Abstract
Surface water is frequently contaminated by the trace metals, in particular lead and zinc, produced by mining activities. The infiltration of this water is likely to pollute surface soils and ground water. The study of the transfer of trace elements, especially lead, under real conditions is difficult to carry out due to the physicochemical and hydrodynamic complexity of real soil (preferential flows, conditions of unsaturation...), of the presence of colloids and of many candidate elements. The objective of the present study was to gain a better understanding of the parameters influencing the migration processes of trace elements in simplified systems; it was based on the study of Pb transfer in laboratory columns filled with soil. The results showed that retention of lead in soil is strongly dependent on feed flow rate, particulate bed tortuosity, bed height, water–soil surface contact and volume of water. Increase in bed height, water–soil surface contact and particulate bed tortuosity leads to higher contact time thus higher lead retention by soil, whereas increase in feed flow rate and volume of water leads to lower contact time thus lower lead retention by soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil, water and bio-diversity are fundamental elements of the ecosystem and are the subject of many agrarian, ecological, biological and hydrological studies. A high percentage of ecosystems consist of arable land which is treated with agrochemical products forming the upper soil layers. Large quantities of chemical elements infiltrate the waters running off the cultivated soils thereby entering the animal and human food chain (Hopkin 1989; Beeby and Eaves 1983; Alexander 2000; Pedersen et al. 2000; Notten et al. 2005).
For many years, industries located near rivers (Anxiang et al. 2005) to facilitate cooling processes and transport, have released their element-charged effluents into the water (Serrano et al. 2005), hence amplifying the transfer of contaminant elements into the water.
In addition to this phenomenon, increasingly limited by the installation of wastewater plants, weathering products, soil leaching and road runoff also contribute to pollutant flows and the spreading of these pollutants over increasingly large surfaces (Marcos 2001).
Water plays a fundamental role in pollution supporting, in the case of trace elements, as for other compounds, many chemical reactions.
Water transports trace elements which accumulate easily in the upper layers of the soil (Baker 1990; Samsoe-Petersen et al. 2002; El Gharmali 2005) from where they enter the food chain by bioaccumulation (Stalikas et al. 1996; Flynn et al. 2002).
Even if only traces of these elements are generally present, they are no less dangerous, contributing to potential problems, such as toxicity of the plants and animals and (Berti and Jacobs 1996; Ma and Liu 1997; Ma et al. 2002) consequently a disturbance in terrestrial ecosystems (Stalikas et al. 1996; Flynn et al. 2002).
Present in water and the terrestrial environment, certain trace elements, such as Zn and Se, are essential to normal plant life. They play an important role in the transformation of matter, mainly in animal enzymatic mechanisms (Stadman 1996; Stadman 2002).
Generally, a weak concentration of these elements in the environment has a positive effect and stimulates the activity of the living organisms (Stadman 1996; Burk and Hill 1999; Gladyshev and Hatfield 1999; Holben and Smith 1999; Stadman 2002). Beyond the maximum threshold, they restrain growth and development and can even be toxic.
It is now clear that our natural environment is a system in which there is a flow of matter; the environment is thus necessarily dynamic with scientific problems which concern the comprehension, the quantification and the modelling of these transfers, and more precisely the transfer of trace elements from contaminated water into soil.
The study of the transfer of trace elements in a porous environment requires a good knowledge of their geochemistry. The phenomena of precipitation–dissolution and adsorption–desorption exert a determining influence on the speciation of trace elements and control their mobility in the environment (Schwab and Banks 1993; Marcos 2001; Schwab et al. 2005).
The distance and speed of migration of a contaminant (Fig. 1) are the result of a very complex system (Marcos 2001), their degree of mobility, activity and bioavailability varying according to several factors such as nature of the soil, properties of the metals (Hellweg et al. 2005), pH (Kuo and McNeal 1984; Stahl and James 1991), cation exchange capacity, organic matter and clay content (McBride and Blasiak 1979), competition with other metal elements (Elliott et al. 1986; Covelo et al. 2004), temperature, concentration in soil solution (Schmidt 2003) and presence of soluble inorganic ligands or soluble organic ligands (McLean and Bledsoe 1992; Bradl 2004) which largely influence trace element adsorption by soil through the formation of stable complexes (Chairidcha and Ritchie 1990; McLean and Bledsoe 1992).
