Abstract
Agricultural soils high in both fluoride (F) and phosphate (P) are common due to long-term accumulation of F from multi-sources and extensive application of phosphate fertilizers. Iron (Fe) and aluminum (Al) (hydro)oxides in acidic soils serve as main geochemical sinks of both P and F, influencing their transport and bioavailability. Though sorption of P and F in their single-ion system has been extensively investigated, studies on co-sorption of F and P on soils are very limited. In this study, the batch technique was used to investigate mutual effects of F and P on their co-sorption/desorption in an acidic red soil with high contents of Fe and Al (hydro)oxides. Results indicate that, in F–P coexisting system, a decrease in pH enhances the sorption of both F and P. An increase in F concentration suppresses P sorption due to competitive effect. However, F sorption can be improved in the presence of P due to surface precipitation of (Al,Fe)–F–P. Sorption of F and P follows both the Langmuir and Freundlich isotherms. Different orders of F and P addition into the soil have no appreciable effect on P sorption, but exert significant impact on F sorption. The presence of F has no measurable effect on P desorption, while the stability of F in the presence of P can be significantly diminished in comparison with that in the absence of P, which would lead to an improvement of F mobility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fluoride (F) is a natural constituent of minerals and soils. Natural concentrations of soluble F tend to be <10 μm (Larsen and Widdowson 1971). Accumulation of F in soils is mainly from weathering of volcanic ashes, primary minerals and strata high in F content (Totsche et al. 2000; Cronin et al. 2003), application of phosphate fertilizers (containing 1.5–4% F) in farming (Loganathan et al. 2001), and several industrial sources, for example, semiconductors, steel/aluminum, phosphate fertilizers and electroplating (Arnesen and Krogstad 1998). Long-term accumulation of F in soils may cause F pollution (Arnesen et al. 1995). Fluoride contents as high as 1,000 mg kg−1 soil were reported in paddy soils in southeastern China (Zhou and Sun 2002). Excessive intake of F may cause dental and/or skeletal fluorosis, which is afflicting many millions of people worldwide (Reardon and Wang 2000; Zhang et al. 2003; Jacks et al. 2005).
As major components in acidic soils, aluminum (Al) and iron (Fe) (hydro)oxides serve as important sinks of F. Due to strong affinity of Al atom for F, F is preferentially adsorbed on Al (hydro)oxides, particularly on amorphous Al solid phases and highly reactive hydroxyaluminum complexes (Khare et al. 2005; Zhu et al. 2006), which are common in acidic soils (Saha et al. 2001). On the other hand, F-facilitated dissolution of Al and Fe (hydro)oxides (so called ligand-induced dissolution) can be significantly enhanced, particularly at low pH, which would exert substantial effects on physical chemistry of the soil solutions and charge density of the soil surface, and hence on sorption/desorption and transport of nutrients and potential pollutants in soils (Polomski et al. 1982; Harrington et al. 2003; Zhu et al. 2004a).
Phosphate (P) is one of major nutrients for plant growth and widely distributed in agricultural soils. Aluminum and Fe (hydro)oxides in soils also serve as the most important sink of P (Peretyazhko and Sposito 2005; Reiner et al. 2005). At low P concentration, P tends to adsorb on Fe (hydro)oxides; at high concentration, however, Al and Fe (hydro)oxides may play equally important roles in P retention (Khare et al. 2004).
Agricultural soils high in both F and P are common due to long-term accumulation of F from multi-sources and extensive application of phosphate fertilizers. The study on P and F co-sorption/desorption is of importance for understanding transport and bioavailability of F and P in F-contaminated agricultural soils, and for assessing mobility of some potential pollutants. Although sorption/desorption behavior of F and P on metal (hydro)oxides, phyllosilicate minerals and soils in their individual systems have been extensively studied (Kau et al. 1997, 1998; Arnesen and Krogstad 1998; Maliyekkal et al. 2006), investigation on co-sorption/desorption of F and P in soils high in both F and P is very limited. To our knowledge, only Ji (1986) investigated co-sorption of F and P on goethite. The objective of this study is to evaluate mutual effects of F and P on their sorption/desorption on/from a red soil in F–P coexisting system. Red soil (Haplic Acrisol) is a typical variable charge soil widely distributed in the south of China, which is high in acidity and Fe and Al (hydro)oxides.
