Abstract
Heavy metals and organochlorine residues were determined in water, sediment, fish muscle, and freshwater shrimps from aquatic environments in urban and peri-urban areas in Morogoro, Tanzania. Most of the water samples had heavy metal concentrations below WHO acceptable water quality guidelines. All sediment samples had comparable heavy metal concentrations that suggest natural rather than anthropogenic origin. Hexachlorobenzene, α-hexachlocychlohexane, cis-chlordane, trans-nonachlordane, cis-nonachlordane, pp′-DDE, op′-DDD, pp′-DDD, op′-DDT, and pp′-DDT in hairy river prawn (Macrobrachium rude), African sharptooth catfish (Clarias gariepinus), and Wami tilapia (Oreochromis urolepis) were detected at significant concentrations above the methods’ detection limits. The ratio of pp′-DDT to ∑DDTs was 0.4 in O. urolepis and 0.3 in C. gariepinus, which indicated previous rather than current use of DDT. In M. rude, only pp′-DDE was detected and in O. urolepis and C. gariepinus there were higher levels of pp′-DDE than pp′-DDT, which demonstrate uptake of pp′-DDE, rather than pp′-DDT, from the environment. Bioaccumulation of organochlorines and mercury was the highest in C. gariepinus, cadmium in M. rude, and lead in both M. rude and O. urolepis. Maximum detected levels of organochlorine pesticides and heavy metals in M. rude, O. urolepis, and C. gariepinus were below the maximum permissible concentrations recommended by FAO/WHO. It is concluded that, at present, the contribution of anthropogenic sources in pollution of aquatic environments in Morogoro urban and peri-urban areas are low and that the concentrations of heavy metals and organochlorine pesticides in water and fish do not indicate a risk to the consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals and organochlorine pesticides are among biosphere pollutants of global concern, due to their ability to bioconcentrate and bioaccumulate up the food chain, environmental persistence, and chronic toxicity. The organic and inorganic pollutants entering water bodies originate from natural and anthropogenic sources (Connell et al. 1999). Whereas weathering of rocks, soils, and volcanic eruptions are among the natural sources, the main anthropogenic sources include industrial, mining, urbanization, and agricultural activities. Once these chemicals enter into aquatic environments, they distribute in water, sediment, and biota compartments. In general, each of these compartments may have a distinct contribution to the ecosystem’s health. From ecological monitoring and human health perspectives, establishing information on presence, distribution, and quantities of contaminants in samples from different matrices such as water, sediments, and edible aquatic biota provides useful information for environmental risk assessment (Boon et al. 2002; van der Oost et al. 2003; Erdogrul et al. 2005).
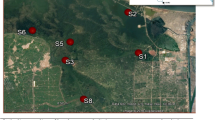
In 2002, Morogoro urban and peri-urban areas, located about 200 km west of Dar es Salaam city in Tanzania, were reported to have a human population of 300,000 (URT 2002). Over 75% of the population receives water for domestic, agriculture, fishing, and industrial purposes from Mindu Dam and rivers in the Mindu Dam Catchment Area (MDCA) (Fig. 1). Because of leaching and runoff from agricultural activities in MDCA, industrial and domestic effluents, oil spills, and traffic emission along the Tanzania–Zambia highway and fishing activities, there have been public concerns for many years about the quality of water and the consumed biota from Mindu Dam and other water bodies in the area. For the time being, there are more concerns on heavy metal pollution due to the illegal small-scale gold mining and associated use of mercury taking place in the Mindu area. Although there are several possible anthropogenic sources of pollution in water bodies in Morogoro urban and peri-urban areas, there is limited information regarding the type and extent of contamination in water, sediments, and biota. Furthermore, there is scanty information about health risks originating from the use of water and consumption of fish from aquatic environments in Morogoro. The objective of this study is to determine the presence and concentrations of heavy metals and organochlorines in water, sediments, and freshwater fish from the study area. Because aquatic organisms can bioconcentrate and bioaccumulate heavy metals and organochlorines at different levels, depending on their habitat and position in trophic levels, C. gariepinus (African sharptooth catfish), O. urolepis (Wami tilapia), and M. rude (hairy river prawn) which are benthic, benthopelagic, and pelagic species, respectively, were included in the study.