Trace metal binding is also described as a time-dependent process (Marcos 2001). The results of Ma and Liu (1997) on the adsorption of zinc on a calcareous soil clearly show the existence of binding kinetics. Christensen (1984a) showed that 95% cadmium is bound in the first 10 min on two different natural soils. Fixing then continues, but very slowly. Zachara et al. (1988) observed exactly the same phenomenon for the adsorption of zinc in micro-molar concentrations on calcite.
The bio-availability of metallic trace elements is directly related to residence time (Plassard et al. 2000). This relation is justified by the effect of time on the reactions between metal ions and soil; these include mainly complexation, adsorption and precipitation of metal ions on the surface, and their diffusion in the mesopores and micropores of soil (Plassard et al. 2000). Under these conditions, the Ci-Cs/Ci ratio known as the extraction-ratio or elimination-ratio measures the rate of extraction (Ke) of a pollutant (Derache 1989). The process of elimination of metallic trace elements is exponential (Derache 1989).
Ct = Ci-Cs is the concentration of metallic trace element fixed by the soil or the resident concentration of metallic trace element:
In addition, expression of pollutant concentration at the exit of the column is:
becomes : \( {\text{Cs}} = {\text{Ci }}{\left( {1 - {\text{exp}}{\left( { - Kt} \right)}} \right)} \)
The constant K is no other than an expression which accounts for all the different parameters which intervene in the transfer of trace elements from contaminated water into soil.
A thorough understanding of all these factors and their interactions is required to take the necessary measures to reduce the risks of soil and ground water pollution.
2 pH Effect
In most cases, trace element adsorption increases with pH. This observation is not necessarily true in competitive systems where trace elements coexist with certain complex agents such as dissolved organic matters. In general, when the pH increases, the bound metal portion passes from 0 to 100% within a very narrow range of pH (Marcos 2001), known as a jump in adsorption. This phenomenon has been clearly observed for trace metal adsorption on amorphous iron oxyhydroxides (Benjamin and Leckie 1981a,b) and on goethite (Coughlin and Stone 1995), for the adsorption of zinc on clays (Farrah and Pickering 1976) and on calcite (Zachara et al. 1988). Two factors could explain this phenomenon: firstly, increased pH decreases competition with other protons. Secondly, new reactional sites could be unprotoned and contribute to an increased reactivity of the environment. Thus, the oxide surface is positively charged at pH values below their isoelectric point and negatively charged when the pH increases beyond this critical value.
3 Dissolved Organic Ligands Effect
Organic ligands present in solution can promote or, on the contrary, significantly hinder the fixation of metal cations (Marcos 2001).
Certain organic acids, generally produced by plant roots and soil micro-organisms behave like chelating agents and can potentially increase or decrease the mobility of trace elements in soil (Shuman 1999; Joner and Leyval 2001; Schmidt 2003; Schwab et al. 2005).
The formation of neutral or negatively charged complexes prevents the adsorption of complex trace elements. The formation of cationic metal complexes can also reduce the adsorption of the complex by the soil (Farrah and Pickering 1976).
Ligands in solution are also susceptible to cause a desorption of the bound metal cations forming dissolved metallic complexes (Christensen 1989).
4 Material and Methods
Throughout this study, soil is conceptualized as a porous medium which is considered as a continuous environment, cohesive or not, presenting internally a volume fraction accessible to a fluid. This “vacuum” volume fraction, composed of caves, cracks, pores..., constitutes the porosity of the environment. It is through these vacuums that the water flow processes in the soil take place (Marcos 2001).
5 Soil Sampling
Bulk samples of surface soil were collected from a mine area located at 10 km to the west of Marrakech city (Morocco), using a 6 cm diameter steel cylinder (Rada 1996). Four uncontaminated soil samples (100 cm width × 20 cm depth) (El Gharmali 2005) were collected and mixed in a plastic basin, to give an average site sample (Rada 1996).
