Abstract
Desorption of Cu, Cr, Pb, and Zn from industrially polluted soils as a result of acidification is in focus. The eight soils of the investigation vary greatly in composition and heavy metal concentration/combination. Three soils had elevated concentrations of Cu, Pb, and Zn; regardless of pollution level, pollution origin, and soil type, the order for desorption as pH decreased was Zn > Cu > Pb. Turning to a single heavy metal in different soils, there was a huge difference in the pH at which the major desorption started. The variation was most significant for Pb where, e.g., less than 10% was desorbed at pH 2.5 from one soil, whereas in another soil 60% Pb was desorbed at this pH. Sequential extraction was made and the soils in which a high percentage of Pb was found in the residual phase (adsorbed strongest) was also the soils where less Pb was desorbed at low pH in the desorption experiments. It was evident that Cu, Pb, and Zn started to desorb at a higher pH from calcareous soils than from soils with low carbonate content. The mechanism responsible for this is co-precipitation of heavy metals in the carbonates. When the carbonates are dissolved at a relatively high pH of about 5, the co-precipitated heavy metals are released. The sequential extraction pattern for Cr differed generally much from the other heavy metals since the majority of Cr was extracted in the last two steps. Cr was also the heavy metal that desorbed the least at high acidification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Different mechanisms are responsible for the adsorption and retention of heavy metals in polluted soils: specific adsorption, cation exchange, organic complexation, and co-precipitation (Alloway 1995). The affinity of different heavy metals for adsorption to different soil particles is a highly complex matter. For example, Pb adsorbed to a high extent as the only heavy metal to Fe oxides, whereas, e.g., Cd, Cr, Cu, Ni, Pb, and Zn all adsorbed to vermiculite in a study of adsorption of different heavy metals to individual soil components (Covelo et al. 2007). Thus, retention of heavy metals in a specific soil depends on the soil composition as well as on the actual heavy metal.
Quite extensive research has been conducted of adsorption phenomena (e.g., Markiewicz-Patkowska et al. 2005; Martínez and Motto 2000), whereas less research has been made to clarify desorption phenomena. Furthermore, desorption has mainly been examined using freshly spiked soils and the findings may not be comparable to old industrially polluted soil where the heavy metals can be adsorbed stronger due to aging. The importance of aging was illustrated in Ottosen et al. (2006) where sequential extraction was used as a tool for comparing the adsorption strength for Cu in spiked soils and industrially polluted soils. The Cu was clearly adsorbed stronger in the industrially polluted soils than in the spiked soils even though the soil types were very similar. Also, the influence on aging on pore-water concentration of Zn has been investigated and the Zn concentration in the pore water was found to be lower in industrially polluted soils than expected from modelling on basis of spiked soils (Lock and Janssen 2003). Thus, aging is a parameter of great importance for the retention of heavy metals in polluted soils and the effect of aging must be taken into account when discussing desorption patterns. The soils included in the present investigation were industrially polluted at least 20 years before the sampling.
The speciation of heavy metals in the soil is determined by the original speciation at the time of pollution. Jensen et al. (2006) showed that Pb speciation in industrially polluted soils depends on the stability of the original speciation. In fact, the original speciation was the major factor determining the actual Pb speciation in the soils. The pollution level was also seen to play a major role, whereas, quite surprisingly, the soil characteristics were found of secondary importance (Jensen et al. 2006). Since the original speciation of the pollutants determines the adsorption pattern, the original speciation is also expected to influence the desorption pattern significantly.
In the present paper, desorption of Cu, Cr, Pb, and Zn from industrially polluted soils is in focus. It was investigated whether the origin of pollution and the pollution level influence the desorption pattern when the soils are acidified. Also, the relation between desorption pattern and some soil characteristics of major importance to adsorption is included in the study. These parameters being carbonate fraction, organic matter, and clay fraction.
