Abstract
Soils in areas of mining and smelting of Pb–Zn ores in Southern Poland are strongly enriched in heavy metals (Zn, Pb, Fe, Cd, Tl, As). The highest concentrations of Zn (<55,506 mg kg−1), Pb (<8,262 mg kg−1), Cd (<220 mg kg−1) and Tl (<67 mg kg−1) are linked to the fine fractions of upper soil layers in sites contaminated by past exploitation and processing of ores. The high stress of metals, and the negative influence of acid waste drainage has limited the development of flora and fauna in these areas. The increasing ability of plants to grow is due to the positive symbiotic action of fungi and bacteria. The mycorrhizal communities were identified in rhizospheres rich in unstable Zn–Pb–Fe sulphides such as sphalerite, galena, pyrite and marcasite and carbonates of Zn (smithsonite) and Pb (cerussite). They occur in associations with sulphates, e.g., gypsum. In parts of fungi, secondary mineral phases containing Zn, Pb, Fe and Mn occur. Metal-bearing aggregates formed during symbiotic action between myccorhiza and bacteria connected with them. They enhance the binding of bio-available ions of Zn, Pb and Mn in the most unstable phases. Metal contents in the mycorrhizal parts of the rhizospheric soils were determined by Atomic Absorption Spectroscopy. Mineralogical investigations involved X-ray diffraction, scanning electron microscopy with energy dispersive spectrometry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In a natural environment unaltered by anthropogenic factors, there is an equilibrium between the release and capture of elements in geological deposits. Anthropogenic activity, e.g., ore-bed exploitation and industrial development, by releasing great quantities of gas and dust into the atmosphere, soils and waters, disturbs that equilibrium. Heavy metals, and especially abiogenic elements such as cadmium, lead and mercury, are ecologically particularly undesirable because even small concentrations are a negative influence on biological processes occurring in both soils and living organisms (Kabata-Pendias and Pendias 1999). The toxicity of a given element depends on its concentration and its biochemical role. Heavy elements cause the growth reduction of roots (Godbold et al. 1998), decrease the integrity of biological films and disturb important enzyme actions such as, e.g., nitrate reductase (Ernst 1996). The toxicity of an element is determined by its availability for transfer between soil solution and living organisms (Juste 1988). In any ecosystem, biological processes causing the binding of heavy metal ions and limiting of their migration in soils and organic components are very valuable.
Biological investigations carried out in recent years have paid particular attention to bacteria and fungi as abiotic soil ligands playing an important role in limiting the effects of plant- and soil contamination by heavy metals. These processes are relatively well studied in trees that, through mutual mycorrhizal symbiosis, form mycorrhizal rhizospheres that store and exchange mineral salts and waters (Read 2002). These investigations have shown that symbiotic relationships between plants and fungi increase their tolerance of heavy metals in soils (Leyval et al. 1997; Krupa 2004; Krupa and Kozdrój 2007; Kozdrój et al. 2007). This may be due to the binding of excess metals in electronegative sites in the cell walls of mycelia (Frey et al. 2000) or the immobilization of elements by precipitation in phosphate phases (Galli et al. 1994; Godbold et al. 1998). According to many, the ability of trees and other long-living plants to grow and develop in polluted environments is possible only because of mycorrhization of their roots (Wilkinson and Dickinson 1995; Krupa 2004). The formation of abiotic relationships and the survivability and vitality of mycorrhizae is very dependent on assisting bacteria (Duponnois and Pienchette 2003; Kozdrój et al. 2007) which points to the influence of microorganisms in limiting metal migration.
Symbiotic fungi occur in soils heavily polluted with heavy metals. As Turnau et al. (2005) show, fungi greatly influence the sequential development of spontaneous plants on waste dumps associated with Pb–Zn mining. Investigations of rhizospheric soils contaminated with heavy metals show that the roots of plants accumulate mineral phases rich in Zn, Pb, Fe, Cd and As (Cabala et al. 2004; Cabala and Teper 2007). Contaminants concentrated in the upper layers of soils play an important role in rhizosphere biochemistry (Courchesne and Gobran 1997).
Some questions arise. Are roots and fungal activity playing an important role in the catalysis of secondary metal-bearing minerals? What are the forms and chemical compositions of metal-bearing minerals occurring in the mycorrhizal zones of rhizospheres? The identification of mineral phases using electron scanning microscopy (ESEM) is important in enabling identification of mineral phases that formed due to fungal activity in roots or in micro-organism secretions.