Part of this soil was air-dried, passed through a 200 μm sieve, homogenized and stored in plastic bags before analysis. Granulometric analysis showed that the studied soil has a sand–mud texture which is rich in clay. The results obtained are shown in Table 1. All physicochemical analyses were carried out on the fine fraction of the soil which contains important metal ratios (El Gharmali 2005).
pH was measured using a pH-meter on a mixture of fine soil and distilled water with a soil to water ratio of 1:2 (Aubert 1978). Electrical conductivity (EC) was determined after 30 min stirring of a mixture of fine soil and distilled water and 2 h of decantation in 1:5 soil/distilled water ratio (Aubert 1978). Cation exchange capacity (CEC) was determined using a 0.1 mol l−1 BaCl2 displacement method (Hendershot et al. 1993; Liu et al. 2001) and organic matter (OM) was measured by K2Cr2O7 digestion method (Nelson and Sommers 1996).
Principal physicochemical parameters of the soil are represented in Table 2.
Metal ion concentrations were measured by flame atomic absorption spectrometry (Perkin Elmer, Model 3100 EDS).
6 Columns
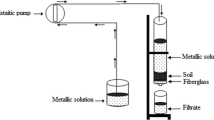
Retention capacity was determined by percolating Pb contamination solution (200 μg/l) through soil columns (Christensen 1989; Selim et al. 1989; Giusquiani et al. 1992; Plassard et al. 2000), 100 cm in length, with various diameters, filled to various heights with soil from the mining area.
All columns were closed at both ends with perforated caps. The upper cap hole was large enough to receive a tube from a peristaltic pump and allow air circulation. A test tube was connected to the hole in the lower cap to collect eluates.
There are several advantages to this dynamic approach compared to a static study on a batch reactor such as a better representation of the transfer conditions during rain water infiltration. Natural proportions of soil mass and water volume in contact with the soil are also respected. Moreover, the porous environment is not dispersed as during stirring in batch experiments. Under these conditions, trace element transfer is controlled by flow and adsorption reactions on the matrix, precipitation–dissolution and complexation in the solution (Marcos 2001).
7 Study of the Soil Tortuosity Effect on Retention of Pb
The space in the porous environment not occupied by the solid phase constitutes the soil’s poral space. It is through these vacuums that the flow processes take place.
Porosity should not be judged only according to its volume and to its distribution, but also to the continuity of the vacuums. Soil pores are differentiated by their opening and their degree of connection (De Marsilly 1981) which define the more or less privileged tortuous ways that water can cross the soil. This is known as “soil tortuosity.”
The study of the effect of soil tortuosity on the lead holding capacity of soil was carried out by adding glass balls (10 mm diameter) to various proportions of volume in the column not occupied by the solid phase (30 and 60% of the total volume of the column).
8 Study of the Particulate Bed Height Effect on Retention of Pb
To study the effect of column filling height on the dynamics of lead and its binding to soil, this part of study was carried out on 3 columns of the same diameter (D = 5 cm; Tony and Pant 2006) filled to different heights with soil; 10, 18 cm (Tony and Pant 2006) and 30 cm (De Matos et al. 2001, Tony and Pant 2006).
9 Study of the Water–Soil Surface Contact Effect on Retention of Pb
10 Study of Water Volume Effect on Retention of Pb
To study the effect of volume on soil lead retention, 3 different volumes of contaminated solution (75, 150 and 250 ml) were passed through three columns of same diameter (D = 5 cm) and same soil filling height (H = 18 cm).
11 Study of Water Flow Speed in Soil on Retention of Pb
The effect of flow speed on soil lead holding capacity was studied by changing the water flow speed at the entry of the column. The upper cap hole was large enough to receive a tube from a peristaltic pump and to permit water injection. Three speeds were used (75, 150 and 250 ml/min).
Fort each study, a representative soil sample of each part of the column was taken. After air drying, 0.5 g of each sample was calcined to 450°C for 2 h in a muffle furnace, made up to 5 ml with hydrofluoric acid (50%) and dried. The residue was dissolved in a mixture of 2.5 ml HNO3 (60%) and 7.5 ml HCl (37%) for 2 h until disappearance of the russet-red vapours. The solution obtained was added to 25 ml of bi-distilled water (Aubert 1978).
Recovered column solutions (eluates) were also recovered in order to follow the evolution of their Pb concentration with the time of their infiltration through the column soil.
