Abstract
This work investigates the solar heterogeneous photocatalytic degradation of three commercial acid dyes: Blue 9 (C.I. 42090), Red 51 (C.I. 45430), and Yellow 23 (C.I. 19140). TiO2 P25 from Degussa was used as the photocatalyst. The dyes were completely degraded within 120 min of treatment in the following increasing order of removal rate: Blue 9 < Yellow 23 < Red 51. The photocatalytic color removal process was well described by a two-first-order in-series reaction, followed by another first-order reaction. Photolytic experiments showed that this process is quite inefficient and highly selective towards Red 51 only. The dyes' solution was completely decolorized and organic matter removals up to 99% were achieved with photocatalysis. The lack of selectivity and the possibility of using solar light to excite the photocatalyst are promising results regarding the feasibility of this technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The discharge of effluents containing high concentrations of dyes is a well-known problem associated with several industries. Due to relatively low fixation rates varying from 89% to 95% in the case of acid dyes and low efficiency of the biological processes usually used for treating those effluents (mainly activated sludge), approximately 5% to 20% of the dyes are discharged to the environment (Chang et al 2003; Hsueh et al. 2005). Due to the carcinogenic, mutagenic, and allergenic character of some dyes, the deleterious effect of color for photosynthetic organisms in water bodies, and the resistance of the dyes to biological degradation (Meriç et al 2004), there is a major need to investigate new adequate alternatives for treating this kind of pollutant.
Acid dyes are a group of anionic dyes presenting one to three sulfonic groups. They are used in acid or neutral baths for fibers such as wool, silk, and polyamide. They may contain azo, anthraquinonic, triarylmethanic, azinic, xanthenic, quinolinic, indigoid, nitro, and nitroso chromophore groups (Guaratini and Zanoni 2000).
During the past few years, photocatalysis has been extensively used, mainly because it has the capacity of degrading a huge number of recalcitrant substances in liquid and gaseous systems using procedures with relatively low cost. Titanium dioxide, TiO2, in its anatase form, is the semiconductor studied the most, as it presents some desired characteristics: high photocatalytic activity, relatively low cost, stability in aqueous systems, and low ecotoxicity (Stafford et al 1996; Linsebigler et al 1995).
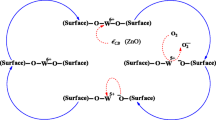
The mechanisms of the photocatalytic oxidation (TiO2/UV) of organic contaminants have been extensively studied (Hagfeldt and Grätzel 1995; Hoffmann et al 1995). Ultraviolet light with λ < 380 nm and, therefore, with energy greater than the TiO2 band gap, induces the transfer of electrons from the valence band to the conduction one. The charged species can recombine with each other (releasing the absorbed energy as heat) or migrate to the photocatalyst particle surface. In the presence of water, hydroxyl radical (OH•) is formed in the photocatalyst surface, as a result of the attack of the H2O molecules from the photogenerated holes, along with other radical species like superoxide (O2 •−) and hydroperoxide (HO2 •). Hydroxyl radical has been pointed out as the main responsible species for the oxidative degradation of organic pollutants.
Heterogeneous photocatalysis has presented several promising results regarding dye degradation (Liu and Chiou 2005; Muruganandham and Swaminathan 2006; Gonçalves et al 1999; Wang 2000). For several compounds, total mineralization has been achieved.
This work assesses the feasibility of degrading a mixture of three acid dyes of distinct chemical structures by heterogeneous photocatalysis using solar irradiation: triarylmethanic Acid Blue 9 (AB9), xanthenic Acid Red 51 (AR51), and azo Acid Yellow 23 (AY23).