The definition of the influence of fungi on plant vegetation in soils formed on metal-bearing wastes is very important to the development of new effective methods for the phytoremediation of areas polluted by metal mining and smelting.
2 Geology and HM Concentration in Soils
The investigated soils occur in an area where carbonaceous rocks of Triassic and Jurassic age are partially covered by Pleistocene fluvioglacial sands. The Triassic rocks host Zn–Pb ores beds of Mississippi Valley Type (Leach et al. 1996). The primary ore minerals are Zn sulphides (sphalerite αZnS and wurtzite βZnS), Pb sulphide (galena PbS) and Fe sulphides (marcasite FeS2 and pyrite FeS2). Concentration levels for Zn, Pb and Fe are 4–6%, 1–3% and 5–8% respectively. Other characteristic elements in the Silesian-Cracovian ores are Cd, Tl, Ag and As (Cabala 1996).
Around Olkusz, the ore bodies are located in epigenetic dolomites that locally outcrop in tectonic horsts (Cabala 2001). Over a period of several million years, supergenic processes resulted in to oxidation of the primary Zn–Pb–Fe sulphides. These processes also resulted in the development of secondary Zn, Pb, Fe and Cd aureoles around the shallow ores (Mayer et al. 2001; Cabala 2001).
Exploitation of Pb and Ag ores started in the twelfth century. More recently, Zn ores have been worked since the nineteenth century. Large scale mining and ore smelting led to the contamination of soils around waste dumps in the second half of the twentieth century (Cabala and Teper 2007; Krzaklewski and Pietrzykowski 2002). Many years of mining and smelting are very clearly reflected in high soil metal contents (Roberts et al. 2002). Pb contents in the soils can range from 10,000–106,000 mg kg−1 (Li and Thornton 2001). The contents of Zn, Pb and Cd in the soils of the Olkusz area place them among the most polluted soils in Europe (Verner et al. 1996; Lis and Pasieczna 1997; Mayer et al. 2001; Cabala et al. 2004). Zn commonly exceeds 10,000 mg kg−1, and Pb 5,000 mg kg−1, in the forest litter and the upper soil layers (Trafas 1996; Lis and Pasieczna 1999). In upper soil layers in the environs of the shallow ore exploitation, Zn, Pb and Cd contents reach 83,400 mg kg−1, 147,700 mg kg−1 and 427 mg kg−1 respectively (Cabala and Teper 2007).
3 Material and Methods of Investigation
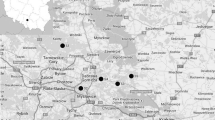
Rhizosphere soils collected from 0–0.5 m depth in areas heavily polluted by mining and smelting of Zn–Pb ores in the Olkusz area (Fig. 1) were the focus of this investigation. Soil samples were collected from the following habitats:
-
Sandy soils with initial xenothermic communities
-
Young afforestations in the vicinity of flotation waste dumps
-
Older forests of mixed type with preponderance of Pinus silvestris and Betula pendula planted during mine-area reclamation
-
Areas of historical exploitation afforested by xenothermic plants.
The investigated rhizosphere soils were collected in the root zones of trees, e.g., Pinus silvestris, Betula pendula and Larix decidua and from plantings of xenothermic grass, e.g., Cardaminopsis arenosa, Calamaglorstis epigeios, Deschampsia caespitosa, Festuca ovina, Hieracium pilosella, Silene vulgaris, Viola tricolor, Dianthus carthusianorum, Biscutella laevigata and Armeria maritima.
3.1 Mineralogical Investigations
The soil samples were examined using X-ray diffraction (XRD) methods. Samples were separated into fractions (<0.45, 0.45–0.63, >0.63 mm) using wet and dry methods of separation. Three to six diffraction patterns were made for each sample. Phase compositions were determined using a Philips PW 3710 Roentgen Diffractometer with graphite monochromator (tube Co kα; 45 kV, 30 mA; impulse counting time 2 s at increments of 0.01–0.02°). X-ray diffraction analysis was carried out using X’Pert software. Quantitative analysis was based on Rietveld’s method.