12 Results and Discussion
12.1 Soil Tortuosity
Soil lead retention in relation to time was monitored in the eluates and then, after drying the soil, in relation to position in the column. Concentrations in eluates and dried soils are shown in Fig. 2 and Table 3, respectively.
Figure 2 shows eluate concentrations over time recovered from three columns with the same filling height but with various porosities. Increased lead concentration was observed in all three solutions. The speed of this increase became increasingly weak and stabilized to reach a maximum equilibrium value (MEV). This value remained lower than the concentration of the contaminated starting solution.
Figure 2 also shows a clear relation between the MEV, reflecting soil holding capacity, and its porosity. As soil ventilation increased, a strong decrease in heavy metal content in column solutions was observed. This finding can be justified by the role of soil ventilation (porosity) in the appearance and exposure of new lead binding sites (El Gharmali 2005).
Table 3 shows soil lead concentrations in different sections of each.
The results obtained show that all soils used in this study had the ability to fix lead from the contamination solution and accumulate it. In all three cases, lead concentrations followed the same pattern; the first soil layers of the column lodged more lead than lower ones. This finding points to the process of lead soil retention as being linked to the saturation of binding sites. Each time sites at one level are saturated, the lead passes to a lower level where it can bind or move on to leave the column, justifying the low values of lead measured in the first solutions recovered at the column ends.
13 Particulate Bed Height
Lead concentrations in recovered solutions and dried soils are shown in Fig. 3 and Table 4, respectively.
Figure 3 shows lead concentrations in recovered solutions after percolation through three columns of the same diameter but of various filling heights in relation to time. The results show that lead soil retention seems to be a two-step process (Mouflih et al. 2005). During the first minutes, lead binding capacity increased rapidly. After this initial binding period, holding capacity increased gradually before reaching an equilibrium of adsorption (Mouflih et al. 2005).
The MEV varied from one case to another; in some cases, it was due to a single varying parameter: filling height. Lead concentration of the recovered solution was inversely related to column filling height.
Table 4 shows the soil bound lead in different sections of the three columns. All three columns had unequal distribution of lead in the various sections of the column: high in the first layers and decreasing with column depth. This finding can be justified by the height effect which provides new lead binding sites.
14 Water–Soil Surface Contact
Lead concentrations in recovered solutions and dried soils are shown in Fig. 4 and Table 5, respectively.
Figure 4 shows lead concentrations in recovered solutions from three different columns with different diameters in relation to time. In all three cases, lead concentrations followed the same pattern, increasing with time to reach the MEV.
The MEV varied from one case to another due to the contact surface effect which provided new lead binding sites leading to a reduction in lead concentrations in the recovered solutions, and thus a reduced MEV.
Table 5 shows soil bound lead in different sections of the three columns. In all three columns, lead concentrations increased with column depth. This finding can be justified by the fact that lead is retained by saturation of the binding sites through the soil layers.
15 Water Volume
Lead concentrations in recovered solutions and dried soils are shown in Fig. 5 and Table 6, respectively.
Figure 5 shows lead concentrations in recovered solutions from three columns of the same dimension. In all three cases, lead concentrations followed the same pattern, increasing with time to reach the MEV. This value was higher for lower volumes of water. This finding can be explained by the effect of gravity which leads to reduced water–soil contact and consequently minimized lead binding capacity.
Table 6 shows soil bound lead in different sections of the three columns. In two columns, lead concentrations increased with column depth when water volume increased. This finding can be justified by the antagonistic effect of gravity (due to the water weight) on lead soil binding. The effect was attenuated with passage through the soil column due to resistance forces which ensured sufficient contact time to establish liaisons between metallic trace elements and soil binding sites.
In this case, the effect of volume is expressed as the effect of gravity leading to a reduction in contact time, and consequently minimized lead holding capacity in soil binding sites.
16 Water Flow Speed in Soil
A distinction must be made between Darcy speed and flow speed in soil. Darcy speed, defined by the Darcy law, is a volumetric flux density, determined by water volumetric flow and total surface of the considered porous section. In fact, water flows only through the pores of the particular bed. A correct definition of flow speed implies a redefinition of the section through which the flow passes.
Lead concentrations in recovered solutions and dried soils are shown in Fig. 6 and Table 7, respectively.