The studied dyes were chosen due to their different chemical structures, presented in Table 1, and their widespread use in several industries (textile, foods, drugs, cosmetics, etc.). Acid Blue 9 (C.I. 42090) was only studied as a probe molecule for assessing the photocatalytic efficiency of air–solid systems (Chin and Ollis 2007; Julson and Ollis 2006), showing no significant direct photolysis (Doushita and Kawahara 2001). The photocatalytic degradation of Acid Red 51 (C.I. 45430) has been scarcely studied by some authors (Hasnat et al 2007; Uddin et al 2007). They have shown that small concentrations of the dye could be efficiently degraded, even with visible light (Zhang et al 1997). Acid Yellow 23 (C.I. 19140) was solely studied as a probe molecule for assessing the influence of metallic species in the photocatalytic process, also showing negligible direct photolysis (Rao et al 2003). Notwithstanding the lack of a significant amount of degradation studies regarding the abovementioned dyes, AB9 has been associated with mutagenic effects and bioaccumulation, AR51 with cancer, and AY23 with allergic reactions (Alsolaiman and Howard 2003; Hess 2002; Sasaki et al 2002; Groten et al 2000; Aziz et al 1997).
2 Materials and Methods
2.1 Reagents
Acid dyes were supplied by Tricon Colors and used as received. The photocatalyst was the TiO2 P25 supplied by Degussa (30 nm particle size, and 50 ± 15 m2 g−1 BET surface area) (Degussa 1990). Solutions were prepared with distilled water.
2.2 Photocatalytic Experiments
A solution with the three dyes at 25 mg L−1 was used. Experiments were carried out in a 250-mL thermostated (30 ± 1°C) cylindrical (7 cm diameter, 9 cm height) Pyrex reactor containing 50.00 mL of the solution at pH = 7.0. The reaction mixture inside the reactor was maintained in suspension by magnetic stirring. In all experiments, air was bubbled continuously through the suspension. The concentration of TiO2 in the suspension was previously optimized and found to be 1.8 g L−1 (Figueiredo et al 2004). In order to remove the photocatalyst particles before analyses, samples were filtered through 0.45-μm-pore-size cellulose acetate filters. Adsorption and photolysis control experiments were also performed.
The experiments with solar light were performed during the month of July of 2007, in days with no clouds, from 11 to 13 h in order to use a period of approximately constant solar radiation, as depicted in Fig. 1. The 24th of July at noon was taken as a typical day and time for the calculations. The hour-based solar radiation was calculated by the software Radiasol (GESTE-PROMEC 2007a). The software input data were: Resende's geographical coordinates (44° 28′ 52″ W and 22° 28′ 41″ S), slope angle (0°), and azimuthal angle (0°).
The spectral distribution of the solar radiation was calculated with the aid of the software Espectro Solar (GESTE-PROMEC 2007b). The software input data were: average altitude of the O3 layer (22 km, a usual value), aerosols concentration coefficient (1.3, also a usual value), temperature (28°C), relative humidity (62%), visibility (10 km) (Folha Online 2007), altitude (407 m), Resende's geographical coordinates (44° 28′ 52″ W and 22° 28′ 41″ S), surface albedo (0.20, for common soil), and scattering albedo (0.90, for mainly agricultural lands). The azimuthal angle (0°) and zenithal angle (42°) were calculated by the software. The spectral distribution obtained is shown in Fig. 2. The irradiance within the UV-B (280–320 nm), UV-A (320–400 nm), and Visible (400–700 nm) regions were 3.48, 31.3, and 313.2 W m−2, respectively.
2.3 Analyses
Samples were irradiated until color and dye removal and mineralization degree were greater than 99% and 95%, respectively. Those criteria were followed by integrating the spectra obtained by scanning the samples from 400 to 700 nm (visible region) with a Shimadzu UV-160A spectrophotometer and measuring dissolved organic carbon (DOC) with a Shimadzu TOC-5000A Carbon Analyzer.
The degradation of each dye was followed by applying the Lambert–Beer equation to the measured absorbances at their respective λ max, shown in Fig. 3, and the previously determined absorptivities, shown in Table 2. The system of linear equations was then solved to obtain the concentration of each dye.
3 Results and Discussion
3.1 UV-Visible Spectrophotometry
Adsorption control experiments showed negligible adsorption of the dyes onto the TiO2 surface. On the other hand, photolysis has been proven to play an important role in the degradation of the studied dyes. Changes in absorbance spectra of irradiated-only samples are shown in Fig. 4a. It can be seen that AR51 is efficiently photolytically degraded; the same cannot be said regarding AB9 and AY23, although some degradation is observed. Figure 4b shows the changes with the photocatalytic system, which presents a much higher performance, giving no signal in the visible region with extended periods of irradiation.