3.2 Chemical Analysis
Heavy metal contents (Zn, Pb, Fe, Mn, Cd and Tl) were analysed by atomic absorption spectroscopy (AAS) using a SOLAAR M6 spectrometer. The sample rhizospheric soils were averaged and dried. Samples (0.2 g) were ground in an agate mortar. A mixture of pure acids was used for the mineralization of each sample: 40% HF (2 ml), 65% HNO3 (3 ml) and 35% HCl (1 ml) and distilled water (2 ml). The mineralization was carried out at (110°C) in a Milestone MLS 1200 microwave furnace. To remove fluorosilicates, 50 ml of 4% H3BO3 was added and each sample again mineralized for a short time. The resulting solution was transferred to a 100 ml flask and filtrated under pressure to plastic bottles using 0.45 μm filters.
3.3 Microscopic Analysis
Electron-probe studies were carried out using an environmental scanning electron microscope Philips XL 30 with EDAX analyser. Back EE (BSE) images were obtained using a Centaurus attachment with a detector resolution of 0.3 Z. The accelerating voltage was 15 kV and the pressure 0.2 Torr. The investigated material was fixed to carbon tapes (1 × 1.5 cm) placed on aluminium stubs. Specimens with fragments of plant roots were cleaned in an air stream to remove loose mineral grains. The samples were carbon coated. EDS spectra analyses were elaborated using Phillips software. All analyses were carried out in the laboratories of the Faculty of Earth Sciences, University of Silesia, Sosnowiec.
4 Results
4.1 Heavy Metal Concentration in Mycorrhizal Top Soil
Zn, Pb, Fe, Mn, Cd and Tl, and related minerals, are concentrated in the fine-grain fractions of the soils (Table 1). A significant enrichment in heavy metals characterizes the fraction <0.18 mm and the highest metal contents the <0.045 mm fraction. Metal concentrations in coarser fractions (>0.71 mm) relate to the presence of metal-bearing polymineral agregates and organic matter (Table 1).
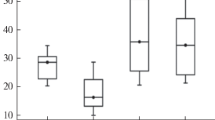
The highest metal concentrations occur in the top soils around areas where supergene ores were formerly exploited (areas B, B2) and in areas where Zn–Pb ores were processed in the past—and at present (Fig. 2). Locally, Zn, Pb, Cd and Tl concentrations are similar to those in the flotation wastes (area A, Fig. 2). The secondary metal concentration takes place in morphological depressions, karst funnels and in areas outside the reach of surface waters running off the mining and flotation wastes.
Average relative heavy metal concentration in top soils. Explanation and location of investigated areas (a–d) on Fig. 1
Particularly high heavy metal concentrations occur in the upper layers of soils to which metal-bearing minerals were transported from the waste dumps by aeolic transport. At several hundred meters from the dumps (area C), average Zn and Pb concentrations in these upper layers are 3,000–10,000 and 1,000–3,000 mg kg−1, respectively. Notably high levels of Cd (20–100 mg kg−1) and Tl (1–20 mg kg−1) are also observed. Heavy metal pollution at 2–7 km from the sources of pollution (area D; Fig. 2) is markedly less.
Areas damaged by the mining processes and waste disposal lie within reclamation works aimed at revitalisation. Soils formed on top of the metal-rich rocks have plants that adjusted to growth under conditions of high metal stress and water deficiency (Wierzbicka and Rostański 2002). Former Zn-Pb mining areas were locally colonized by xenothermic plant communities. Symbiotic fungi are an important influence on the progress of plant colonization. Hence, this attempt to identify these fungi in rhizospheric soils heavily polluted with metal-bearing minerals.
4.2 Metalliferous Minerals in Mycorrhizal Rhizospheres
Among the primary mineral components of soils and rhizospheres formed on the Pleistocene sands, quartz, feldspars, clay minerals (illite, kaolinite) predominate. In some cases, zircon, magnetite, Ti oxides and apatite also occur. The intensive mining and processing of Zn–Pb ores resulted in the transport of allochthonous mineral components to the upper soil layers. These latter include carbonates (calcite and dolomite) and metalliferous minerals genetically linked to the ores (Table 2). Soils formed on Triassic limestones and dolomites are characterized by high carbonate contents that make them highly alkaline. Top soils polluted by historical or present mining are enriched in Zn–Pb–Fe sulphides, Fe oxides, Zn–Pb carbonates, secondary Ca, Mg, Fe, Pb and Zn sulfates, Zn and Pb carbonates and Fe and Mn oxides. Chemical transformations of the metalliferous minerals are associated with the release of active ions, Zn2+, Pb2+, Fe3+, Cd2+, Tl+, Mn4+ and SO4 2- into soil solutions.