Figure 6 shows lead concentrations in recovered solutions from three columns of the same dimension. In all three cases, lead concentrations followed the same pattern, increasing with time to reach the MEV. This value was higher for lower water speeds, a finding which can be explained by the fact that high speeds reduce the contact time necessary for liaisons between lead and soil binding sites, thereby decreasing soil retention capacity.
Table 7 shows soil bound lead in different sections of the three columns. Lead concentrations increased with column depth confirming that water speed hindered soil lead retention. The effect was attenuated with passage through the soil column due to resistance forces, such as friction, which decreased water speed and ensured sufficient contact time. Consequently, soil lead binding capacity was higher in lower layers.
17 Conclusion
Soil is one of the key elements of all terrestrial ecosystems and is a very complex heterogeneous medium consisting of soil matrix, soil water and soil air. Heavy metal ions are the most toxic inorganic pollutants which occur in soils and can be of natural or of anthropogenic origin.
Retention of lead on soil in a mining area has stronger dependence on feed flow rate, particulate bed tortuosity, bed height, water–soil surface contact and water volume. Increase in bed height, water–soil surface contact and particulate bed tortuosity leads to higher contact time thus higher lead retention by soil, whereas increase in feed flow rate and water volume leads to lower contact time thus lower lead retention by soil.
References
Alexander, M. (2000). Aging, bioavailability, and overestimation of risk from environmental pollutants. Environmental Science & Technology, 34, 4259–4264.
Anxiang, L., Shuzhen, Z., & Xiao-quan, S. (2005). Time effect on the fractionation of heavy metals in soils. Geoderma, 125(3–4), 225–234.
Aubert, G. (1978). Méthodes d’analyses des sols. Marseille: G.R.D.P., (p 191).
Baker, D. E. (1990). Copper. In B. J. Alloway (Ed.), Heavy metals in soils (pp. 151–176). London, UK: Blackie.
Beeby, A., & Eaves, S. L. (1983). Short-term changes in Ca, Pb, Zn and Cd concentrations of the garden snail Helix aspersa Müller from a central London car park. Environmental Pollution (series A), 30, 233-244.
Benjamin, M. M., & Leckie, J. O. (1981a). Multiple-site adsorption of Cd, Cu, Zn et Pb on amorphous iron oxyhydroxide. Journal of Colloid and Interface Science, 79(1), 209–221.
Benjamin, M. M., & Leckie, J. O. (1981b). Conceptual model for metal-ligand surface interactions during adsorption. Environmental Science & Technology, 15(9), 1050–1056.
Berti, W. R., & Jacobs, L. W. (1996). Chemistry and phytotoxicity of soil trace elements from repeated sewage sludge applications. Journal of Environmental Quality, 25, 1025–1032.
Bradl, H. B. (2004). Adsorption of heavy metal ions on soils and soils constituents. Journal of Colloid and Interface Science, 277, 1.
Burk, R. F., Hill, K. E. (1999). Orphan selenoproteins. BioEssays, 21, 231–237.
Chairidcha, P. I., & Ritchie, G. S. P. (1990). Zinc adsorption by a lateritic soil in the presence of organic ligands. Soil Science Society of America Journal, 54, 1242–1248.
Christensen, T. H. (1984a). Cadmium soil sorption at low concentrations: III. Prediction and observation of mobility. Water, Air and Soil Pollution, 26(3), 255–264.
Christensen, T. H. (1989). Cadmium soil sorption at low concentrations: VII. Effect of stable solid waste leachate complexes. Water, Air and Soil Pollution, 44, 43–56.
Coughlin, B. R., & Stone, A. T. (1995). Nonreversible adsorption of divalent metal ion (Mn(II), Co(II), Ni(II), Cu(II) and Pb(II)onto goethite: Effects of acidification, Fe(II) addition, and picolinic acid addition. Environmental Science & Technology, 29, 2445–2455.
Covelo, E. F., Andrade, M. L., & Vega, F. A. (2004). Heavy metal adsorption by humic umbrisols: Selectivity sequences and competitive sorption kinetics. Journal of Colloid and Interface Science, 280, 1–8.
De Marsilly, G. (1981). Hydrogéologie quantitative (p. 215). Paris: Masson. (Collection Sciences de la Terre).
De Matos, A. T., Fontes, M. P. F., Da Costa, L. M., & Martinez, M. A. (2001). Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environmental Pollution, 111(3), 429–435.