2 Materials and Methods
2.1 Soil Samples
One red soil, naturally developed on Quaternary red earths without disturbance of land-use activities, was collected from surface A horizon (0–20 cm) in a level area of Yintan, Jiangxi Province, China (116°17′E, 28°23′N), on which Masson pines were sparsely planted. After air-drying, the soil sample was gently ground to pass through a 60-mesh sieve for use. Selected properties of the soil are given in Table 1. Soil pH was determined in water (soil:water 1:2.5 w/w′). Content of organic carbon was determined using a Carlo Erba elemental analyzer. Cation exchange capacity (CEC) was determined using the ammonium acetate method (Zhu et al. 2004b). Exchangeable hydrogen (Ex-H) and exchangeable Al (Ex-Al) were determined using the KCl leaching–NaOH titration method (Yu and Wang 1988). The soil sample has low organic matter content. The ratio of Ex-Al to CEC is high (0.52), indicative of low saturation of base cations and high acidity, which is consistent with low pH of the soil. The contents of free Fe and Al (hydro)oxides (FeDCB, AlDCB) in the soil were determined using the DCB (dithionite-citrate-bicarbonate) method (Heron et al. 1994).
2.2 Experimental Procedures and Analytical Methods
Fluoride and P stock solutions were prepared by dissolving NaF and KH2PO4 in deionized water, respectively. Fluoride and P solutions of desired pH values were obtained by adjusting using dilute HCl and NaOH solutions.
Sorption of F and P
Experiments of F and P sorption were conducted using a batch method. 2.00-g soil samples were weighed into a series of 100-ml polyethylene centrifuge tubes, to which 50-ml mixing solutions with varying concentrations of F (0∼190 mg l−1) and P (0∼100 mg l−1) were added. Each mixing solution contained 1.49 g l−1 (0.02 mol l−1) KCl as background electrolyte. After being capped and vigorously shaken by hand, the tubes were placed in a water bath at 27 ± 1°C and gently shaken until equilibrium. Then the suspensions were centrifuged (3.46 × 104 g) and filtrated (0.45 μm). Filtrate pH was measured immediately after filtration. The filtrates were then stored at 4°C until determination of F and P concentrations. Fluoride was determined by the F-electrode method (Zhu et al. 2004a). Prior to measurement of F, 10.0 ml total ionic strength adjusting buffer (TISAB) was added to each filtrate. After 20 min, F concentrations were determined using a F-electrode and a calomel reference electrode. The same volume of TISAB was also added to the standard F solutions. TISAB was used to adjust solution pH to about 5.5 and to liberate F complexed with Al. Phosphate was determined by the molybdate blue method. Amounts of F and P sorbed were determined by difference. Our preliminary experiments showed that the equilibrium for F sorption could be reached within 48 h, but 120 h was needed for the equilibrium of P sorption. In this study, 120 h was chosen as equilibrium time for both F and P sorption, unless otherwise stated. Effects of initial pH and concentrations of F and P on their sorption were evaluated. Different ways of F and P addition, i.e. (1) F addition after P, (2) simultaneous addition of F and P, and (3) P addition after F, on their sorption were also assessed. To minimize the variation of solid/liquid ratio before and after addition of the second ion in each sequential addition, 5-ml concentrated solution of the second ion was added to achieve a desired total concentration of the ion.
Desorption of F and P
Desorption of F and P was carried out by extraction using 1.49 g l−1 KCl solution immediately after sorption experiments (initial F and P concentrations 190 mg l−1 and 50 mg l−1, respectively). Briefly, after the suspensions were centrifuged at the end of sorption (120 h) and the supernatants decanted carefully, 50-ml 1.49 g l−1 KCl solution was added and mixed by ultrasonic dispersion for 10 min, then the suspensions were gently shaken in a rotary shaker for predetermined time periods (30–240 min). At the end of each extraction time, the suspension was centrifuged for 10 min and the supernatant was separated by filtration (0.45 μm) for F and P analysis. The entrapped solution at the end of the sorption step was corrected on basis of original solution density and weight difference between the initial dry samples and the wet ones after sorption step.
All the experiments were run in duplicate and all the data presented were the averages of duplicate analysis.