4.3 XRD Investigations
XRD data show that the fine fractions of the rhizospheric soils contain high contents of crystalline phases that compare with the paragenetic assemblages seen in the sulphide and oxidized Zn–Pb ores (Cabala 2001). These are mainly Fe sulphides and oxides, Zn, Pb sulphides and carbonates, and Zn and Fe sulfates (Table 2). Among unstable sulphides, marcasite dominates and pyrite is rare. Trace elements such as Cd, Tl, As, Ag, Sr and In occur in the crystalline structures of the ore minerals (Cabala 1996; Leach et al. 1996; Mayer et al. 2001). The common presence of gypsum CaSO4·2H2O and bassanite CaSO4 0.5H2O in the soils indicates the high activity of sulfate ions that in limestones are stabilised in hydrated calcium sulfates that include anglesite PbSO4, jarosites KFe3 (SO4)2 (OH)6, hydronium jarossite (H3O)Fe3(SO4)2(OH)6 and hexahydrites MgSO4 6H2O (Table 2). During the oxidizing stage of chemical transformation, Zn and Pb carbonates (smithsonite ZnCO3, monheimite (Zn,Fe)CO3, cerussite PbCO3) formed. Locally Zn silicates, e.g., hemimorphite Zn4Si2O7(OH)2 H2O, formed (Table 2).
4.4 ESEM Data
Assemblages of hyphae (Fig. 3a–f) are evident in heavily polluted top soil layers and on associated plant roots. Massive hyphae of parenchymatic character form mycorrhizal hyphae; fragments are seen in BSE images (Fig. 3a,b). Such hyphae geometry is typical of ectomycorrhizal symbiosis with trees. Loose assemblages of mycelia and rhizomorphs suggest perytrophic mycorrhizal that commonly occurs in association with xenothermic plants. The morphological forms of mycorrhizal rhizospheres of external mycorrhizal, e.g., dychotomic branching of roots, indicate symbiosis with trees of Pinus type (Fig. 3c). The ESEM investigations reveal that metalliferous minerals commonly occur on mycorrhizal roots (Fig. 3a–f). These rhizosphere morphologies and associated mineral phases deserve particular attention, as does the way their occurrence indicates that they formed during a secondary stage. Their origin can be linked with the biotic impact of mycorrhiza, roots or symbiotic bacteria exudates. Identification of the metalliferous phases proves that rhizosphere solutions contain ions of metals such as Zn2+, Pb2+, Fe3+, Mn4+.
BSE images show mycelia of Basidomycetes typical of tree ectomycorrhiza (Fig. 3b). Hyphae were also identified in the areas with historical dumps inhabitated by mosses and initial communities of xenothermic plants. These occur in fresh soils rich in Fe oxides, Zn and Pb carbonates, relicts of Zn, Pb and Fe sulphides and gypsum (Fig. 3d).
The structural features of some mineral aggregates indicate that they formed during the period of root vegetation or that their origin was stimulated by mycorrhizal rhizosphere exudates (Fig. 3e,f). The origin of such forms is caused by heavy metal biostabilisation. They comprise polymineral aggregates composed of such unstable phases as Fe–Mn oxides, Zn and Pb oxides and aluminosilicates. Among the components of minerals occurring on hyphae, in their vicinity or on root rhizoplans, heavy metals such as Zn (Fig. 4a–f), Fe (Fig. 4a–d), Pb (Fig. 4c,e,f) and Mn (Fig. 4e,f) are significant.
EDS spectra of metalliferous components in mycorrhizal rhizospheres. Locations as in Fig. 3a–f
In rhizospheres polluted with heavy metals, metalliferous minerals were identified on the surface of hyphae (I), in their vicinity (II) and forming secondary covers on hyphae (III):
-
I.
Metalliferous minerals are located among hyphae of mycorrhizae (Fig. 3a,c). Mycelium lines (rhizomorphs) have metalliferous mineral aggregates built into their structure (Fig. 3b) and contain Fe and Zn (Fig. 4b). Concentrations (0.1–2 μm in size) of such metalliferous minerals occur in the external zones of mycorrhizal hyphae. EDS microanalyses reveal that they have complicated chemical formulas (Fig. 3a,b). The presence of Si, Al, Mg, and Ca indicates the presence of clay minerals and dolomite. Clear carbon peaks may indicate a carbonate composition or that they are organometallic compounds.