Figure 5 shows the degradation profiles obtained after solving the linear system of Lambert–Beer equations. Photolysis is able to easily destroy AR51 within 120 min of irradiation, retaining only 2% of the initial absorbance, as can be seen in Fig. 5a. The behavior of AB9 and AY23 is completely different. In fact, approximately no degradation is observed during the first 75 min. Their photolysis only begins after AR51 is practically removed. It seems that AR51 acts like an inner filter impairing photons to reach the AB9 and AY23 molecules. Even after 120 min of irradiation, only 7% of each dye is degraded, approximately. By observing Fig. 5b, one can see that, during photocatalysis, the behavior of the system is absolutely different. Regarding AR51, the degradation was approximately three times faster. AB9 and AY23 presented a much more pronounced difference between photolysis and photocatalysis: they were both completely degraded with 120 min of irradiation. Comparing the three dyes, one would say that xanthenic dyes are the easiest to degrade, followed by azo and triarylmethanic ones.
3.2 Kinetic Modeling and Decolorization Rates
The primary goal of this work is to assess the feasibility of solar heterogeneous photocatalysis for color removal of the dye bath. Therefore, the decolorization kinetics and the respective rates were investigated.
A previous work has shown that the photocatalytic degradation of AR51 could be described by zero-order kinetics (Doushita and Kawahara 2001). Therefore, it was checked if the same model could be applied to the photolytic color removal data, as the other two dyes did not undergo significant degradation. Figure 6 shows that color removal indeed followed zero-order kinetics. Moreover, it can be seen that the data presented two different slopes: one up to 75 min of irradiation and another after that. A least square fit to the data gave the following parameters: k 0–75 = 0.0033 ± 0.00047 ACU min−1 (R 2 = 0.9995) and k 75–120 = 0.0015 ± 0.00025 ACU min−1 (R 2 = 0.9998), where ACU stands for arbitrary color units. Therefore, the initial decolorization rate, after 75 min, is decreased by one half. It is worth mentioning that this change matches the beginning of AB9 and AY23 photolysis. With fewer photons reaching AR51 molecules, it is expected a decrease in its decolorization rate. Figure 6 also shows that approximately 32% of the initial color was removed by the photolytic process.
Other works indicate that photocatalytic dye degradation in liquid systems can be described by first-order kinetics (Julson and Ollis 2006; Tarasov et al 2003; Lachheb et al 2002; Tanaka et al 2000; Vinodgopal et al 1996). Then, it was also checked if this model could be applied to the data for the photolytic color removal of the dye bath. However, the semilog data plot, shown in Fig. 7a, did not produce a single straight line. Therefore, a simple first-order model is not suited for the entire period of irradiation.
In fact, the semilog plot of absorbance vs time presents three regions (dashed lines), suggesting a two-first-order in-series reaction (k 1 and k 2) during the first 75 min, as depicted by Eq. 1, followed by another first-order reaction (k 3) up to 120 min. The two-first-order in-series approach has been used to model the photocatalytic degradation of AB9 in solid phase (Julson and Ollis 2006).
As both reactions are first-order, the batch model for the concentration of the dyes, C D, and of the intermediates, C I, with time can be described by Eqs. 2 and 3:
Measured absorbances are the sum of contributions from the dyes and intermediates; therefore, the Lambert–Beer equation and Eqs. 2 and 3 were used to obtain Eq. 4, where a D and a I are the absorptivities of the dyes and the intermediates, respectively.
The parameters k 1 and k 2 and the ratio a I/a D were first estimated by evaluating the limiting values of Abs t . For long periods, it was assumed that k 1 is greater than k 2 \(\left( {e^{ - k_2 t} \gg e^{ - k_1 t} } \right)\), yielding Eq. 5. Data obtained for degradation times greater than 45 min were fit to Eq. 5 to obtain k 2 and the slope (S) of the best fitted line.
For short periods, the exponential terms in Eq. 4 were expanded to provide the first two terms in the series and rearranged to give Eq. 6. Data obtained for degradation times up to 15 min, together with k 2 and S estimates, were fitted to Eq. 6, yielding an estimate of k 1.