Derache, P. (1989). Toxicologie & sécurité des aliments: Mécanisme d’action des toxiques. Lavoisier, Tec & Doc 1986, deuxième tirage. pp. 39–64.
El Gharmali, A. (2005). Impact des résidus miniers et des eaux résiduaires sur la contamination métallique des écosystèmes aquatiques et terrestres de la région de Marrakech, Maroc. Thèse de 3ème cycle.
Elliott, H. A., Liberati, M. R., & Haung, C. P. (1986). Competitive adsorption of heavy metals by soil. Journal of Environmental Quality, 15, 214–219.
Farrah, H., & Pickering, F. (1976). The sorption of zinc species by clay minerals. Australian Journal of Chemistry, 29, 1649–1656.
Flynn, H., Mc Mahon, V., Diaz, G. C., Demergasso, C. S., Corbisier, P., Meharg, A. A., et al. (2002). Assessment of bioavailability of arsenic and copper in soils and sediments from the Antofagasta region of Northern Chile. Science of the Total Environment, 286(1–3), 51–59.
Giusquiani, P. L., Gigliotti, G., & Businelli, D. (1992). Mobility of heavy metals in urban waste-amended soil. Journal of Environmental Quality, 21, 330–335.
Gladyshev, V. N., & Hatfield, D. L. (1999). Selenocysteine-containing proteins in mammals. Journal of Biomedical Science, 6, 151–160.
Hellweg, S., Ulrich, F., Thomas, B., & Hungerbühler, K. (2005). Site-dependent fate assessment in LCA: Transport of heavy metals in soil. Journal of Cleaner Production, 13, 341–361.
Hendershot, W. H., Lalande, H., & Duquette, M. (1993). Ion exchange and exchangeable cations. In M. R. Carter (Ed.), Soil sampling and methods of analysis (pp. 167–175). Boca Raton, FL, USA: Lewis Publisher.
Holben, D. H., & Smith, A. M. (1999). The diverse role of selenium within selenoproteins: A review. Journal of the American Dietetic Association, 99(7), 836–843 (July 1999).
Hopkin, S. P. (1989). Ecophysiology of metals in terrestrial invertebrates. New York: Elsevier Applied Science.
Joner, E. J., & Leyval, C. (2001). Time-course of heavy metal uptake in maize and clover as affected by root density and different mycorrhizal inoculation regimes. Biology and Fertility of Soils, 33, 351–357.
Kuo, S., McNeal, B. L. (1984). Effect of pH and phosphate on cadmium sorption by hydrous ferric oxide. Soil Science Society of America Journal, 48, 1040–1044.
Liu, D., Yang, S., Xie, J., & Zhao, J. (2001). Physiology and development of two Roegneria kamoji populations stressed by copper. Ying Young Sheng Tai Xue Bao, 12(3), 455–457.
Ma, Y., Dickinson, N. M., & Wong, M. H. (2002).Toxicity of Pb/Zn mine tailings to earthworm Pheretima and the effect of burrowing on metal availability. Biology and Fertility of Soils, 36, 79–86.
Ma, Y. B., & Liu, J. F. (1997). Adsorption kinetic of zinc in a calcaneous soil as affected by pH and temperature. Communications in Soil Science and Plant Analysis, 28(13&14), 1117–1126.
Marcos, L. (2001). Etude expérimentale et modélisation du transfert du Zinc et du Plomb dans des milieux sableux modèles, thèse à l’école des mines de Nantes. N° ED 0376-001.
Mc Bride, M. B., & Blasiak, J. J. (1979). Zinc and copper solubility as a function of pH in an acid soil. Soil Science Society of America Journal, 49, 866–870.
McLean, J. E., & Bledsoe, B. E. (1992). Behavior of metals in soils. EPA Groundwater Issue, EPA/540/S-92/018. Washington, DC: US EPA.
Mouflih, M., Aklil, A., & Sebti, S. (2005). Removal of lead from aqueous solutions by activated phosphate. Journal of Hazardous Materials, 119(1–3), 183–188.
Nelson, D. W., & Sommers, L. E. (1996). Total carbon, organic carbon and organic matter. In D. L. Sparks (Ed.), Methods of soil analysis, part 3 (pp. 961–1010). Madison, WI, USA: Chemical Methods, Soil Science Society of American.