-
II.
Grains and aggregates of Zn and Pb sulphides and carbonates, and Fe oxides, are incorporated into ectomycorrhizal networks of fungi (Fig. 3c). Metalliferous phases occurring in assemblages with hyphae commonly have spherical forms (Fig. 3a,c) typical of smelting emissions (Cabala and Teper 2007).
-
III.
Glazings and dripstones of metalliferous phases occur on hyphae or filling spaces between mycelia lines (Fig. 3e,f). They form thin (0.1–2 μm) irregular covers over areas a few tens of μm in scale. They have the form of amorphic- or cryptocrystalline aggregates. These phases contain Pb, Zn, Fe and Mn (Fig. 4e,f). Clear carbon peaks in EDS spectra (Fig. 4f) indicate that heavy metals can be chemically bound in organometallic phases.
5 Summary and Conclusions
Zn, Pb, Fe sulphides and carbonates were deposited in abundance on the ground surface as a result of many years of exploitation of sulphide- and supergene Zn–Pb ores in the Olkusz area (Verner et al. 1996; Cabala et al. 2004; Cabala and Teper 2007). Metalliferous mineral phases, of a size rarely >45 μm, occur in association with plant roots, hyphae and micro- and mezofauna. This study shows that the finest fractions are typically the most enriched in Zn, Pb, Mn, Cd and Tl (Table 1). That is why the development of symbiotic mycorrhiza is possible in conditions of strong metal stress with levels of Zn, Pb and Cd exceeding 20,000, 3,000 and 100 mg kg−1, respectively (Fig. 2).
In areas around Olkusz strongly polluted by Zn-Pb, assemblages of fungi occur symbiotically with trees (Pinus) and xenothermic plant roots. Symbiotic fungi and bacteria, interacting with plant roots, influence the soil biochemistry. In their ability to limit the translocation of Zn2+, Cd+ and Pb2+ ions from solutions to roots, they protect plants from excess metal stress (Leyval et al. 1997; Krupa 2004). During this process of biochemical stabilisation of metal ions in mycorrhizal rhizospheres, new metal-rich phases are formed.
Investigations aiming to identify the products formed during biotic interactions of ligands with metal-bearing solutions should be carried out in rhizosphere zones heavily polluted with metals. The mineral components of rhizospheres are particularly sensitive to the influence of root- and fungi exudates and the products of bacterial metabolism (Courchesne and Gobran 1997; Hinsinger et al. 2005).
ESEM studies of rhizosphere mineral phases lead to interesting results on the chemical composition, forms and origin of minerals occurring on roots and symbiotic fungi. The present study confirms that, by using ESEM methods, secondary phases formed during biological interactions of fungi can be identified. The occurrence of secondary metalliferous phases in the form of covers or fillings of spaces between hyphae indicate the existence of a direct relationship between the interactions of symbiotic fungi or bacteria and the crystallization of mineral phases rich in Zn, Pb, Fe and Mn. Metal mobilization can reflect the filtration of soil solutions through mycorrhiza—a process that traps a significant amount of heavy metals on the surfaces of mycelia or their structures (Leyval et al. 1997; Jentschke and Godbold 2000; Krupa 2004). Submicron metalliferous phases occurring on the surface of hyphae can also form due to the ability of mycelia to bind heavy metals through pigments deposited on their surface (Sommer et al. 2001). The influence of symbiotic fungi on minerals rich in K, Mg, Na results in the delivery of biogenic elements to plants and waters (Hees et al. 2005; Conn and Dighton 2000; Baxter and Dighton 2005). Crystallization of stable metalliferous minerals, by decreasing the bioavailability of metals, limits their toxic influence and, by doing so, promotes plant vegetation and soil-forming processes. Mycorrhization, and the biological activity of soils polluted with heavy metals, stimulate the spontaneous stabilisation of mine waste dumps.
References
Baxter, J. W., & Dighton, J. (2005). Phosphorus source alters host plant response to ectomycorrhizal diversity. Mycorrhiza, 15, 513–523. doi:10.1007/s00572-005-0359-0.
Cabala, J. (1996). Concentrations of trace elements in Zn–Pb ores and possibilities of their transfer to waste deposits. Prace Naukowe GIG, 13, 17–32 (in Polish with English summary).