Finally, Origin® software was used to perform a nonlinear curve fit to the absorbance data, using the Levenberg–Marquardt algorithm. Equation 4 was inserted into the software and the obtained estimates were used as initial guesses for the kinetic parameters. The values determined for k 1 and k 2 were 0.087 ± 0.0055 min−1 and 0.022 ± 0.0016 min−1, respectively, with a determination coefficient, R 2, of 0.998. Figure 7b shows the kinetic model fitted to the experimental data. It can be seen that the proposed model is able not only to represent the solid-phase photocatalytic degradation of a single acid dye but also the photolytic color removal of a mixture of them.
After applying a first-order reaction model to the data after 75 min, the estimated value for k 3 was 0.070 ± 0.00041, with an R 2 around 1.00. Figure 7b also shows that approximately 100% of the initial color was removed by the photocatalytic process.
By comparing the photolytic and photocatalytic processes, although the kinetic constants reflect different orders, one can see that the photocatalytic process is approximately one order of magnitude faster than the photolytic one, with the role of hydroxyl radicals in the oxidation becoming clear. In fact, whereas photolysis removed 32% of the initial color in 120 min, photocatalysis presented the same performance in only 7 min, approximately.
3.3 DOC and Mineralization Degree
In order to obtain a suitable concentration profile vs time for both processes, lumps were defined as follows: lump 1, dyes; lump 2, intermediates; and lump 3, CO2. Dye concentrations, C D, in milligrams of dye per liter, determined by spectrophotometry, were converted into milligrams of DOC per liter by Eq. 7, where m(C) stands for the weight of carbon in the dye molecule and m(Dye) stands for the dye molar weight.
The intermediate concentrations, C I, were determined by Eq. 8, where C C stands for CO2 concentrations.
Finally, C C is calculated by Eq. 9, where DOC0 is the initial organic carbon concentration and DOC t is that during degradation.
Figure 8 shows the obtained profiles using the above methodology. In Fig. 8a, related to photolysis, it becomes clear that intermediate concentrations were quite low, ranging from 3% to 4% of the initial DOC. This means that degraded dyes were successfully mineralized, reaching a 24% mineralization degree in 120 min. Figure 8b presents the photocatalytic profiles. It can be seen that it really resembles an in-series reaction. Intermediate concentrations were also low, reaching a maximum of 13% of the initial DOC at 60 min of irradiation. However, 95% of mineralization was achieved with photocatalysis after 120 min of irradiation, a much higher performance when compared to photolysis.
4 Conclusions
The studied acid dyes presented different behaviors during photolysis and photocatalysis.
Although the photocatalytic degradation rate of Acid Red 51 is approximately four times greater than the photolytic one, times are short enough to state that it is efficiently degraded by both processes. However, Acid Blue 9 and Acid Yellow 23 were only sparingly degraded by photolysis. Therefore, one can say that, if an effluent contains only xanthenic dyes, photolysis may be a cost-effective alternative.
On the other hand, heterogeneous solar photocatalysis proved to be a feasible alternative technology to degrade all of the studied dyes. Although they have completely different chemical structures (with different chromophores), they were all degraded with reasonable rates. In fact, this lack of selectivity is a desired advantage when treating complex industrial colored effluents. This is a promising result as the process can be driven by solar light, considerably decreasing the cost involved with the excitation of the photocatalyst.
The order of removal rates was: Acid Blue 9 < Acid Yellow 23 < Acid Red 51. Color was removed somewhat faster than organic matter, although they presented comparable rates. The effluent from photocatalysis was completely colorless, and mineralization degrees above 99% were achieved within 120 min of treatment.
The photocatalytic degradation of the dyes was successfully modeled by a model of two first-order in-series reactions followed by a third first-order reaction. In a 95% confidence level, all of the kinetic constants estimated were statistically significant with appropriate determination coefficients.
References
Alsolaiman, M. M., & Howard, L. (2003). FD&C blue dye no. 1 and blue nail discoloration: Case report. Nutrition (Burbank, Los Angeles County, Calif.), 19(4), 395–396. doi:10.1016/S0899-9007(02)00994-2.