Carbonate Fraction
A soil with high carbonate content can generally contain a higher amount of Pb than a non-calcareous soil (García-Delgado et al. 1996). The formation of heavy metal carbonates can be an important retention mechanism in calcareous soils and thus also determines the desorption pattern. Precipitation of malachite (Cu2CO3(OH)2) may be significant in Cu-polluted calcareous soils (Lund and Fobian 1991). Smithsonite (ZnCO3) (Madrid and Diaz-Barrientos 1992) and cerrusite (PbCO3) (Zhang et al. 1998) are likely to be formed when calcareous soils are polluted with zinc and lead, respectively. Saeed and Fox (1977) investigated the relation between pH and Zn solubility in spiked acid and calcareous soils. Zn was solubilized at a slightly higher pH in the calcareous soils than in the acid soils. A study by Martínez and Motto (2000) confirmed this. From spiked calcareous soil, the heavy metals were desorbed at higher pH values (slightly below neutral) compared to the spiked non-calcareous soils.
Content of Organic Matter
The content of solid-phase humic substances is greatly affecting the adsorption capacity for heavy metals by cation exchange and formation of chelate complexes. Carboxyl groups play a predominant role in metal binding in both humic and fulvic acids (Alloway 1995). Presence of dissolved organic matter may, on the contrary, also decrease heavy metal adsorption, as found for Cu by Mesquita and Carranca (2005). Schnitzer and Kerndorff (1980) investigated the sorption of heavy metals (including Cu, Cr, Pb, and Zn) to humic acid at different pH values and found that Pb was adsorbed to the highest extent in the pH range investigated (2.4 to 5.8) and Zn to the least extent. Thus, the influence from organic matter on the overall adsorption is dependent on the actual heavy metal.
Clay Fraction
The term clay fraction in this paper covers the particles with sizes less than 2 µm. The clay fraction includes both mineral particles (e.g., clay minerals and oxides) and organic particles as small humic particles. The clay fraction contributes significantly to an increased sorption capacity of soils, due to the large surface area (Alloway 1995) with relatively high cation exchange capacity and sites for specific adsorption.
When removal of heavy metals from the polluted soil matrix is the issue, knowledge on desorption of heavy metals from the different adsorption sites is essential. In general, soil pH seems to have the greatest effect of any single factor on the desorption processes, generally with a higher desorption at low soil pH (Alloway 1995). The focus of the present paper is desorption of Cu, Cr, Zn, and Pb from different industrially polluted soils as a result of acidification. The relations between desorption, pH, and the soil parameters carbonate content, organic matter, and clay fraction are investigated in the course of chemical extraction experiments in HNO3 with varying concentrations. It must be stressed that the pH range in the investigation is not relevant for any risk assessment for the sites since the soils are strongly acidified, but knowledge about desorption at different pH values is relevant to remediation processes that aim at removing the heavy metals from soils.
2 Materials and Methods
2.1 Origin of Soils
The investigation covers eight Danish industrially polluted soils. Three of the soil samples were sampled in the upper 30 cm at different polluted sites (soils 1, 2, and 4). Five soil samples were sampled in soil piles after excavation of other polluted sites (soils 3, 5, 6, 7, and 8). The samples from the soil piles cannot be related to a specific sampling depth. The origin of the pollution in the different soil samples is given in Table 1.
2.2 Soil Characterization
The heavy metal concentration was measured after pretreatment of the soil as described in Danish Standard 259: 1.0 g of dry soil and 20.0 ml (1:1) HNO3 were heated at 200 kPa (120°C) for 30 min. The liquid was separated from the solid particles by vacuum through a 0.45-μm filter and diluted to 100 ml. Heavy metal concentration was measured with atomic absorption spectrophotometry (AAS). Five measurements were made for each soil. The unit of metal concentration in soil used in this paper is mg/kg dry matter.
Soil pH was measured by mixing 10.0 g dry soil and 25 ml 1.0 M KCl. After 1 h of contact time, pH was measured using a radiometer pH electrode. The carbonate content was determined by a volumetric calcimeter method as described in Loeppert and Suarez (1995). CEC was measured with a method comparable to the acid–NaCl method described in EPA Standard Method 9080. The grain size distribution was found by a combined sieve and pipette method. An estimate of the organic content was found as loss of ignition at 550°C.
The sequential extraction procedure used is the one recommended by the “Standards, Measurements and Testing Programme of the European Union” (Mester et al. 1998). A residual fraction was however added to the procedure in this investigation. The conditions of the sequential extraction procedure are outlined in Table 2.