3 Results and Discussion
3.1 Effects of Initial pH on Sorption of Fluoride and Phosphate
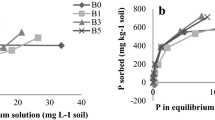
Solution pH generally exerts significant effect on sorption of both cations and anions on metal (hydro)oxides, phyllosilicate minerals and soils. The effects of initial pH on F and P sorption on the red soil in F–P mixing system are presented in Figs. 1 and 2. Also presented in the figures are F and P sorption in their single-ion systems for comparative purpose. At 100 mg l−1 of initial P concentration, the amount of sorbed P decreased slightly with increasing initial pH at low F concentration (76 mg l−1) and in the absence of F; at a high F concentration (190 mg l−1), however, an increase in initial pH markedly decreased P sorption. At 40 mg l−1 of initial P concentration, initial pH (3.5–7.5) exerted only slight effect on P sorption, regardless of the presence of F. Phosphate sorption was progressively suppressed with increasing F concentration, indicating competitive effect of F on P. Similar to the effect of pH on F sorption in single-ion system, F sorption in the presence of P also decreased with increasing initial pH. The increase in F sorption with increasing P concentration indicated that the presence of P promoted F sorption. It is inconsistent with the common sense that coexisting anions are competitive with each other for binding sites.
Surface adsorption of both P and F on metal (hydro)oxides and clay minerals is mainly via ligand exchange, that is, F and P exchange surface –OH/OH2 groups, forming inner-sphere complexes (Kraemer et al. 1998; Shin and Han 2004). Adsorption on positively charged sites via electrostatic force, forming outer-sphere complexes, may also contribute partly to F and P retention (Valdivieso et al. 2006). In F–P coexisting system, the increases in amounts of F and P sorption with decreasing solution pH could be attributed partly to an increase in positively charged sites due to protonation. The red soil studied is a typical variable charge soil high in free Fe and Al (hydro)oxides, whose pHzpc (pH at zero point of charge) are between 7.5 and 9.5 (Stumm and Morgan 1996). Therefore, protonation in the experimental pH conditions was an important factor governing P and F surface adsorption. At low concentrations of P (40 mg l−1) and F (76 mg l−1), slight effect of increasing pH on both P and F sorption was due probably to low surface coverage of the soil particles. In this case, even though deprotonation have decreased the density of positively charged sites, available remaining positively charged binding sites were still sufficient for P and F adsorption. As a result, pH exerted only slight effect on the overall sorption of P and F. At high concentrations of P (100 mg l−1) and F (190 mg l−1), however, the total number of positively charged binding sites was probably far inadequate for monolayer surface coverage by the two ions. In this case, an enhancement in positive surface charges due to protonation would elevate the adsorption of P and F to different extents depending on solution pH and concentrations of the two ions.
Given the similar mechanisms of F and P surface adsorption aforementioned, it is expected that P and F would be competitive with each other in P–F coexisting system, which would have resulted in a reduction in retention of one ion with an increase in concentration of the other, and vice versa. On the contrary, the enhancement of F sorption in the presence of P indicated that, besides surface adsorption, other more important mechanism(s) must have been involved and played a critical role in F sorption.
It is recognized that, besides surface adsorption via ligand exchange and electrostatic force mechanism (two-dimensional surface adsorption), P may also be retained via surface precipitation at a condition well below the saturation with respect to metal phosphate solid phase (Li and Stanforth 2000; Zhao and Stanforth 2001), which has been confirmed by X-ray adsorption near-edge structure (XANES) spectrum (Khare et al. 2005), 31P and 27Al nuclear magnetic resonance (NMR) spectrum (Lookman et al. 1997), and zeta potential measurements (Li and Stanforth 2000; Zhao and Stanforth 2001; Ler and Stanforth 2003). Zhao and Stanforth (2001) proposed a four-step surface precipitation model for P surface precipitation on goethite, which was lately illustrated in more detail by Ler and Stanforth (2003) (cf. Fig. 3), that is, (1) formation of mono- or bidentate complexes on A hydroxyls, (2) the adsorbed P as a new binding site for dissolved Fe ions, forming a ternary complex (surface precipitation) and reducing Fe concentration in solution, (3) goethite dissolving to replenish the Fe in solution, which can then adsorb on P adsorbed on the surface, (4) the adsorbed Fe acting as a new binding site for dissolved P. The above four-step processes proceed continuously, leading to occurrence of surface precipitation at a condition well below saturation with respect to poorly crystalline iron phosphate solid phases. The dissolution of goethite to provide a continuous supply of dissolved iron for precipitating the P is one of critical steps in the model. Besides H+-promoted dissolution, Fe dissolution can be anion-facilitated, that is, ligand-induced dissolution. In the same way as iron phosphate precipitation on goethite, aluminum phosphate precipitation may also occur on Al (hydro)oxides at a suitable P concentration level and physiochemical conditions.
