423. doi:10.1016/S0043-1354(98)00462-X.
Marchant, R., Barmutta, L. A., & Chessman, B. C. (1994). Preliminary study of the ordination and classification of macroinvertebrate communities from running waters in Victoria, Australia. Australian Journal of Marine and Freshwater Research, 45, 945–962. doi:10.1071/MF9940945.
Marqués, M. J., Martínez-Conde, E., & Rovira, J. V. (2003). Effects of zinc and lead mining on the benthic macroinvertebrates of a fluvial ecosystem. Water, Air, and Soil Pollution, 148, 363–388. doi:10.1023/A:1025411932330.
McCord, S. B., Grippo, R. S., & Eagle, D. M. (2007). Effects of silviculture using best management practices on stream macroinvertebrate communities in three ecoregions of Arkansas, USA. Water, Air, and Soil Pollution, 184, 299–311. doi:10.1007/s11270-007-9417-x.
Metzeling, L., Perriss, S., & Robinson, D. (2006). Can the detection of salinity and habitat simplification gradients using rapid bioassessment of benthic invertebrates be improved through finer taxonomic resolution or alternatives indices? Hydrobiologia, 572, 235–252. doi:10.1007/s10750-005-9004-3.
Napier, G. M. (1992). Application of laboratory-derived data to natural aquatic ecosystems. PhD thesis. Graduate School of the Environment, Macquarie University.
Norris, R. H. (1986). Mine waste pollution of the Molonglo River, New South Wales and the Australian Capital Territory: Effectiveness of remedial works at Captains Flat mining area. Australian Journal of Marine and Freshwater Research, 37, 147–157. doi:10.1071/MF9860147.
Norris, R. H., Lake, P. S., & Swain, R. (1982). Ecological effects of mine effluents on the South Esk River, north-eastern Tasmania (III). Benthic macroinvertebrates. Australian Journal of Marine and Freshwater Research, 33, 789–809. doi:10.1071/MF9820789.
NPWS (National Parks and Wildlife Service). (1999). Blue Mountains National Park, Walking Track Guide. Walking Tracks in the Grose Valley. Blue Mountains Heritage Centre, Blackheath, NSW: self-published.
NPWS (National Parks and Wildlife Service). (2001). Blue Mountains National Park Plan of Management. Blue Mountains Heritage Centre, Blackheath, NSW: self-published.
Pinder, L. C. V., & Far, I. S. (1987). Biological surveillance of water quality. 3. The influence of organic enrichment on the macroinvertebrate fauna of small chalk streams. Archiv fuer Hydrobiologie, 109, 619–637.
Plafkin, J. L., Barbour, M. T., Porter, K. D., Grosse, S. K., & Hughes, R. M. (1989). Rapid bioassessment protocols for use in streams and rivers: Benthic macroinvertebrates and fish. Washington D.C.: United States Environmental Protection Agency.
Resh, V. H., & Jackson, J. K. (1993). Rapid assessment approaches to biomonitoring using benthic macroinvertebrates. In D. M. Rosenberg, & V. H. Resh (Eds.), Freshwater Biomonitoring and Benthic Macroinvertebrates (pp. 195–223). New York, London: Chapman & Hall.
Rosenberg, D. M., & Resh, V. H. (1993). Freshwater biomonitoring and benthic macroinvertebrates. New York, London: Chapman & Hall.
Say, P. J., & Whitton, B. A. (1981). Changes in flora down a stream showing a zinc gradient. Hydrobiologia, 76, 255–262. doi:10.1007/BF00006217.
Sheehan, P. J. (1984). Effects on community and ecosystem structure and dynamics. In P. J. Sheehan, et al. (Ed.), Effects of Pollutants at the Ecosystem Level (pp. 51–100). Chichester, New York: John Wiley & Sons.
Short, T., Black, J. A., & Birge, W. J. (1990). Effects of acid-mine drainage on the chemical and biological character of an alkaline headwater stream. Archives of Environmental Contamination and Toxicology, 19, 241–248. doi:10.1007/BF01056093.
Sloane, P. I. W., & Norris, R. H. (2003). Relationship of AUSRIVAS-based macroinvertebrate predictive model outputs to a metal pollution gradient. Journal of the North American Benthological Society, 22, 457–471. doi:10.2307/1468274.
Sydney Water Corporation. (2004). Annual Environmental Indicators Compliance Report 2003/2004. Sydney, NSW: Sydney Water Corporation.
Underwood, A. J. (1991). Beyond BACI: Experimental designs for detecting human environmental impacts on temporal variations in natural populations. Australian Journal of Marine and Freshwater Research, 42, 569–587. doi:10.1071/MF9910569.
Walsh, C. J., Sharpe, A. K., Breen, P. F., & Sonneman, J. A. (2001). Effects of urbanisation on streams of the Melbourne region, Victoria, Australia. I. Benthic macroinvertebrate communities. Freshwater Biology, 46, 535–551. doi:10.1046/j.1365-2427.2001.00690.x.
Warwick, R. M. (1993). Environmental impact studies on marine communities: Pragmatical considerations. Australian Journal of Ecology, 18, 63–80. doi:10.1111/j.1442-9993.1993.tb00435.x.
Whitehurst, I. T., & Lindsey, B. I. (1990). The impact of organic enrichment on the benthic macroinvertebrate communities of a lowland river. Water Research, 24, 625–630. doi:10.1016/0043-1354(90)90195-C.
Winner, R. W., Van Dyke, J. S., Caris, N., & Farrell, M. P. (1975). Response of the macroinvertebrate fauna to a copper gradient in an experimental polluted stream. Vereiningung für theoretische und angewandte Limnologie, 19, 2121–2127.
Winterbourn, M. J. (1998). Insect faunas of acidic coal mine drainages in Westland, New Zealand. New Zealand Journal of Entomology, 21, 65–72.
Wright, I. A. (1994). The ecological impacts of Wentworth Falls sewage treatment plant on Blue Mountains Creek. MSc thesis. Graduate School of the Environment, Macquarie University.
Wright, I. A. (2006). Australian Chironomidae (Diptera) in upland streams and freshwater lakes, PhD thesis, University of Western Sydney.
Wright, I. A., Chessman, B. C., Fairweather, P. G., & Benson, L. J. (1995). Measuring the impact of sewage effluent on the macroinvertebrate community of an upland stream: The effect of different levels of taxonomic resolution and quantification. Australian Journal of Ecology, 20, 142–149. doi:10.1111/j.1442-9993.1995.tb00528.x.
Acknowledgements
This study formed part of the senior author’s PhD research at the University of Western Sydney. Sydney Water made laboratory facilities and sampling apparatus available for use for this study. Susan Alexandra Wright and other volunteers generously assisted with field work, and Oleg Nicetic conducted univariate data analysis. We are grateful for the comments by Dr Debbie Rae, Dr Helen Nice and anonymous referees on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, I.A., Burgin, S. Comparison of Sewage and Coal-Mine Wastes on Stream Macroinvertebrates Within an Otherwise Clean Upland Catchment, Southeastern Australia. Water Air Soil Pollut 204, 227–241 (2009). https://doi.org/10.1007/s11270-009-0040-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0040-x