2.3 Desorption Experiments
The soil samples were dried at 105°C and crushed lightly by hand in a mortar to crush lumps formed during drying. Soil suspensions of 5 g dry soil and 10 ml of different concentrations of HNO3 were shaken for 48 h. A duplicate was made for each acid concentration. The concentration range of the acid was chosen for each soil from knowledge of the carbonate content, so the buffering capacity of the soil was exceeded in some of the extractions with each soil. After 48 h, pH was measured in the suspensions with a combined radiometer pH electrode. The suspension was filtered through a 0.45-μm filter and the heavy metal concentration was measured in the solution by AAS.
3 Results and Discussion
By visual inspection of the soils, observations that could influence the data evaluation were made for soils 2 and 5. Soil 2 contained small wood pieces/needles of a few millimeters in size. Since this soil was sampled at a wood preservation site, the wood pieces can be impregnated and thus contain Cu and Cr, but whether this is the case is unknown. In soil 5, few small light green particles of up to 3 mm in size were present. In another soil sample from the same site, malachite has been identified by XRD (Kliem and Hansen 2000) and the green particles in soil 3 are also expected to be malachite.
In Table 3, the concentrations of heavy metals in the experimental soils are shown together with the measured characteristics for each soil: carbonate content, organic content, fraction of clay, silt, and sand, CEC, and original soil pH.
In Denmark, there are two sets of limiting concentrations in relation to heavy metals in soils (Miljøstyrelsen 2003). The first is for the most sensitive area use: 500 mg/kg of Cr, Cu, and Zn and 40 mg Pb/kg. The second set is the concentration above which human contact with the soil must be avoided: 1,000 mg/kg of Cr, Cu, and Zn and 400 mg Pb/kg. All experimental soils exceed the highest of the limiting values with at least one heavy metal (see Table 3).
The pollution level of these industrially polluted soils is expected related to the spillage of the pollutant rather than the retention capacity of the soil. The retention capacity is probably not exceeded for any of the soils at the time of sampling, because all pollutions are old and mobile parts of heavy metals had been washed out to deeper soil layers in the years between spill of pollutants and sampling date.
3.1 Comparison of Experimental Soils
In Table 3, the percentages of carbonates, organic content, and clay fraction of the different soils are shown. The soils differ relatively much in the content of these three components. The content of organic matter (estimated as loss on ignition) was between 2% and 10%. Loss on ignition is not an exact measure for the organic content since, e.g., crystalline water of clay particles will also add to this factor. Nevertheless, the method is sufficiently precise to see that neither of the soils is rich in organic matter, though soils 2 and 7 had an organic content that can significantly influence the adsorption behavior. The carbonate content was less than 0.6% in soil samples 1, 2, and 4, which were the soils sampled at polluted sites (top soils). The carbonate content of these soils was lower than the carbonate content in the soils sampled from excavated soil, in which the carbonate content was between 3.3% and 14.4%. The difference in carbonate content is due to the natural weathering processes of the upper layers of the soil, where soils 1, 2, and 4 were sampled. In the samples from already excavated soils, the upper part of the soil was mixed with soils from deeper layers with higher carbonate content and thus the overall carbonate content was higher. The clay fraction of the three top soils was also higher than for the mixed soils. Especially soil 2 and 4 had high content of clay (19% and 11%, respectively). The lower content of carbonates and the higher content of clay fraction in the top soils make it difficult to predict the retention capacity of these soils relative to the other soils since the lower carbonate content is related to less adsorption capacity whereas, on the contrary, the high clay content is linked to a higher adsorption capacity. The CEC of the soils 2 and 4 was high compared to the other soils and thus these soils had more sites for non-specific adsorption. The large clay fraction of the two soils is responsible for this high CEC. It can be noticed that the soil with highest percentage of carbonates (soil 8) has the lowest CEC of the studied soils, and this may be due to the limitations of the measurement for soils containing minerals that are partly dissolved during the procedure of CEC measurement as calcareous particles (Dohrmann 2006). However, soil 8 has low organic content and low clay content and can be expected to have a low CEC.