Schematic of (Al,Fe)–F–P surface precipitation process, adopted from Ler and Stanforth (2003) with some modifications (Me is Al and/or Fe). Step 1, surface complexation. Step 2, ternary adsorption of (Al,Fe)–F–P (surface precipitation). Step 3, dissolution of aluminum and/or iron (hydro)oxides and formation (Al,Fe)–F complexes. Step 4, adsorption of phosphate on sorbed (Al,Fe)–F complexes (surface precipitation, continued)
Dissolution of Fe or Al (hydro)oxides and silicate minerals can be facilitated by some inorganic and organic anions (Stumm and Morgan 1996; Kraemer et al. 1998). Under an acidic condition, F can effectively promote Al dissolution due to high affinity of Al atom for F (Nordin et al. 1999; Zhu et al. 2004a). Iron dissolution can also be promoted by F but to a much less extent due to much low affinity of Fe atom for F (Wilcke et al. 2000). The facilitating effect can be greatly improved with elevated F concentration and solution acidity. According to the model of Zhao and Stanforth (2001), it is expected that an enhancement in concentrations of dissolved Fe and/or Al would favor surface precipitation of metal phosphate. In the presence of F, soluble Al is present dominantly as Al–F complexes (\( {\text{AlF}}^{{{\left( {3 - x} \right)}}}_{x} ,x = 1 \sim 6 \)) of various species depending on solution pH and the concentration of total F, among which AlF2+ and \( {\text{AlF}}^{ + }_{2} \) are the main species (Moore and Ritchie 1998). Accordingly, positively charged ions AlF2+ and \( {\text{AlF}}^{ + }_{2} \), instead of Al3+, would participate in the formation of ternary complexes with adsorbed P (Fig. 3). Similarly, \( {\text{FeF}}^{{{\left( {3 - x} \right)}}}_{x} \) (x = 1 or 2) may also participate in the formation of surface precipitation, but with much lower contribution to the total surface precipitants due to lower concentration of Fe–F complexes than that of Al–F complexes. One may conclude that it is the formation of (Al,Fe)–F–P precipitant that has outweighed the competitive effect of P on F in governing F sorption, and thus lead to the overall increase in retention with an increase in P concentration. In this case, the overall sorption capacity of the red soil for P and F is expected to be well beyond that predicted by the density of surface binding sites available. This phenomenon was reported by Ji (1986), who found that the total amount of P and F sorbed was in excess of the amount of A hydroxyl sites on the goethite based on his theoretical modeling. Abnormally high sorption density of \( {\text{AsO}}^{{3 - }}_{4} \) on Fe and Al (hydro)oxides under acidic conditions has been explained as the result of Fe– or Al–As coprecipitation in literature (Gräfe et al. 2004; Jia et al. 2006; Violante et al. 2006).
Considering the increase in dissolved Al and Fe facilitated by F with a decrease in pH and therefore an increase in (Al,Fe)–F–P precipitation, surface precipitation, besides surface protonation aforementioned, was also partly responsible for the enhancement of F sorption with decreasing pH.
It should be pointed out that the surface complexes and mechanism shown in Fig. 3, although being partly supported by the literature, need to be further validated by spectroscopic and other surface techniques, such as XANES, NMR, and zeta potential measurement.
3.2 Isotherms for Flouride and Phosphate Sorption
Isotherm for P sorption in the presence of F is showed in Fig. 4 (pH 6.0 ± 0.02). An increase in F concentration reduced P adsorption, similar to the result shown in Fig. 1. The isotherm data were fitted to two commonly used isotherm equations, the Langmuir (Eq. 1) and Freundlich (Eq. 2):
where Q e is the amount of F or P sorbed at equilibrium, Q m the theoretical maximum monolayer sorption capacity, C e the equilibrium concentration of F or P in solution, and K L, n and K F are empirical constants. The calculated parameters are given in Tables 2 and 3. As can be seen, P sorption could be well described by both the Langmuir and Freundlich equations. The values of 1/n calculated from the Freundlich isotherm can provide information about affinity of an adsorbent for adsorbates studied: a decrease in the value of 1/n indicates an increase in affinity, and 1/n <1 indicates favorable sorption and 1/n >1 unfavorable sorption (Tsai et al. 2004). As shown in Table 2, all the values of 1/n <1 imply favorable sorption of the red soil for P, which is believed the corollary of high contents of Fe and Al (hydro)oxides in the soil. With an increase in F concentration, the values of 1/n for P sorption increased, indicating a reduction in affinity, which is probably a quantitative indicative of competitive effect of F on P sorption.