Aziz, A. H. A., Shouman, S. A., Attia, A. S., & Saad, S. F. (1997). A study on the reproductive toxicity of erythrosine in male mice. Pharmacological Research, 35(5), 457–462. doi:10.1006/phrs.1997.0158.
Chang, D. J., Chen, I. P., Chen, M. T., & Lin, S. S. (2003). Wet air oxidation of a reactive dye solution using CoAlPO4 5- and CeO2 catalysts. Chemosphere, 52(6), 943–949.
Chin, P., & Ollis, D. F. (2007). Decolorization of organic dyes on Pilkington Activ (TM) photocatalytic glass. Catalysis Today, 123(1–4), 177–188. doi:10.1016/j.cattod.2007.01.069.
Degussa (1990). Degussa Technical Bulletin Pigments. Highly dispersed metallic oxides produced by the AEROSIL® process.
Doushita, K., & Kawahara, T. (2001). Evaluation of photocatalytic activity by dye decomposition. Journal of Sol-Gel Science and Technology, 22(1–2), 91–98. doi:10.1023/A:1011220505116.
Figueiredo, A. M., Andrade, P. R., & Azevedo, E. B. (2004). Abstracts of the 13a Semana de Iniciação Científica. Rio de Janeiro: UERJ.
Folha Online (2007). Folha Online homepage. http://www1.folha.uol.com.br/folha/tempo/br-resende.shtml. Accessed 14 July 2007.
GESTE-PROMEC (2007a). http://www.solar.ufrgs.br/#radiasol. Accessed 14 July 2007.
GESTE-PROMEC (2007b). http://www.solar.ufrgs.br/#espectro. Accessed 14 July 2007.
Gonçalves, M. S. T., Oliveira-Campos, A. M. F., Pinto, E. M. M. S., Plasência, P. M. S., & Queiroz, M. J. R. P. (1999). Photochemical treatment of solutions of azo dyes containing TiO2. Chemosphere, 39(5), 781–786. doi:10.1016/S0045-6535(99)00013-2.
Groten, J. P., Butler, W., Feron, V. J., Kozianowski, G., Renwick, A. C., & Walker, R. (2000). An analysis of the possibility for health implications of joint actions and interactions between food additives. Regulatory Toxicology and Pharmacology, 31(1), 77–91. doi:10.1006/rtph.1999.1356.
Guaratini, C. C. I., & Zanoni, M. V. B. (2000). Textile dyes. Química Nova, 23(1), 71–78. doi:10.1590/S0100-40422000000100013.
Hagfeldt, A., & Grätzel, M. (1995). Light-induced redox reactions in nanocrystalline systems. Chemical Reviews, 95(1), 49–68. doi:10.1021/cr00033a003.
Hasnat, M. A., Uddin, M. M., Samed, A. J. F., Alam, S. S., & Hossain, S. J. (2007). Adsorption and photocatalytic decolorization of a synthetic dye erythrosine on anatase TiO2 and ZnO surfaces. Journal of Hazardous Materials, 147(1–2), 471–477. doi:10.1016/j.jhazmat.2007.01.040.
Hess, E. V. (2002). Environmental chemicals and autoimmune disease: cause and effect. Toxicology, 181, 65–70. doi:10.1016/S0300-483X(02)00256-1.
Hoffmann, M. R., Martin, S. T., Choi, W. Y., & Bahnemann, D. W. (1995). Environmental applications of semiconductor photocatalysis. Chemical Reviews, 95(1), 69–96. doi:10.1021/cr00033a004.
Hsueh, C. L., Huang, Y. H., Wang, C. C., & Chen, C. Y. (2005). Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere, 58(10), 1409–1414. doi:10.1016/j.chemosphere.2004.09.091.
Julson, A. J., & Ollis, D. F. (2006). Kinetics of dye decolorization in an air–solid system. Applied Catalysis B Environmental, 65(3–4), 315–325. doi:10.1016/j.apcatb.2005.12.021.
Lachheb, H., Puzenat, E., Houas, A., Ksibi, M., Elaloui, E., Guillard, C., & Herrmann, J. M. (2002). Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue). Applied Catalysis B Environmental, 39(1), 75–90. doi:10.1016/S0926-3373(02)00078-4.