Between the upper soils, the retention capacity of soil 1 is considered low compared to soils 2 and 4 due to the lower carbonate content, organic content, and clay fraction. Of the excavated soils, it is not possible to arrange the soils after expected retention capacity, since the influence of the adsorption on carbonates, organics, and clays is not known relatively to each other.
3.2 Sequential Extraction
The results from sequential extraction are shown in Fig. 1a–e as the concentrations extracted in each step. Compared to the initial concentration, 89–110% of the heavy metals were recovered through the sequential extraction, which is considered acceptable, since industrially polluted soils are inhomogeneous both in what regards soil matrix and pollution level. Caution must always be taken when evaluating sequential extraction results and each step cannot be related solely to one retention mechanism. Still, sequential extraction provides valuable information on the retention strength of the heavy metals and the overall adsorption pattern.
Results from sequential extraction. a Cu distribution in different soils, b Cr distribution in different soils, c Cu and Cr distribution in soil 2 (the concentrations are too high to be shown together with the other Cu- and Cr-polluted soils), d Pb distribution in different soils, e Zn distribution in different soils
The sequential extractions showed few points to be generalized. Except from Cr being associated little to the first two fractions and Zn most to these fractions, the sequential extraction pattern differed significantly between soils and heavy metals. There was no correlation between the concentration and distribution found in the sequential extraction. Further, there was no correlation between organic content and the fraction of heavy metals extracted in step 3, which is the step linked to the organic fraction. There was no correlation between CEC and the part of heavy metals extracted in the first step of the sequential extraction, e.g., soil 5 has a low CEC but more than 1,000 mg Cu/kg is extracted in this step. This might be explained for soil 5 with the 9.7% carbonates and the Cu co-precipitated in the carbonates are expected to be extracted in this phase, too. The retention mechanisms contributing to the first step are complex.
In the industrially polluted soils, the adsorption mechanisms can influence each other, complicating consistent conclusions on the basis of sequential extraction. For example, step 2 generally indicates the amount of metal bound to oxides. However, the actual type of oxides present is influencing the adsorption. For example, Covelo et al. (2007) showed in a study where different soil components were polluted with heavy metals that Pb adsorb to Fe oxides to a much higher extent than the other heavy metals, that Pb and Cu adsorb to Mn oxides, whereas Cr and Zn did not adsorb to these oxides to a significant extent. In the present investigation, 32–39% Zn, 23–42% Cu, and 6–9% Cr were extracted in step 2 of the sequential extraction. For Pb, the pattern is more complex and the sequential extraction showed between 5% (soil 3) and 55% (soil 4) in step 2. Except for only little Cr extracted in step 2, the sequential extraction pattern for these industrially polluted soils did not follow the pattern for the spiked single soil components of Covelo et al. (2007). Especially Zn, which was not found to be adsorbed significantly to oxides in the single soil component experiments, was extracted to a relatively high percentage in these industrial soils.
Overall, it is noticed from the sequential extraction that the pattern for Cr differs from the three other heavy metals. Cr is mainly extracted in steps 3 and 4 (which is consistent with Köleli 2004 in Cr-polluted agricultural soils), whereas Pb, Cu, and Zn are generally extracted during steps 1 and 2 to the highest extent. The difference may be related to the difference in possible oxidation steps under normal soil conditions. Cr can be found as Cr(III) and Cr(IV), whereas for the three other pollutants, only Pb(II), Cu(II), and Zn(II) are likely. In the soils of the present investigation, where the pollution is old, the prevailing form of Cr is expected to be Cr(III), since Cr(VI) is relatively mobile and would most likely have been washed out to deeper layers during the years. The total Cr concentrations in soils 1 and 2 were 220 and 8,400 mg/kg, respectively. Still, the result from the sequential extraction in percentage was very similar for the two soils. Chromium was present in soils 1 and 2 together with Cu and, in these two soils, Cr seems adsorbed stronger than Cu. In soil 6, most Cr was found in step 3, which is different from the two other soils. The difference may be related to the form Cr had during the spill. Soils 1 and 2 are polluted from wood preservation where Cr(VI) was used and soil 6 is from a car-painting facility. A soil sample from the same site as soil 6 was investigated using SEM-EDX in Jensen et al. (2006) (where it was named soil 3). Pb was consistently found associated with Cr in that investigation, which reveals the presence of lead chromate (PbCrO4), a yellow pigment previously used in paint. Lead chromate is, however, extremely stable during digestion (Jensen et al. 2006) and the concentrations of Pb and Cr from Table 3 do not probably include the lead chromate. Since Cr is released during the oxidation step, Cr is either adsorbed to particles that can be oxidized (e.g., organic matter) or in a form where Cr(III) can be oxidized to Cr(VI) and thus desorbed.