2 Materials and Methods
2.1 Study Area
This study was carried out in Mindu Dam, rivers, and swampy areas located in Morogoro urban and peri-urban areas at 6.82°S and 37.66°E (Fig. 1). Mindu Dam is a dependable source of water supply and freshwater fishery in Morogoro urban and peri-urban areas. Since the most important species for fishery in Mindu Dam are M. rude, O. urolepis, and C. gariepinus, screening for heavy metals and organochlorines were carried out in these species that were representative from an ecological point of view. In fish, only the muscles, which is the main part of fish consumed, was used. Other species available in Mindu Dam include Petrocephalus steindacheri, Brycinus imberi, Haplochromi sp., Anguilla mossambica, A. bengsenis labiata, Barbus kersteni, Synodontis sp., and Labeo cylindicus, but these are not included in the study.
2.2 Sample Collection, Shipment, and Storage
Water and bottom sediment samples were collected from 14 different sampling points (Fig. 1) that included Mzinga river at SUA intake (1), Mzinga river (2), Ngerengere river (3), Likulunge river (4), Mlali river (5), entrance of rivers in Mindu Dam (6), center at Mindu Dam (7), exit point of water in Mindu Dam (8), upstream at Ngerengere river bridge (9), downstream Ngerengere river bridge (10), Kichangani swampy area (11), Mwembesongo river (12), exit point at Mafisa sewage ponds (13), and Ngerengere river at VETA Kihonda (14). Additional water samples were collected from tap water originating from Mzinga River at Sokoine University of Agriculture (SUA) and at Mindu Dam. All samples were collected in duplicates from each of the sampling points. Bottom sediment samples were collected using a stainless steel shovel and soon after their collection, they were placed in sterile sampling glass bottles, about three-quarters full. In order to prevent oxidation of collected samples, the remaining space was filled with water, collected using the same stainless steel sampling shovel, from the same sampling point. Samples were placed in cool boxes with ice packs and were transported to the laboratory within 2 h of their collection. In the laboratory, samples were stored at 4°C until analysis. Water samples were collected from same sampling points and were collected by dipping sterile sampling bottles straight into the water. During collection of tap water, the tap was left running for about 2 min, in order to empty standing water, after which water samples were tapped straight into sterile sampling bottles. The pH was determined and then adjusted to 2, using analytical quality concentrated nitric acid. Collected water samples were stored and transported as described above for sediments. O. urolepis and C. gariepinus with body weights of between 200 and 400 g were caught in Mindu Dam. Fish were transported to the laboratory alive. Muscle samples, approximately 2 cm3, were collected from just below the dorsal fin, above the midline just caudal to the pectoral fins. Collected samples from each of the sacrificed fish were stored at −20°C soon after their collection until analysis. In addition, whole fish species of M. rude were also collected from Mindu Dam and were stored at −20°C until analysis.
2.3 Analysis of Heavy Metals in Water and Sediments
Heavy metal analysis in water and sediment samples were carried out at the Southern and Eastern African Mineral Centre (SEAMIC) in Dar es Salaam, Tanzania. Water was decanted from sediment samples and sediments were air dried in an air-conditioned room set at 25°C and 65% relative humidity. Sediments were further dried in an oven at 45°C for 48 h and then milled using an Agate Planetary Micro-mill (Fritsch). The resulting fine dry powder was digested for analysis of heavy metals. For analysis of copper (Cu), lead (Pb), zinc (Zn), chromium (Cr), and cadmium (Cd), the sediment samples were digested using the aqua regia digestion system (3:1 parts of HCl to HNO3). Dry powdered sediments (0.5 g) were mixed with 1.5 mL concentrated HCl and 0.5 mL concentrated HNO3 in a graduated test tube and digested on a hotplate at 95°C. The digest was cooled to room temperature and diluted with deionized water to 10 mL. After settling overnight, samples were ready for analysis. For analysis of mercury (Hg) and arsenic (As), the sediment samples were digested in the same manner but using a water bath at 70°C and with a final volume of 20 mL. The analytical procedure for these two heavy metals is described below.