Isotherm for F sorption in the presence of P is shown in Fig. 5 (pH 6.0 ± 0.02). In comparison with the case of at the absence of P, the presence of P at low concentration (20 mg l−1) exerted only slight effect on F sorption. The presence of P with high concentration (100 mg l−1), however, enhanced appreciably F retention at both high concentrations of F (> 100 mg l−1) and P (100 mg l−1). The isotherm data for F sorption can also be well fitted to the Langmuir and Freundlich equations (Table 3), and all the values of 1/n <1 suggest favorable sorption of the red soil for F. An increase in the values of 1/n with an increase in initial P concentration indicates a decrease in affinity of the soil for F. It seemingly conflicts with the observation of the overall increase in experimental F sorption amount with an increase in P concentration. As a matter of fact, besides precipitation of (Al,Fe)–F–P, surface adsorption also contributed partly to F sorption. Increasing P adsorption would impart more negative surface charge, which would suppress electrostatic adsorption of F. In addition, an increase in negative surface charges would further repel P approaching to the sites of ternary complexes (step 4 in Fig. 3), which is unfavorable for (Al,Fe)–F–P precipitation. These two factors are probably the main reasons for the decrease in affinity of the soil for F with increasing P concentration. It should be pointed out that, even though the decrease in affinity of the soil for F sorption with an increase in P concentration as evidenced by the values of 1/n, no experimental manifestation of competitive effect of P on F sorption was exhibited. A possible explanation is that continuous formation of new binding sites on surface precipitant phase has improved F retention, and outcompeted the effect of decreasing affinity on F sorption. The values of 1/n for P sorption were smaller than those for F sorption, probably a quantitative indicative of higher affinity of the soil for P than for F.
Similar information on physical mechanism of F and P sorption can also be obtained from the Langmuir affinity parameter K L, as obtained from 1/n, which will not be readdressed here. The theoretical maximum monolayer sorption capacity (Q m) for both F and P sorption increased when the concentration of coexisting ion (P or F) increased, suggesting that, unlike many normal adsorption processes, the total surface binding sites and probably other surface properties were not fixed during F and P sorption process due to the involvement of precipitation. Therefore, Q m only could not provide unambiguous information about physical mechanism of F or P sorption.
Li and Stanforth (2000) observed that the change from adsorption into surface precipitation during P retention on goethite was not discernible in the adsorption isotherm. It implies that, in some cases, sorption isotherm is of no use in distinguishing surface precipitation from adsorption. It therefore comes as no surprise that an abrupt break of isotherm curves was not observed in whole F or P concentration ranges of the present study even though surface precipitation was believed to have occurred.
3.3 Mutual Effects of Sequential Addition of Fluoride and Phosphate on Their Sorption
In natural environments, it is likely that F and P are sequentially introduced into and accumulated in soils, i.e., (1) P addition after F, (2) F addition after P, (3) simultaneous addition of F and P. The different ways of addition may exert different impacts on P and F co-sorption on soils. Effects of the different ways of addition on co-sorption of F and P were evaluated. As shown in Fig. 6, the orders of P and F application made no appreciable difference of P sorption. It is not unexpected because stronger affinity of the soil for P than for F could guarantee P to preferentially adsorb on active binding sites or to be able to exchange previously adsorbed F, regardless of the orders of F and P application. As shown in Fig. 7, the amount of F sorption is the highest in the case of simultaneous introduction of F and P, followed by that in the case of F introduction first; F sorption is the lowest in the case of P introduction first.
Fluoride can more easily diffuse into meso- and micro-pores than P due to small ionic radius of F (0.14 nm) than that of P (0.22 nm). Given the stronger affinity of the soil for P than for F, when P was first introduced, preferentially adsorbing P and metal phosphate precipitation would block the pores and limit F diffusion into the inner pores and, as a result, most binding sites in deep micro-pores became inaccessible to F, inevitably leading to a significant decrease in F sorption (Wang and Xing 2002, 2004). In the case of F addition first, F would diffuse easily into and be retained in deep micro-pores, which was not able to be exchanged by lately added P due to inaccessibility of those binding sites to P. Following this reasoning, F sorption in the case of F addition first would have been highest among the three ways of introduction, but our results did not support the theoretical reasoning. The slightly larger F retention in the case of simultaneous application of F and P than that in the case of F addition first was believed to be the result of more surface precipitation in the former case, because, in the former case, the formation of (Al,Fe)–F–P ternary complexes and following surface precipitation are more favored due to both high concentrations of P and Al–F complexes than in the other two ways of addition.
