Abstract
A bioretention media is a stormwater treatment option designed to reduce peak runoff volumes and improve water quality through soil infiltration and plant mitigation. To investigate the heavy metal removal in a bioretention media in a cold climate setting, a small pilot sized bioretention box was built in Trondheim, Norway. The system was sized using the Prince Georges County bioretention design method from 1993. Three runoff events, created using historical data, were undertaken in April 2005 and then again in August 2005. Both the peak flow reduction and the total volume reduction were significantly lower in April compared to August. Peak flow reduction was 13% in April versus 26% in August and the total volume reduction was 13% in April versus 25% in August. Metal retention was good for both seasons with 90% mass reduction of zinc, 82% mass reduction of lead and 72% mass reduction of copper. Plant uptake of metals was documented between 2 to 7%; however adsorption and mechanical filtration through the mulch and soil column were the most dominant metal retention processes. The metal retention was independent of the selected hydraulic loading rates (equivalent to 1.4–7.5 mm h−1 precipitation) showing that variable inflow rates during this set of events did not affect the treatment efficiency of the system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A wide range of inorganic compounds have been identified in stormwater. Heavy metals (copper (Cu), zinc (Zn), cadmium (Cd), and lead (Pb)) together with polycyclic aromatic hydrocarbons (PAHs), sediments and de-icing salts are the most common pollutants in road runoff (Makepeace et al. 1995). Vehicle brake emissions and tire wear have been identified as the most important sources of copper and zinc respectively. Building sidings were important sources of copper, zinc, lead, and cadmium, while atmospheric deposition was a source of copper, cadmium, and lead (Davis et al. 2001a). Highways have been found to contribute more than 75% of total metal loadings, even though they often occupy a relatively small percentage of the total area (Ellis et al. 1987). Toxicity screenings have also found as much as 20% of samples from urban highways to be severely toxic, compared to only 1% of urban stormwater samples as a whole (Marsalek et al. 1999). Hence controlling and treating highway runoff can involve treating a relatively small portion of the flows for a large environmental benefit.
Bioretention areas, also called rain gardens, have become a frequently used best management practice (BMP) to retain pollutants from road runoff. Bioretention areas consist of a sandy loam soil medium, a mulch layer, and plants designed for retention and treatment of stormwater through infiltration, filtration, adsorption, ion-exchange, volatilization, decomposition, and plant uptake (Clar et al. 2004; USEPA 2000). A long-term continuous field scale rain garden project in Connecticut, collecting roof runoff in a residential area, monitored the performance of two rain gardens. Metals and nutrients were sampled, but only nutrients were analyzed, as most of the metal samples were below detection limits (Dietz and Clausen 2005). The rain gardens were found to be performing well with respect to flow reduction, with a 98% infiltration of the inflow and a geometric average weekly inflow divided by weekly outflow of 0.95 ± 0.35 and 0.99 ± 0.53 weeks for the two gardens (Dietz 2005). Laboratory and event based field testing of bioretention performance with respect to metal retention have shown an above 90% concentration reduction of copper, zinc, and lead. However plant uptake accounted for only approximately 5% removal by mass. In comparison the mulch layer accounted for 20% of the copper, 10% of the lead, and 34% of the zinc (Davis et al. 2001b). Field test of existing established bioretention areas had a wider performance range with respect to metal removal. The field test reported from 42–70% metal removal for a less developed bioretention facility to over 90% in well developed bioretention facility with good vegetation (Davis et al. 2003).
Metal retention in the soil media can be related to cation-exchange (non-specific adsorption), co-precipitation, and organic complexation (Alloway 1995). In addition, plant uptake of metals also contributes to the retention of dissolved metals, however this is a slower process compare soil adsorption. Plants however, are important in the interaction between the plant roots, the rhizosphere, and the surrounding soil interaction by improving infiltration, soil texture, and preventing clogging (Gregory 2006). Metal retention is determined by the size fraction of the particles to which the metals are adsorbed. Depending on particle size, metals are retained by trapping the suspended sediment, soil sorption or by plant uptake and biological activity in the soil medium. Copper and zinc in stormwater from highway and residential areas have been found to be associated with particles > 5 μm or dissolved (defined by passing through a 10 kilo Daltons (kDa) ultrafilter), while lead has been found to be associated only with particles > 5 μm (Tuccillo 2006). Uptake of metals in plants commonly used for stormwater installations have also been demonstrated, but with higher uptake in aquatic plants where both roots and shoots are in direct contact with the polluted water (Fritioff and Greger 2003). Aquatic plants (Elodea canadensis and Potamogeton natans L.) uptake of metals (Cu, Zn, Cd, and Pb) at different temperature and salinity concentrations have shown that metal uptake in the plant tissue increased with increasing temperature and decreasing salinity. This can be a concern for cold climate bioretention facilities during snowmelt, which creates runoff with low temperatures and high salinity concentrations. The concentrations of metals were also higher in plants with lower total biomass (Fritioff et al. 2004), but the effect of temperature on terrestrial plant uptake of metals has not been documented. The zinc, lead and total petroleum hydrocarbons uptake were compared for five emergent species; Iris, T. latifolia, Sparganium, S. acutus, and Eleocharis, in a constructed wetland. Typha latifolia and Sparganium were found to be the most suited for metal uptake and storage. It was also found that Iris, a very common plant in stormwater treatment systems, was the least favorable of the five species (Ellis et al. 1994).