Water samples were measured directly for Cu, Pb, Zn, Cr, and Cd following aspiration of samples without filtration and therefore were analyzed as dissolved metals. Samples were analyzed for these heavy metals using a GBC 906 flame atomic absorption spectrophotometer (ICP Ultima 2, Horiba Jobin Yvon, France). Analysis for total Hg was carried out using the atomic absorption spectrophotometer cold vapor generation technique (ICP Ultima 2, Horiba Jobin Yvon). Briefly, 50 mL of the sample was mixed with 10 mL 1:1 H2SO4 to 2% potassium permanganate solution. The mixture was allowed to stand for 15 min, then 1 mL of 5% potassium sulfate was added. The mixture was heated in a water bath at 95°C for 1 h. After cooling, 3% hydroxylamine solution was added, until the permanganate turned colorless. The digested sample (10 mL) was acidified, using 10 mL 6 M HCl. Samples were then analyzed for total Hg by atomic absorption spectrophotometry (AAS), with a hydride generation system, against standards which were digested together with the samples. Similarly, dissolved As in water and in aqua regia soluble solutions from sediments were measured using AAS with a hydride generation system. Briefly, 10 mL of water or sediment was mixed with 10 mL 6 M HCl in a 50-mL test tube. After adding 1 mL of KI–ascorbic acid solution, the mixture was vortexed thoroughly and measured using the AAS hydride generation (HG) system against standards, which were digested together with the samples.
2.4 Metal Analysis in Fish and Shrimps
Muscle samples from fish and whole freshwater shrimps were homogenized and analyzed for Hg, Cd, and Pb at the National Veterinary Institute in Norway, in the Chemistry Section of the Department of Feed and Food Hygiene. This laboratory is accredited for these analyses, in accordance with the requirements of ISO/IEC 17025:2005. Approximately 0.8 g of the samples were treated with nitric acid and hydrogen peroxide, and digested by microwave (Milestone, Ethos Plus) under pressure at 180°C. The homogenate was then diluted with deionized water to 25 mL. Samples were analyzed for Cd and Pb using a graphite furnace atomic absorption spectrophotometer (ETAAS), and for Hg using a cold vapor generation system (CVAAS) with SnCl2 reduction. Modifiers that included mixtures of palladium/magnesium nitrate for Cd and palladium/ammonium phosphate for Pb were used. Both elements were analyzed using a L’VOV platform on a Varian Instrument (Spectrophotometer AA 600 Varian, Australia) equipped with deuterium background compensation. Mercury was analyzed on the same instrument, but equipped with a continuously flow system through a quartz cell.
2.5 Analysis of Organochlorines
The analysis of organochlorines was performed at the Laboratory of Environmental Toxicology at the Norwegian School of Veterinary Science, Oslo, Norway. The analytical method is described in Brevik (1978) with slight modifications. In brief, fish muscles and whole freshwater shrimps were thawed and about 8 g of sample were homogenized using an Ika Ultra Turrax. Samples were transferred into glass centrifuge tubes and PCB 29, 112, and 207 were added as internal standards (Promochem, Wesel, Germany). Acetone and cyclohexane were used for extraction of lipids and percentage fat content was determined gravimetrically. The organic layer, containing fat and organochlorine compounds, was concentrated by evaporation. A more detailed description of the methods used for extraction, fat determination, concentration, and clean-up is given in Murvoll et al. (2005). The samples were analyzed for polychlorinated biphenyls (PCBs) (PCB 28, 52, 47, 74, 66, 101, 99, 149, 118, 114, 153, 105, 137, 138, 187, 183, 128, 156, 157, 180, 170, 189, and 194) and organochlorine pesticides (hexachlorobenzene (HCB), β-hexachlorocyclohexane (β-HCH), oxychlordane, p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE) and mirex) using a gas chromatography–electron capture detector (GC–ECD). For GC analysis, 2 µL of the extracts were injected by autosampler (Agilent 7683 series auto sampler) to a GC (Agilent 6890 series) with an EC detector (Agilent 6890 Series) (all from Agilent Technologies, Avondale, PA, USA). Further modifications in the organochlorine analysis methodology included pulse pressure set to 60 psi, with a temperature of 270°C, a dual column (SPB 5 and SPB-1701), and total run time of 69.6 min (90°C–2 min, increase to 180°C by 25°C/min, 180°C–2 min, increase to 220°C by 1.5°C/min, 220°C–2 min, increase to 275°C by 3°C/min, 275°C–15 min). The laboratory is accredited for these analyses, in accordance with the requirements of ISO/IEC 17025:2005. Standard procedures were used to ensure adequate quality assurance and control, and the results were within the laboratory’s accredited requirements. Five-point linear calibration curves of the analyzed standard solutions were used. The detection limit for different congeners was 0.15–1.1 ng/g w/w. Two blank samples consisting of solvents were analyzed for every batch of ten samples. The mean percentage recovery and coefficient of variation (% CV) of the relevant congeners in the spiked samples were 85–91% and 5–7%, respectively.