Cabala, J. (2001). Development of oxidation in Zn–Pb deposits in Olkusz area. In A. Piestrzyński et al. (Ed.), Mineral deposits at the beginning of the 21st century (pp. 121–124). Lisse: Balkema.
Cabala, J., & Teper, L. (2007). Metalliferous constituents of rhizosphere soils contaminated by Zn–Pb mining in southern Poland. Water, Air, and Soil Pollution, 178, 1–4 351–362. doi:10.1007/s11270-006-9203-1.
Cabala, J., Teper, E., Teper, L., Małkowski, E., & Rostański, A. (2004). Mineral composition in rhizosphere of plants grown in the vicinity of a Zn–Pb ore flotation tailings pond. Preliminary Study. Acta Biologica Cracoviensia. Series; Botanica, 46, 65–74.
Conn, C., & Dighton, J. (2000). Litter quality influences on decomposition, ectomycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biology & Biochemistry, 32, 89–496. doi:10.1016/S0038-0717(99)00178-9.
Courchesne, F., & Gobran, G. R. (1997). Mineralogical variations of bulk and rhizosphere soils from a Norway spruce stand. Soil Science Society of America Journal, 61(4), 1245–1249.
Duponnois, R., & Pienchette, C. (2003). A mycorrhiza helper bacterium enhances ectomycorrhizal and endomycorrhizal symbiosis of Australian Acacia species. Mycorrhiza, 13, 85–91.
Ernst, W. H. (1996). Schwermetalle. In C. Brunold, A. Rüegesegger, & R. Brändle (Eds.), Stress bei Pflanzen. Wissenschaft (pp. 191–220). Stuttgart: Verlag Paul Haupt.
Frey, B., Zierold, K., & Brunner, I. (2000). Extracellular complexation of Cd in the Hartig net and cytosolic Zn sequestration in the fungalmantle of Picea abies Hebeloma crustuliniforme ectomycorrhizas. Plant, Cell & Environment, 23(11), 1257–1265. doi:10.1046/j.1365-3040.2000.00637.x.
Galli, U., Schuepp, H., & Brunold, C. (1994). Heavy metal binding by mycorrhizal fungi. Physiologia Plantarum, 92, 364–368. doi:10.1111/j.1399-3054.1994.tb05349.x.
Godbold, D. L., Jentschke, G., Winter, S., & Marschner, P. (1998). Ectomycorrhizas and amelioration of metal stress in forest trees. Chemosphere, 36, 757–762. doi:10.1016/S0045-6535(97)10120-5.
Hees, P., Jones, D. L., Jentschke, G., & Godbold, D. L. (2005). Organic acid concentrations in soil solution: Effects of young coniferous trees and ectomycorrhizal fungi. Soil Biology & Biochemistry, 37, 771–776. doi:10.1016/j.soilbio.2004.10.009.
Hinsinger, P., Gobran, G. R., Gregory, P. J., & Wenzel, W. W. (2005). Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. The New Phytologist, 168, 293–303. doi:10.1111/j.1469-8137.2005.01512.x.
Jentschke, G., & Godbold, D. (2000). Metal toxicity and ectomycorrhizas. Physiologia Plantarum, 109(2), 107–116. doi:10.1034/j.1399-3054.2000.100201.x.
Juste, C. (1988). Appreciation de la mobilité et de la biodisponbilité des éléments en trace du sol. Science du Sol, 26, 103–112.
Kabata-Pendias, A., & Pendias, H. (1999). Trace metals biogeochemistry. Warsaw, PWN, 2 Edit. (pp. 398, in Polish).
Kozdrój, J., Piotrowska-Seget, Z., & Krupa, P. (2007). Mycorrhizal fungi and ectomycorrhiza associated bacteria isolated from an industrial desert soil protect pine seedlings against Cd(II) impact. Ecotoxicology (London, England), 16, 449–456. doi:10.1007/s10646-007-0149-x.
Krupa, P. (2004). Ectomycorrhises and their significance for the trees growing in place polluted with heavy metals. (p. 92). Katowice: University of Silesia Publ. (in Polish with English summary).
Krupa, P., & Kozdrój, J. (2007). Ectomycorrhizal fungi and associated bacteria provide protection against heavy metals in inoculated Pine (Pinus sylvestris L) seedlings. Water, Air, and Soil Pollution, 182, 83–90. doi:10.1007/s11270-006-9323-7.