Notten, M. J. M., Oosthoek, A. J. P., Rozema, J., & Aerts, R. (2005). Heavy metal concentrations in a soil–plant–snail food chain along a terrestrial soil pollution gradient. Environmental Pollution, 138(1), 178–190 (November 2005).
Pedersen, M. B., Kjar, C., & Elmegaard, N. (2000). Toxicity and bioaccumulation of copper to black bindweed (Fallopia convolvulus) in relation to bioavailability and the age of soil contamination. Archives of Environmental Contamination and Toxicology, 39, 431–439.
Plassard, F., Winiarski, T., & Petit-Ramel, M. (2000). Retention and distribution of three heavy metals in a carbonated soil: comparison between batch and unsaturated column studies. Journal of Contaminant Hydrology, 42(2–4), 99–111 (31 March 2000).
Rada, A. (1996). Etude de la contamination métallique des sols de la zone d’épandage de la ville de Marrakech (Maroc): Contribution du fond géochimique et des apports anthropiques dans le transfert du cadmium dans le système sol-plante. Thèse Doct. D’état, Univ. Cadi Ayyad, Fac. Semlalia, Marrakech.
Samsoe-Petersen, L., Larsen, E. H., Larsen ,P. B., & Bruun, P. (2002). Uptake of trace elements and PAHs by fruit and vegetable from contaminated soils. Environmental Science & Technology, 36, 3057–3063.
Schmidt, U. (2003). Enhancing phytoextraction: The effect of chemical soil manipulation on mobility, plant accumulation, and leaching of heavy metals. Journal of Environmental Quality, 32, 1939–1954.
Schwab, A. P., & Banks, M. K. (1993). The impacts of vegetation on the leaching of heavy metals from mine tailings: solubilization of zinc by organic acids. In: Proc. AWMA, Denver, CO, Paper 93-WA-89.06.
Schwab, A. P., Ying hong, H., & Banks, M. K. (2005). The influence of organic ligands on the retention of lead in soil. Chemosphere, 61(6), 856–866 (November 2005).
Selim, H. M., Amarcher, M. C., & Iskandar, I. K. (1989). Modeling the transport of chromium (IV) in soil columns. Soil Science Society of America Journal, 53, 996–1004.
Serrano, S., Garrido, F., Campbell, C. G., & García-González, M. T. (2005). Competitive sorption of cadmium and lead in acid soils of Central Spain. Geoderma, 124(1–2), 91–104 (January 2005).
Shuman, L. M. (1999). Organic waste amendments e.ect on zinc fractions of two soils. Journal of Environmental Quality, 28, 1442–1447.
Stadman, T. C. (1996). Selenocysteine. Annual Review of Biochemistry, 65, 83–100.
Stadman, T. C. (2002). Discoveries of vitamin B12 and selenium enzymes. Annual Review of Biochemistry, 71, 1–16.
Stahl, R. S., & James, B. R. (1991). Zinc sorption by B horizon soils as a function of pH. Soil Science Society of America Journal, 55, 1592–1597.
Stalikas, C. D., Mantalovas, A., & Pilidis, G. A. (1996). Multielement concentration in vegetable species grown in two typical agricultural areas of Greece. Science of the Total Environment, 206, 17–24
Tony, S. S., & Pant, K. K. (2006). Experimental and modelling studies on fixed bed adsorption of As(III) ions from aqueous solution. Separation and Purification Technology, 48(3), 288–296 (15 March 2006).
Zachara, J. M., Kittrick, J. A., & Harsh, J. B. (1988). The mechanism of Zn2+ adsorption on calcite. Geochimica et Cosmochimica Acta, 52, 2281–2291.
Acknowledgements
The authors would like to thank Y. François for his invaluable technical assistance. They gratefully acknowledge the assistance provided by Mme Nadira Talbi and Mohamed Barkouch for their assistance and Mme Susan Gamon in proof-reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barkouch, Y., Sedki, A. & Pineau, A. A New Approach for Understanding Lead Transfer in Agricultural Soil. Water Air Soil Pollut 186, 3–13 (2007). https://doi.org/10.1007/s11270-007-9450-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9450-9