2.6 Comparison of the Detected (Analyzed) Levels with Tolerable Daily Intakes
The tolerable daily intakes (TDI) for total chlordane, DDT, HCB, HCH, cadmium, lead, and mercury are 0.0005, 0.005, 0.0006, 0.0003, 0.001, 0.0035, and 0.002 mg/kg body weight, respectively (WHO 2006). Maximum acceptable residue levels (MARL) were calculated from the TDI values (Henry and Kishimba 2006), with an assumption that a 60-kg person in the Mindu fishing community consumes about 0.2 kg of fresh fish per day. Fish consumption in the Mindu community is one to three times a day and 7 days a week. This assumption was based on information collected from fishermen and the actual weight of fish taken home for family consumption during sampling days. Generally, one to three fish (total average weight of about 1.2 kg minus 50% of non-edible parts were consumed per day in a household of three to five people.
Therefore, the calculation for MARL was as follows:
-
Assumptions: Fish consumption pattern was 0.2 kg fresh weight fish/60 kg person/day.
-
\({\text{The}}\;{\text{MARL}} = \frac{{{\text{TDI}}\;\left( {{{{\text{mg}}} \mathord{\left/ {\vphantom {{{\text{mg}}} {{\text{kg}}\;{\text{body}}\;{\text{weight}}}}} \right. \kern-\nulldelimiterspace} {{\text{kg}}\;{\text{body}}\;{\text{weight}}}}} \right) \times {\text{Body}}\;{\text{weight}}\;\left( {{\text{kg}}} \right)}}{{{\text{Amount}}\;{\text{of}}\;{\text{fish}}\;{\text{consumed}}\;{\text{per}}\;{\text{day}}\;\left( {{\text{kg}}} \right)}}\)
-
The MARL obtained was then compared with the maximum concentrations of heavy metals and organochlorine pesticides that were detected in the samples (Table 6).
3 Statistical Analysis
Due to the limited number of samples analyzed and the results for analyzed samples that had residues BDL, only a few statistical comparisons were possible. Concentrations of heavy metals and organochlorines were compared between species using non-parametric multiple comparison tests. The Kruskal–Wallis and the Mann–Whitney–Wilcoxon tests were used to detect significant differences of contamination levels among fish species and freshwater shrimp, using JMP® Software for Statistical Visualization version 6 (SAS Institute Inc.).
4 Results and Discussion
Most of the water samples from different aquatic environments had heavy metals concentrations below WHO (2006) accepted drinking water quality standards (Table 1). Water samples from Kichangani swampy area (Fig. 1) had 29-, 20-, and 2.2-fold higher levels of Pb, Cd, and Hg, respectively, than WHO (2006) accepted drinking water quality standards. Railway workshops were the most probable sources of the high heavy metal concentrations detected in this area. Higher levels of Hg compared to WHO (2006) accepted drinking water quality standards were also detected in water samples from Mlali River (2.4-fold) and below the bridge in Ngerengere River (4-fold) (Fig. 1). High levels of lead were detected only in water samples from the center of Mindu Dam (Fig. 1), at levels that were five times higher than the WHO (2006) accepted drinking water quality standards. Since none of the rivers flowing into the dam had high concentrations of Pb, the possible source of the detected concentrations is probably traffic emissions and oil spills along the Tanzania–Zambia highway. The total Cr levels were 1.6-fold higher at Mzinga River near SUA intake (Fig. 1) than the WHO standards. Due to minimal industrial activities undertaken in the river basin, the levels of total Cr detected reflect presence or use of chromium-containing compounds such as wood preservatives and fungicides in the area. The center of the Mindu Dam had higher concentrations of lead, both in water and in the sediment, that suggested their origin from anthropogenic activities. However, the treated tap water from Mindu Dam and Mzinga river at SUA had levels below the detection limit or very low concentrations.