3.4 Desorption of Fluoride and Phosphate
The rate and extent of ion release from soils are closely dependent on their binding mechanisms (surface adsorption, surface precipitation, and formation of solid solution with the adsorbents, etc). It is accepted that chloride ions can release anions adsorbed via outer-sphere surface complexation, but cannot readily exchange anions adsorbed via inner-sphere surface complexation. Ions incorporated in surface precipitants and solid solutions are generally non-exchangeable. Using 1.49 g l−1 KCl as an extractant, the kinetic release of F and P from the red soil pre-sorbing F and P is shown in Fig. 8. The desorption percentages of P and F in their single-ion systems were very low, only 16 and 5%, respectively, at extraction time of 4 h, which could be attributed to strong sorption of the two ions via inner-sphere surface complexation plus possible surface precipitation of P. As shown in Fig. 8, co-sorption of F and P exerted no marked effect on P desorption, which is probably a combined result of the following two aspects: (1) the surface complexation structure of P is not substantially affected by the presence of F due to higher affinity of the soil for P than for F, (2) regardless of the presence of F, surface precipitation of P would occur at a suitable P concentration level. In other words, the presence of F has no significant influence on the mechanisms of P sorption, and therefore exerts no marked effect on the stability of co-sorbing P. Contrasting to P desorption, the co-sorption of P and F greatly enhanced F desorption, for example, 48% of F desorption after 4-h extraction compared to only 5% in F single-ion system. (Al,Fe)–F–P precipitant phase was believed not to be the source of F desorption. Were it not the case, P release would have concomitantly increased, but this phenomenon was not observed (Fig. 8). Therefore, the increase in F desorption should be mainly from exchangeable F.
As addressed in the previous section, although (Al,Fe)–F–P precipitation could be attributed to the main reason of increased F retention, surface adsorption also partly contributed to F sorption. The blocking of micro-pores by preferentially adsorbing P and (Al,Fe)–F–P precipitant would hinder F diffusion into the inner micro-pores. Therefore, the inner binding sites would be inaccessible to F, rendering F adsorption mainly on the surficial binding sites (including newly formed sites on (Al,Fe)–F–P precipitant). Furthermore, some active binding sites were occupied preferentially by P via inner-sphere surface complexation, leaving less active binding sites for F adsorption probably mainly via outer-sphere surface complexation. According to the surface complexation model (SCM) (Stumm and Morgan 1996), ions in outer-sphere complexes are much more easily released than those in inner-sphere complexes. These two aspects could be attributed to the improvement of F desorption.
To sum up, (Al,Fe)–F–P precipitation mainly contributed to the overall increase in F retention; the blocking of adsorbed P and (Al,Fe)–F–P precipitation rendered more F adsorption on surficial binding sites, which could be attributed to an increase in F desorption in F–P coexisting system. It should be pointed out that, due to the formation of (Al,Fe)–F–P precipitation, most F immobilized on the soil still remained stable enough, not to be released (>50%) by ion exchange.
4 Concluding Remarks
In F–P coexisting system, the amounts of F and P sorbed on the red soil increased with decreasing initial pH due to increasing surface protonation and (Al,Fe)–F–P precipitation. As a competing ion, the presence of F with increasing concentration could progressively suppress P sorption. Contrarily, the presence of P can markedly enhance F sorption, which can be attributed to the formation of surface (Al,Fe)–F–P precipitation. Isotherms for F and P sorption in F–P coexisting system can be satisfactorily fitted to both the Langmuir and Freundlich equations. As suggested by the values of 1/n obtained from the Freundlich equation, the sorption of both F and P on the red soil is favorable, which is probably the corollary of high contents of Fe and Al (hydro)oxides in the soil. The values of 1/n also suggest that the red soil has stronger affinity for P than for F. Different orders of F and P addition in the pre-sorption almost had no effect on P sorption, but had substantial impact on F sorption: the amount of F sorption is the highest in the case of simultaneous application of F and P due probably to large quantity of (Al,Fe)–F–P ternary complexes formation, followed by that in the case of F application first; F sorption is the lowest in the case of P application first. The co-sorption of F and P exerts no appreciable effect on desorption of P but can significantly enhance F desorption, which is environmentally important for assessing bioavailability and transport of F.
References
Arnesen, A. K. M., Abrahamsen, G., Sandvika, G., & Krogstad, T. (1995). Aluminum-smelter and fluoride pollution of soil and soil solution in Norway. The Science of the Total Environment, 163, 39–53.
Arnesen, A. K. M., & Krogstad, T. (1998). Sorption and desorption of fluoride in soil polluted from the aluminum smelter at Ardal in western Norway. Water, Air, and Soil Pollution, 103, 357–373.