Krzaklewski, W., & Pietrzykowski, M. (2002). Selected physico-chemical properties of zinc and lead ore tailings and their biological stabilisation. Water, Air, and Soil Pollution, 141, 125–142. doi:10.1023/A:1021302725532.
Leach, D. L., Viets, J. G., Kozlowski, A., & Kibitlewski, S. (1996). Geology, geochemistry, and genesis of the Silesia–Cracow zinc–lead district, southern Poland. Society of Economic Geologists. Special Publication, 4, 171–181.
Leyval, C., Turnau, K., & Haselwandter, K. (1997). Effect of heavy metal pollution on mycorrhizal colonization and function: Physiological, ecological and applied aspects. Mycorrhiza, 7, 139–153. doi:10.1007/s005720050174.
Li, X., & Thornton, I. (2001). Chemical partitioning of trace and major elements in soils contaminated by mining and smelting activities. Applied Geochemistry, 16, 1693–1706. doi:10.1016/S0883-2927(01)00065-8.
Lis, J., & Pasieczna, A. (1997). Pb–Zn–Cd geochemical anomalies in soil of Upper Silesia. Przegląd Geologiczny, 2(45), 182–189 (in Polish, with English summary).
Lis, J., & Pasieczna, A. (1999). Detailed geochemical map of Upper Silesia 1:25000. Pilot sheet Sławków. Polish Geological Institute, Warsaw.
Mayer, W., Sass-Gustkiewicz, M., Góralski, M., Sutley, S., & Leach, D. L. (2001). Relationship between the oxidation zone of Zn–Pb sulphide ores and soil contamination in the Olkusz ore district (Upper Silesia, Poland). In A. Piestrzyński, et al. (Ed.), Mineral deposits at the beginning of the 21st century (pp. 165–168). Lisse: Balkema.
Read, D. J. (2002). Towards ecological relevance–progress and pitfalls in the path towards an understanding of mycorrhizal functions in nature (Ecological studies, 157). In M. G. A. van der Heiden, and I.R. Sanders (Eds.). Mycorrhizal ecology (pp. 3–24). Berlin: Springer.
Roberts, D. R., Scheinost, A. C., & Sparks, D. L. (2002). Zinc speciation in a smelter-contaminated soil profile using bulk and microspectroscopic techniques. Environmental Science & Technology, 36, 1742–1750. doi:10.1021/es015516c.
Sommer, P., Burgera, G., Wieshammer, G., Wenzel, W. W., & Strauß, J. (2001). Effects of mycorrhizal associations on the metal uptake by willows from polluted soils: Implication for soil by phytoextraction. Leben und Überleben – Konzepte für die Zukunft. BOKU Wien, 18.–21. Nov. Open file access 2007 http://www.rhizo.at/download.asp?id=690.
Trafas, M. (1996). Changes in the properties of post-flotation wastes due to vegetation introduced during process of reclamation. Applied Geochemistry, 11, 181–185. doi:10.1016/0883-2927(95)00062-3.
Turnau, K., Jurkiewicz, A., Lingua, G., Barea, J. M., & Gianinazzi-Pearson, V. (2005). Role of arbuscular mycorrhiza and associated microorganisms in phytoremediation of heavy metal-polluted sites. In M. N. V. Prasad, K. S. Sajwan, & R. Naidu (Eds.), Trace elements in the environment (pp. 229–246). Boca Raton: Taylor & Francis.
Verner, J. F., Ramsey, M. H., Helios-Rybicka, E., & Jędrzejczyk, B. (1996). Heavy metal contamination of soils around a Pb–Zn smelter in Bukowno, Poland. Applied Geochemistry, 11, 11–16. doi:10.1016/0883-2927(95)00093-3.
Wierzbicka, M., & Rostański, A. (2002). Microevolutionary changes in ecotypes of calamine waste heap vegetation near Olkusz, Poland: A review. Acta Biologica Cracoviensia. Series; Botanica, 44, 7–19.
Wilkinson, D. M., & Dickinson, N. M. (1995). Metal resistance in trees: the role of mycorrhizae. Oikos, 72, 298–300. doi:10.2307/3546233.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cabala, J., Krupa, P. & Misz-Kennan, M. Heavy Metals in Mycorrhizal Rhizospheres Contaminated By Zn–Pb Mining and Smelting Around Olkusz in Southern Poland. Water Air Soil Pollut 199, 139–149 (2009). https://doi.org/10.1007/s11270-008-9866-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9866-x