The sequential extraction result is a clear indication of the complexity of heavy metal adsorption in industrially polluted soils. The adsorption is both soil and heavy metal specific; and further, the origin of the pollution plays a significant role.
3.3 Buffering of Soils Against Acidification
There are different buffering systems in soils that act upon acidification. In calcareous soils, acidification is neutralized by dissolution of the carbonates. Organic matter also influences the acidification by ion exchange and the affinity for adsorption of H+ is generally high. During further acidification, the Al buffer range is reached (pH below about 4.5) (De Vries et al. 1989). For evaluation of the buffering ability of the experimental soils, pH of the soil suspensions at different HNO3 concentrations is shown in Fig. 2. The acid demand for acidification of the soils correlates well with the carbonate content as expected. The acid demand actually follows exactly the order of increasing carbonate content. Soils 2 and 4 had a carbonate content of 0.6% and 0.5%, respectively. Meanwhile, the acidification curves did not follow each other as close as expected from the carbonate content. Soil 4 was acidified faster than soil 2. From Table 2, it is seen that the main difference between these two soils is the higher organic content in soil 2 and this may be responsible for the difference in buffering capacity of the two soils.
Acidification of soil directly influences the different types of adsorption to both organic and inorganic soil particles. The H+ ions are exchanging with heavy metals in the cation exchange sites, thus desorbing the non-specifically bound heavy metals (Alloway 1995). Also, the specific adsorption is influenced by a decrease in pH, with a release of heavy metals as a result. Acidifying calcareous soils causes the carbonates to dissolve and among these are the carbonates where heavy metals are co-precipitated. Breakdown of other soil constituents occurs at strong acidification, e.g., Al and Fe hydroxides and this dissolution also results in release of the heavy metals bound in these phases.
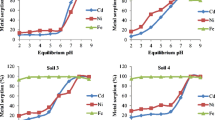
3.4 Heavy Metal Desorption from Soils Polluted with Three Heavy Metals
Desorption of heavy metals from each of the soils with three pollutants (soils 3, 5, 6, and 8) is seen in Fig. 3a–d. For all four soils, Zn starts desorbing at the highest pH. In the three soils (3, 5, and 8) containing Cu and Pb as well, Cu desorbs at a higher pH than Pb, and thus the desorption order was Zn > Cu > Pb, which is in agreement with the findings of Martínez and Motto (2000) for spiked soils.
It can be seen from Fig. 3 that only Zn desorption reaches about 100% in the investigated pH ranges. In soil 3, the lowest pH was just below 2 and at this pH, less than 10% Pb was desorbed whereas about 100% Zn was desorbed. This clearly illustrates that the desorption pattern is metal specific.
3.5 Desorption Pattern for Each of Cu, Cr, Pb, and Zn
The dependency of desorption for each of Cu, Cr, Pb, and Zn on the acidification in the different soils is shown in Fig. 4a–d. The general tendency for all the experimental soils is that the pH where desorption starts is in the order Zn > Cu > Pb > Cr. Thus, the desorption pattern in general follows the pattern from the soils with combined pollutions (Fig. 3a–d) regardless of soil type.