All heavy metals analyzed were detected at comparable concentrations in sediments from different sampling sites (Table 2). This and the low concentrations suggest natural background sources such as soil erosion, weathering of rocks, and deposition from air rather than anthropogenic sources. Although pollution of water bodies in Morogoro has over the years been speculated to originate from various sources, such as industrial discharges, agricultural activities, sewage, and domestic wastes (Bwathondi et al. 1992), metal levels detected in sediments in this study suggest minimal input from anthropogenic sources to the total pollution loading. For instance, Mzinga Corporation (an industry using heavy metals) has, over the years, been suspected of discharging industrial effluents to Mindu Dam via Mzinga River. Findings from this study, however, did not indicate a significant contribution from this point source, because heavy metal concentrations in water and sediment samples collected at Mzinga River at SUA intake (upstream of Mzinga Corporation) were not significantly different from and downstream of Mzinga river (below Mzinga Corporation). Similar findings were also reported in previous studies (Bwathondi et al. 1992).
Sediment quality control regulations in aquatic ecosystems varies considerably within and between countries and locations (ANZ 2000), thus most countries use country-specific reference values. Tanzania however lacks it own sediment quality guideline for heavy metals. In this study, measured heavy metal concentrations in sediment were therefore compared with the consensus-based sediment quality guideline values referred to as the probable effect concentration (PEC) proposed by MacDonald et al. (2000). The PEC guideline was selected for comparison because various evaluations have demonstrated that these guidelines provide a unifying synthesis of the existing sediment quality guidelines and reflect causal rather than correlative effects (MacDonald et al. 2000). Based on this comparison, all sediment samples had heavy metal levels lower than the PEC values.
Different concentrations of Cd, Pb, and Hg were detected in three different aquatic species (Fig. 2) and the accumulation orders were inconsistent (Fig. 3a). The accumulation orders Pb were C. gariepinus > M. rude > O. urolepis; for Hg M. rude > O. urolepis > C. gariepinus; and for Cd M. rude > C. gariepinus > O. urolepis. Accumulation of Hg was significantly higher in C. gariepinus than in O. urolepis (P < 0.01). Variations in accumulation order for heavy metal concentrations other than Hg and the observed significant difference in concentrations of Hg between species were probably due to differences in trophic levels, habitat preferences, and feeding behaviors (Barron 1990; Traunspurger and Drews 1996). Aquatic organisms are good indictors of environmental pollution because they concentrate pollutants in their tissues directly from water and sediments, and through them, it is also possible to assess the bioaccumulation of pollutants in the food chain (Boon et al. 2002). The ability of aquatic organisms to concentrate heavy metals is clearly demonstrated in Table 3. Although Cd and Hg were not detected in water samples from Mindu Dam, possibly due to insignificant concentrations, they were detected in all organisms studied. This suggests the relevance of using samples from different matrices when assessing the status of pollution in environments.
The percentage of fish samples in which the organochlorine pesticides were detected is shown in Table 4 and their concentrations in Table 5. Organochlorine pesticides detected included HCB, γ-HCH, cis-chlordane, cis- and trans-nonachlordane, p,p′-DDE, o,p′-DDD, p,p′-DDD, o,p′-DDT, and p,p′-DDT. PCBs were not detected. Generally, concentrations of organochlorine pesticides detected in fish and freshwater shrimp were low. This might have been due to the ban on the use of most of the organochlorine pesticides in Tanzania, in response to the Stockholm Convention on the use of Persistent Organic Pollutants (POPs) that came into implementation in the late 1990s (Madete and Enock 2005). Use of organochlorine pesticides has been replaced by the less persistent organophosphates, carbamates, and pyrethroids (Ngowi et al. 2007). Although these pesticides are relatively more expensive and toxic compared to organochlorines, they are recommended for use because of their shorter half-life and low persistence in the environment.