Linsebigler, A. L., Lu, G. Q., & Yates, J. T. (1995). Photocatalysis on TiO2 surfaces—principles, mechanisms, and selected results. Chemical Reviews, 95(3), 735–758. doi:10.1021/cr00035a013.
Liu, H. L., & Chiou, Y. R. (2005). Optimal decolorization efficiency of reactive red 239 by UV/TiO2 photocatalytic process coupled with response surface methodology. Chemical Engineering Journal, 112(1–3), 173–179. doi:10.1016/j.cej.2005.07.012.
Meriç, S., Kaptan, D., & Ölmez, T. (2004). Color and COD removal from wastewater containing Reactive Black 5 using Fenton's oxidation process. Chemosphere, 54(3), 435–441. doi:10.1016/j.chemosphere.2003.08.010.
Muruganandham, M., & Swaminathan, M. (2006). Photocatalytic decolourization and degradation of reactive orange 4 by TiO2-UV process. Dyes and Pigments, 63(2–3), 133–142. doi:10.1016/j.dyepig.2005.01.004.
Rao, K. V. S., Lavédrine, B., & Boule, P. (2003). Influence of metallic species on TiO2 for the photocatalytic degradation of dyes and dye intermediates. Journal of Photochemistry and Photobiology A Chemistry, 154(2–3), 189–193. doi:10.1016/S1010-6030(02)00299-X.
Sasaki, Y. F., Kawaguchi, S., Kamaya, A., Ohshita, M., Kabasawa, K., Iwama, K., Taniguchi, K., & Tsuda, S. (2002). The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 519(1–2), 103–119. doi:10.1016/S1383-5718(02)00128-6.
Stafford, U., Gray, K. A., & Kamat, P. V. (1996). Photocatalytic degradation of organic contaminants: Halophenols and related model compounds. Heterogeneous Chemistry Reviews, 3(2), 77–104. doi:10.1002/(SICI)1234-985X(199606)3:2<77::AID-HCR49>3.0.CO;2-M.
Tanaka, K., Padermpole, K., & Hisanaga, T. (2000). Photocatalytic degradation of commercial azo dyes. Water Research, 34(1), 327–333. doi:10.1016/S0043r-1354(99)00093r-7.
Tarasov, V. V., Barancova, G. S., Zaitsev, N. K., & Dongxiang, Z. (2003). Photochemical Photocherical kinetics of organic dye oxidation in water. Process Safety and Environmental Proterction, 81(B4), 243–249. doi:10.1205/095758203322299761.
Uddin, M. M., Hasnat, M. A., Samed, A. J. F., & Majumdar, R. K. (2007). Influence of TiO2 and ZnO photocatalysts on adsorption and degradation behaviour of erythrosine. Dyes and Pigments, 75(1), 207–212. doi:10.1016/j.dyepig.2006.04.023.
Vinodgopal, K., Wynkoop, D. E., & Kamat, P. V. (1996). Environmental photochemistry on semiconductor surfaces: Photosensitized degradation of a textile azo dye, acid orange 7, on TiO2 particles using visible light. Environmental Science & Technology, 30(5), 1660–1666. doi:10.1021/es950655d.
Wang, Y. Z. (2000). Solar photocatalytic degradation of eight commercial dyes in TiO2 suspension. Water Research, 34(3), 990–994. doi:10.1016/S0043r-1354(99)00210rr-9.
Zhang, F., Zhao, J., Zang, L., Shen, T., Hidaka, H., Pelizzetti, E., & Serpone, N. (1997). Photoassisted degradation of dye pollutant in aqueous TiO2 dispersions under irradiation by visible light. Journal of Molecular Catalysis A Chemical, 120(1–3), 173–178. doi:10.1016/S1381-1169(96)00405-r-0.
Acknowledgments
The authors want to thank FAPERJ for financial support and Degussa for providing the photocatalyst.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dias, M.G., Azevedo, E.B. Photocatalytic Decolorization of Commercial Acid Dyes using Solar Irradiation. Water Air Soil Pollut 204, 79–87 (2009). https://doi.org/10.1007/s11270-009-0028-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0028-6