Desorption of Pb, Zn, and Cu occurs at a higher pH in the three calcareous soils (soils 3, 5, and 7) than in the soil with a low content of carbonates (Fig. 4). This is in consistency with both Martínez and Motto (2000) and Ottosen et al. (2001). Martínez and Motto (2000) showed that each heavy metal exhibited an approximate pH value at which the solubility increased markedly (6.2 for Zn, 5.5 for Cu, and 5.2 for Pb), except for the calcareous soil where the metals were dissolved at higher pH values compared to the non-calcareous soils (6.8 for Zn, 6.2 for Cu, and 6.0 for Pb). In the present study for the non-calcareous soils, pH must be less than 3 before Pb desorption starts (soil 4). For Cu, the pH must be slightly higher, between 4.2 and 5 (soils 1 and 2). Thus, for these non-calcareous industrially polluted soils, the pH at which Cu and Pb desorbs is clearly lower than for the spiked soils from the investigation of Martínez and Motto (2000). In the calcareous soils of the present investigation, Zn desorption starts at pH of about 6 (soils 3 and 5), Cu at pH of about 5 (soils 3 and 5), and Pb of about 4.3 (soils 3, 5, and 7). Again, this is lower than the pH values found by Martínez and Motto (2000) for the spiked calcareous soil. This may indicate the importance of aging of heavy metal polluted soil on the desorption processes or presence of less soluble species; however, the general pattern of desorption of Cu, Zn, and Pb at a higher pH in calcareous soils than in non-calcareous soils is confirmed.
The percentage of all heavy metals desorbed after strong acidification differs from soil to soil, though the tendency is most pronounced for Pb. At pH of about 1, only 25% Pb was desorbed from soil 6 whereas 95–100% Pb desorbed at this pH from soil 7. This pattern correlates well to the sequential extraction result (Fig. 1b) where Pb was found in the residual fraction to a higher extent in soil 6 than in soil 7. In soil 3, most Pb (80%) was found in the residual fraction of the sequential extraction, and desorption of Pb from soil 3 also showed the lowest desorption (<10%) in the pH range investigated.
For the Zn-polluted soils, the sequential extraction patterns were quite similar (Fig. 1d) and the pH-dependent desorption was also relatively similar in comparison to Pb, though desorption is higher from the calcareous soils as discussed above.
4 Conclusions
Eight industrially polluted soils with elevated concentrations of one or more of the heavy metals Cu, Cr, Pb, and Zn were acidified and the desorption pattern was investigated. Three soils had the pollution combination Cu, Pb, and Zn. From these, desorption occurred in the order Zn > Cu > Pb as pH decreased. Comparison between the soils showed that desorption occurred at a higher pH in calcareous soils compared to soils with low carbonate content. This reveals that the heavy metals were adsorbed/co-precipitated in the carbonate fraction of these soils to a relatively high extent. The pattern of desorption could not be linked to the heavy metal concentration. Likewise, sequential extraction showed that, except from the majority of Zn being extracted in the first two steps and Cr being extracted in the last two fractions, there were no trends to be generalized. The sequential extraction pattern was probably influenced by the heavy metal speciation at the time of spillage rather than the soil characteristics, since there was no correlation with soil characteristics. There was, however, correlation between high percentages of heavy metals extracted in the last step of sequential extraction (residual fraction) and low desorption even at very low pH, so the sequential extraction can overall be used for evaluation of adsorption strength.
References
Alloway, B. J. (1995). Soil processes and the behaviour of heavy metals. In B. J. Alloway (Ed.), Heavy metals in soils. London: Blackie Academic & Professional.
Covelo, E. F., Vega, F. A., & Andrade, M. L. (2007). Competitive sorption and desorption of heavy metals by individual soil components. Journal of Hazardous Materials, 140, 308–315. doi:10.1016/j.jhazmat.2006.09.018.
De Vries, W., Posch, M., & Kämäri, J. (1989). Simulation of the long-term soil response to acid deposition in various buffer ranges. Water, Air, and Soil Pollution, 48(3–4), 349–390. doi:10.1007/BF00283336.
Dohrmann, R. (2006). Cation exchange capacity methodology I: an efficient model for the detection of incorrect cation exchange capacity and exchangeable cation results. Applied Clay Science, 34, 31–37. doi:10.1016/j.clay.2005.12.006.
García-Delgado, R. A., García-Herruzo, F., Rodríguez-Maroro, J. M., & Vereda, C. (1996). Influence of soil carbonates in lead fixation. Journal of Environmental Science and Health. Part A, Environmental Science and Engineering & Toxic and Hazardous Substance Control, 31(9), 2099–2109.