Cronin, S. J., Neall, V. E., Leconintre, J. A., Hedley, M. J., & Loganathan, P. (2003). Environmental hazards of fluoride in volcanic ash: A case study from Ruapehu volcanic, New Zealand. Journal of Volcanology and Geothermal Research, 121, 271–291.
Gräfe, M., Nachtegaal, M., & Sparks, D. L. (2004). Formation of metal-arsenate precipitates at the goethite-water interface. Environmental Science and Technology, 38, 6561–6570.
Harrington, L. F., Cooper, E. N., & Vasudevan, D. (2003). Fluoride sorption and associated aluminum release in variable charge soil. Journal of Colloid and Interface Science, 267, 302–313.
Heron, G., Cromzel, C., Bourg, A. C. M., & Christensen, T. H. (1994). Speciation of Fe(II) and Fe(III) in contaminated aquifer sediments using chemical extraction techniques. Environmental Science and Technology, 28, 1698–1705.
Jacks, G., Bhattacharya, P., Chaudhary, V., & Singh, K. P. (2005). Controls on the genesis of some high-fluoride groundwaters in India. Applied Geochemistry, 20, 221–228.
Ji, G.-L. (1986). Competitive adsorption of phosphate and fluoride on iron oxide. Acta Pedologica Sinica, 23, 220–227 (in Chinese with English abstract).
Jia, Y., Xu, L., Fang, Z., & Demopoulos, G. P. (2006). Observation of surface precipitation of arsenate on ferrihydrite. Environmental Science and Technology, 40, 3248–3253.
Kau, P. M. H., Smith, D. W., & Binning, P. (1997). Fluoride retention by kaolin clay. Journal of Contaminant Hydrology, 28, 267–288.
Kau, P. M. H., Smith, D. W., & Binning, P. (1998). Experimental sorption of fluoride by kaolinite and bentonite. Geoderma, 84, 89–108.
Khare, N., Hesterberg, D., Beauchemin, S., & Wang, S.-L. (2004). XANES determination of adsorbed phosphate distribution between ferrihydrite and boehmite in mixtures. Soil Science Society of American Journal, 68, 460–469.
Khare, N., Hesterberg, D., & Martin, J. D. (2005). XANES investigation of phosphate sorption in single and binary systems of iron and aluminum oxide minerals. Environmental Science and Technology, 39, 2152–2160.
Kraemer, S. M., Chiu, V. Q., & Hering, J. G. (1998). Influence of pH and competitive adsorption on the kinetics of ligand-promoted dissolution of aluminum oxide. Environmental Science and Technology, 32, 2876–2882.
Larsen, S., & Widdowson, A. E. (1971). Soil fluoride. Journal of Soil Science, 22, 210–221.
Ler, A., & Stanforth, R. (2003). Evidence for surface precipitation of phosphate on goethite. Environmental Science and Technology, 37, 2694–2700.
Li, L., & Stanforth, R. (2000). Distinguishing adsorption and surface precipitation of phosphate on goethite (α-FeOOH). Journal of Colloid and Interface Science, 230, 12–21.
Loganathan, P., Hedley, M. J., Wallace, G. C., & Roberts, A. H. C. (2001). Fluoride accumulation in pasture forages and soils following long-term applications of phosphorus fertilizers. Environmental Pollution, 115, 275–282.
Lookman, R., Gorbet, P., Merckx, R., & Van Riemsdijk, W. H. (1997). Application of 31P and 27Al MAS NMR for phosphate speciation studies in soil and aluminum hydroxide: Promises and constraints. Geoderma, 80, 369–388.
Maliyekkal, S. M., Sharma, A. K., & Philip, L. (2006). Manganese-oxide-coated alumina: A promising sorbent for defluoridation of water. Water Research, 40, 3497–3506.
Moore, C. S., & Ritche, G. S. P. (1998). Aluminum speciation and pH of an acid soil in the presence of fluoride. Journal of Soil Science, 39, 1–8.
Nordin, J. P., Sullivan, D. J., Phililips, B. L., & Casey, W. H. (1999). Mechanisms for fluoride-promotion dissolution of bayerite [β-Al(OH)3(s)] and boehmite[γ-AlOOH]: 19F-NMR spectroscopy and aqueous surface chemistry. Geochimica et Cosmochimica Acta, 63, 3513–3524.
Peretyazhko, T., & Sposito, G. (2005). Iron(III) reduction and phosphorous solubilization in humid tropical forest soils. Geochimica et Cosmochimica Acta, 69, 3643–3652.