Hexachlorobenzene, which in the past was commonly used as a fungicide for seed treatment, is no longer registered for use in Tanzania. Thus, the low concentrations of HCB that were detected in fish might have originated from other sources, such as solvents, dyes, plastics, incinerators, etc. Since HCBs can be carried by air over long distances, the sources could be other contaminated areas, away from Morogoro urban and peri-urban areas. In the current study, γ-HCH was the only isomer that was detected at low concentrations in samples from O. urolepis and C. gariepinus, suggesting past use of γ-HCH in horticultural crops in the area. In the past, chlordane was used extensively as a broad spectrum insecticide to control termites in agriculture. The isomer that was detected in all species was trans-nonachlordane that also suggested past use. The p,p′-DDE, a metabolite of p,p′-DDT, was detected at higher concentrations than other DDT metabolites in samples from all species investigated in Mindu Dam. This finding was similar to that reported in other related studies (Mwevura et al. 2002; Erdogrul et al. 2005). The ratio of p,p′-DDT to ∑DDTs in O. urolepis was 0.4 and in C. gariepinus was 0.3. Since in both fish species the ratios were less than 0.5, this suggested past, rather than recent, use of DDT in the study areas. In M. rude, p,p′-DDE was the only DDT metabolite that was detected and in O. urolepis and C. gariepinus there were higher levels of p,p′-DDE than p,p′-DDT. This indicated uptake of p,p′-DDE from the environment, rather than p,p′-DDT.
The accumulation order for organochlorine pesticides detected was C. gariepinus > O. urolepis > M. rude for all pesticides, except total chlordane, for which the order was C. gariepinus > M. rude > O. urolepis (Fig. 3b). The observed difference in concentrations of organochlorine pesticide could be attributed to chemical factors such as water solubility, polarity, shape, and size as well as organisms’ factors such as lipid content, age, feeding behavior, and habitat (Esser and Moser 1982). Other studies observed a higher bioaccumulation of organochlorine pesticides in benthic than in pelagic fish species (Pazou et al. 2006). Similarly, in this study, pesticide levels were higher in C. gariepinus, which is benthic, than in O. urolepis and M. rude, which are benthopelagic and pelagic species, respectively.
The TDI for organochlorine pesticides and heavy metals are shown in Table 6. The levels detected in M. rude, O. urolepis, and C. gariepinus were below the maximum acceptable residual limit (MARL). These findings suggest that there is no indication of risks to the consumers of fish in Mindu Dam.
5 Conclusion
Findings from this study have demonstrated insignificant levels and concentrations of heavy metals and organochlorine residues in water, sediments, and fish in aquatic environment in Morogoro urban and peri-urban areas. Given such insignificant levels and concentrations, it is concluded that leaching and runoff from agricultural activities in MDCA, industrial and domestic effluents, oil spills and traffic emission along the Tanzania–Zambia highway, fishing activities, and illegal small-scale gold mining have no significant contribution to the pollution load. Levels of heavy metals and organochlorine pesticides in water and fish are low and there is no indication of risks to the consumers of fish and users of water in the area. In order to maintain this status, stringent measures against mining operations, in particular gold, in the area and use of persistent organochlorines as well as proper disposal of industrial, animal, and domestic wastes are recommended. Since this study focused on the chemistry of heavy metals and organochlorines in water sediments and fish as well as public health concerns, further studies are recommended in particular on the ecological risk assessments for these chemicals at various levels of biological organizations.
References
ANZ. (2000). Sediment quality guidelines. In: Australian and New Zealand Guidelines for Fresh and Marine Water Quality. Vol 2. Aquatic Ecosystems—Rationale and Background Information. Report, pp 41.
Barron, M. (1990). Bioconcentration. Environmental Science & Technology, 24, 1612–1618. doi:10.1021/es00081a001.
Boon, J. P., Lewis, W. E., Tjoen-A-Choy, M. R., Allchin, C. R., Law, R. J., De Boer, J., et al. (2002). Levels of polybrominated diphenyl ether (PBDE) flame retardants in animals representing different trophic levels of the North Sea food Web. Environmental Science & Technology, 36, 4025–4032. doi:10.1021/es0158298.
Brevik, E. M. (1978). Gas chromatographic method for the determination of organochlorine pesticides in human milk. Bulletin of Environmental Contamination and Toxicology, 19, 281–286. doi:10.1007/BF01685799.
Bwathondi, P. O. J., Fupi, V., Nanyaro, G., Mgini, J., Manduta, M. C., Busumbiro, S., Siafu, D. K., Mhije, Z.J., Masallaand, J., & Mafie, N. Y.(1992). Investigations on the presence of heavy metals (lead and mercury) in Mindu Dam, Morogoro. Report, pp 22.