Jensen, P. E., Ottosen, L. M., & Pedersen, A. J. (2006). Speciation of Pb in industrially polluted soils. Water, Air, and Soil Pollution, 170, 359–382. doi:10.1007/s11270-005-9008-7.
Kliem, B. K., & Hansen, L. (2000). Electrodialytic soil remediation in a small pilot plant (Part II). Extractions, IR, XRD, TEM and SEM investigations on untreated and EDR-treated Cu-polluted soil. In: B. K. Kliem, Bonding of heavy metals in soil, Ph.D. thesis, Technical University of Denmark, August 2000.
Köleli, N. (2004). Speciation of chromium in 12 agricultural soils from Turkey. Chemosphere, 57, 1473–1478. doi:10.1016/j.chemosphere.2004.08.068.
Lock, K., & Janssen, C. R. (2003). Influence of ageing on zinc bioavailability in soils. Environmental Pollution, 126, 371–374. doi:10.1016/S0269-7491(03)00232-X.
Loeppert, R.H., Suarez, D.L. (1995) Methods of Soil Analysis. Part 3. Chemical Methods—SSSA Book series No. 5, 451–455.
Lund, U., & Fobian, A. (1991). Pollution of two soils by arsenic, chromium and copper, Denmark. Geoderma, 49, 83–103. doi:10.1016/0016-7061(91)90093-9.
Madrid, L., & Diaz-Barrientos, E. (1992). Influence of carbonate on the reaction of heavy metals in soils. Journal of Soil Science, 43, 709–721.
Markiewicz-Patkowska, J., Hursthouse, A., & Przybyla-Kij, H. (2005). The interaction of heavy metals with urban soils: sorption behaviour of Cd, Cu, Cr, Pb and Zn with a typical mixed brownfield deposit. Environment International, 31, 513–521. doi:10.1016/j.envint.2004.09.004.
Martínez, C. E., & Motto, H. L. (2000). Solubility of lead, zinc and copper added to mineral soils. Environmental Pollution, 107, 153–158. doi:10.1016/S0269-7491(99)00111-6.
Mesquita, M. E., & Carranca, C. (2005). Effect of dissolved organic matter on copper zinc competitive adsorption by a sandy soil at different pH values. Environmental Technology, 26(9), 1065–1072. doi:10.1080/09593332608618493.
Mester, Z., Cremisini, C., Ghiara, E., & Morabito, R. (1998). comparison of two sequential extraction procedures for metal fractionation in sediment samples. Analytica Chimica Acta, 359, 133–142. doi:10.1016/S0003-2670(97)00687-9.
Miljøstyrelsen (2003). Liste over kvalitetskriterier i relation til forurenet jord (A list of quality criteria in relation to polluted soil) In Danish.
Ottosen, L. M., Hansen, H. K., Ribeiro, A. B., & Villumsen, A. (2001). Removal of Cu, Pb and Zn in an applied electric field in calcareous and non-calcareous soils. Journal of Hazardous Materials, B85, 291–299. doi:10.1016/S0304-3894(01)00231-X.
Ottosen, L. M., Kubal, M., & Lepkova, K. (2006). Comparison of electrodialytic removal of Cu from spiked kaolinite, spiked soil and industrially polluted soil. Journal of Hazardous Materials, 137(1), 113–120. doi:10.1016/j.jhazmat.2005.04.044.
Saeed, M., & Fox, R. L. (1977). Relations between suspension pH and Zn solubility in acid and calcareous soils. Soil Science, 124, 199–204. doi:10.1097/00010694-197710000-00002.
Schnitzer, M., & Kerndorff, H. (1980). Effects of pollution on humic substances. Environ Sci Health B, 15(4), 431–456.
Zhang, P., Ryan, J. R., & Yang, J. (1998). In Vitro soil Pb solubility in the presence of hydroxyapatite. Environmental Science & Technology, 32, 2763–2768.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ottosen, L.M., Hansen, H.K. & Jensen, P.E. Relation Between pH and Desorption of Cu, Cr, Zn, and Pb from Industrially Polluted Soils. Water Air Soil Pollut 201, 295–304 (2009). https://doi.org/10.1007/s11270-008-9945-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9945-z