Polomski, J., Flühler, H., & Blaster, P. (1982). Fluoride-induced mobilization and leaching of organic matter, iron, and aluminum. Journal of Environmental Quality, 11, 452–456.
Reardon, E. J., & Wang, Y. (2000). A limestone reactor for fluoride removal from wastewaters. Environmental Science and Technology, 34, 3247–3253.
Reiner, G. R., Andersson, T., Lövgren, L., & Persson, P. (2005). Phosphate sorption in aluminum- and iron-rich humus soils. Soil Science Society of American Journal, 69, 77–86.
Saha, U. K., Taniguchi, S., & Sakurai, K. (2001). Adsorption behavior of cadmium, zinc, and lead on hydroxyaluminum- and hydroxyaluminosilicate-montmorillonite complexes. Soil Science Society of American Journal, 65, 694–703.
Shin, E. W., & Han, J. S. (2004). Phosphate adsorption on aluminum-impregnated mesoporous silicates: surface structure and behavior of adsorbents. Environmental Science and Technology, 38, 912–917.
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry: Chemical equilibria and rates in natural waters (3rd ed.). New York: Wiley.
Totsche, K. U., Wilcke, W., Korbus, M., Kobza, J., & Zech, W. (2000). Evaluation of fluoride-induced metal mobilization in soil columns. Journal of Environmental Quality, 29, 454–459.
Tsai, W. T., Chang, C. Y., Ing, C. H., & Chang, C. F. (2004). Adsorption of acid dyes from aqueous solution on activated bleaching earth. Journal of Colloid and Interface Science, 275, 72–78.
Valdivieso, A. L., Bahena, J. L. R., Song, S., & Urbina, R. H. (2006). Temperature effect on the zeta potential and fluoride adsorption at the α-Al2O3/aqueous solution interface. Journal of Colloid and Interface Science, 298, 1–5.
Violante, A., Ricciardella, M., Gaudio, S. D., & Pigna, M. (2006). Coprecipitation of arsenate with metal oxides: Nature, mineralogy, and reactivity of aluminum precipitates. Environmental Science and Technology, 40, 4961–4967.
Wang, K., & Xing, B. (2002). Adsorption and desorption of cadmium by goethite pretreated with phosphate. Chemosphere, 48, 665–670.
Wang, K., & Xing, B. (2004). Mutual effects of cadmium and phosphate on their adsorption and desorption by goethite. Environmental Pollution, 127, 13–20.
Wilcke, W., Totsche, K. U., Körber, M., Kobza, J., & Zech, W. (2000). Fluoro-mobilization of metals in a Slovak forest soil affected by the emissions of an aluminum smelter. Journal of Plant Nutrient and Soil Science, 163, 503–508.
Yu, T.-R., & Wang, Z.-Q. (1988). Soil Analytical Chemistry (in Chinese). Beijing: Science Press.
Zhang, B., Hong, M., Zhao, Y., Lin, X., Zhang, X., & Dong, J. (2003). Distribution and risk assessment of fluoride in drinking water in the west plain region of Jilin Province, China. Environmental Geochemistry and Health, 25, 421–431.
Zhao, H., & Stanforth, R. (2001). Competitive adsorption of phosphate and arsenate on goethite. Environmental Science and Technology, 35, 4753–4757.
Zhou, Q., & Sun, T. (2002). Effects of chromium(VI) on extractability and plant uptake of fluorine in agricultural soils of Zhejiang Province, China. Water, Air, and Soil Pollution, 133, 145–160.
Zhu, M.-X., Jiang, X., & Ji, G.-L. (2004a). Interactions between variable charge soils and acidic solutions containing fluoride: An investigation by using repetitive extractions. Journal of Colloid and Interface Science, 276, 159–166.
Zhu, M., Jiang, X., & Ji, G. (2004b). Experimental investigation on aluminum release from haplic acrisols in southeastern China. Applied Geochemistry, 19, 981–990.
Zhu, M.-X., Xie, M., & Jiang, X. (2006). Interaction of F with hydroxyAl-montmorillonite complexes and implications for F-contaminated acidic soils. Applied Geochemistry, 21, 675–683.
Acknowledgements
This work was jointly supported by the State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences (No. 055111), and the National Natural Science Foundation of China (No. 49904005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, MX., Ding, KY., Jiang, X. et al. Investigation on Co-sorption and Desorption of Fluoride and Phosphate in a Red Soil of China. Water Air Soil Pollut 183, 455–465 (2007). https://doi.org/10.1007/s11270-007-9394-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9394-0