Connell, D. M., Lam, P., Richardson, B., & Wu, R. (1999). Introduction to ecotoxicology (p. 170). London: Blackwell.
Erdogrul, Ö., Covaci, A., & Schepens, P. (2005). Levels of organochlorine pesticides, polychlorinated biphenyls and brominated diphenyl ethers in fish species from Kahramanmaras, Turkey. Environment International, 31, 703–711. doi:10.1016/j.envint.2005.01.002.
Esser, H. O., & Moser, P. (1982). An appraisal of problems related to the measurement and evaluation of bioaccumulation. Ecotoxicology and Environmental Safety, 6, 131–148. doi:10.1016/0147-6513(82)90001-X.
Henry, L., & Kishimba, M. A. (2006). Pesticide levels in Nile tilapia (Oreochromis niloticus) and Nile perch (Lates niloticus) from Southern Lake Victoria, Tanzania. Environmental Pollution, 140, 348–354. doi:10.1016/j.envpol.2005.06.029.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus based sediment quality guidelines for fresh water ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31. doi:10.1007/s002440010075.
Madete, A. E., & Enock, J.(2005). The Stockholm Convention on Persistent Organic Pollutants (POPs): Overview and Implementation Status in Tanzania. Proceedings of the Multi Stakeholder Workshop on Pesticides and Poverty. Implementing Chemical Conventions for Safe and Just Development. Centre for Continuing Education, University of Dar Es Salaam, Dar Es Salaam, 3rd–5th /08/2005.
Murvoll, K. M., Jenssen, B. M., & Skaare, J. U. (2005). Effects of pentabrominated diphenyl ether (Pbde-99) on vitamin status in domestic duck (Anas platyrhynchos) hatchlings. Journal of Toxicology and Environmental Health, 68A, 515–533.
Mwevura, H., Othman, O. C., & Mhehe, G. L. (2002). Organochlorine pesticide levels in sediments and biota from the coastal area of Dar es Salaam city, Tanzania. Marine Pollution Bulletin, 45, 262–267. doi:10.1016/S0025-326X(01)00331-9.
Ngowi, A. V. F., Mbise, T. J., Ijani, A. S. M., London, L., & Ajayi, O. C. (2007). Pesticides use by smallholder farmers in vegetable production in Northern Tanzania. Crop Protection (Guildford, Surrey), 26, 1617–1624. doi:10.1016/j.cropro.2007.01.008.
Pazou, E. Y. A., Lalèyè, P., Boko, M., van Gestel, C. A. M., Ahissou, H., Akpona, S., et al. (2006). Contamination of fish by organochlorine pesticide levels in the Ouémé River catchment in the Republic of Bénin. Environment International, 32, 594–599. doi:10.1016/j.envint.2006.01.007.
Traunspurger, W., & Drews, C. (1996). Toxicity analysis of freshwater and marine sediments with meio- and macro-benthic organisms: a review. Hydrobiology, 328, 215–261. doi:10.1007/BF00017632.
URT.(2002). The United Republic of Tanzania, Population and Housing Census, http://www.tanzania.go.tz/census
Van der Oost, R., Beyer, J., & Vermeulen, P. E. (2003). Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology, 13, 57–149. doi:10.1016/S1382-6689(02)00126-6.
WHO. (2006). Chemical aspects. In: World Health Organization. Guidelines for Drinking-Water Quality Standards Incorporating First Addendum. Vol. 1, Recommendations. 3rd ed, 145–196.
Acknowledgements
This study was kindly funded by the Norwegian Universities’ Committee for Development Research and Education (NUFU) PRO 08/2002. We express our sincere thanks to technical personnel in particular Mr. Thor Waaler in the Chemistry Department at the National Veterinary Institute in Oslo, Norway, the Laboratory of Environmental Toxicology at Norwegian School of Veterinary Science in Oslo, Norway and SEAMIC in Dare es Salaam, Tanzania.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mdegela, R.H., Braathen, M., Pereka, A.E. et al. Heavy Metals and Organochlorine Residues in Water, Sediments, and Fish in Aquatic Ecosystems in Urban and Peri-Urban Areas in Tanzania. Water Air Soil Pollut 203, 369–379 (2009). https://doi.org/10.1007/s11270-009-0019-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0019-7