The objectives of this study were to compare the metal retention in a bioretention media for different hydraulic loading rates and during two seasons in 2005; late winter/early spring (frozen soil, dormant vegetation and low biological activity) and full summer (maximum vegetation cover and high biological activity). The experiments sought to investigate to what extent the cold climate and dormant above ground biomass affected the system performance both with respect to hydraulic loading rate and metal retention. A qualitative comparison of the results from the two seasons was performed.
2 Experimental Section
2.1 Design and Construction
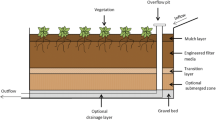
A bioretention box was constructed in the summer of 2004 using design specifications from the first Prince George’s County bioretention design manual (Prince George’s County 1993). The box was designed to receive runoff from a 20 m2 impervious surface area utilizing 5% of the drainage area for the bioretention box. This resulted in a bioretention box with a surface area of 0.95 m2. The system consists of a water-tight plastic box (width: 88.2 cm, length 109.1 cm, and height: 90 cm). The box was filled with a sandy soil (55 cm), between a layer of gravel at the bottom (10 cm) and mulch on the top (10 cm), leaving 15 cm freeboard for standing water. The total soil volume in the box was approximately 0.57 m3. The bioretention box was suspended with four cables attached to the wooden frame. Strain gages (S-shaped) were connected on each cable to weigh the box. At the bottom a 25 mm PVC tube drained the outflow from the box into a storage tank in the bottom of the measuring station (Fig. 1). Outflow rates were measured with a pressure transducer (0–160 mbar), and water was pumped into the box with a hose pump (Bredel SPX 15) controlled by a frequency inverter.
2.2 Soil and Vegetation
The vegetation in the bioretention box was comprised of hardy cold resistant plants with minimal maintenance requirements. Native species were used in the project. Based on these criteria Lythrum salicaria., Iris pseuacorus, Vinca minor and Hippophaë rahmnoides were chosen to give a good mixture of small shrubs, evergreens and perennials. Topsoil with a high organic matter (OM) content (8.7%) mixed with sand was used as retention medium, creating a sandy soil with the following composition; 2.6% clay, 92.7% sand, and 4.7% slit. The high sand content was chosen to ensure adequate infiltration during the winter months to avoid standing water in the media which would freeze to a concrete frost in the soil (Stoecker and Weitzman 1960). Stähli et al. (1999) showed that the rate at which infiltrated water refreezes in a sandy soil can greatly affect the infiltration rate, especially in the upper 50 cm of the soil, making adequate drainage very important for a cold climate bioretention area. The soil cation exchange capacity (CEC) was 45 (cmolc kg−1). The soil pH was 6.88, and the mulch pH was 5.5, all measured at the start of the study period.
2.3 Hydraulic Loading Rate
The hydraulic loading rates were chosen based on historic precipitation records from the Risvollan Urban Hydrologic Research station (RUHR), where the bioretention box was located. Average annual precipitation in the watershed is 900 mm year−1, out of which 30–40% is snow. Average 24 h temperature in April is 4°C, and 13.2°C in August (Thorolfsson et al. 2003). The precipitation events chosen for the study were based on historical data recorded from September 2004. Historic precipitation records were used to simulate a real runoff situation as closely as possible. Different duration and intensities were chosen to investigate the influence of hydraulic loading rate on the performance of the system. The first event, E1, had a duration of 4.2 h with 151 mm total runoff (max rainfall intensity 2.2 mm h−1 and average 1.3 mm h−1). The second event, E2, lasted 4.6 h with 179 mm runoff (max intensity 7.4 mm h−1 and average 3.5 mm h−1), while the last event, E3, lasted 6.6 h with 251 mm runoff (max intensity 2 mm h−1and average 1.4 mm h−1). The three events were first used for the set of runs on April 12th, 13th and 14th, 2005. And then again for the second set on August 30th and 31st and September 1st, 2005.
2.4 Pollutant Loadings
Various sources of stormwater for the inflow were considered. For the April events, the final choice fell on tunnel wash water from the Hell Tunnel, a 4 km long highway tunnel. The tunnel is cleaned twice a year, and the spring cleaning coincided with the time of the planned study and had the advantage of being representative road runoff. Based on typical pollutant concentrations in runoff from Scandinavian (Petterson et al. 1999; Roseth et al. 2003; Westerlund 2005) and European roads (Boller 1997; Crabtree et al. 2005), and typical concentration levels in the tunnel wash water (Roseth et al. 2003), a wash water dilution of 1:6 (mixture A, Table 1) was used for the first event (April, E1). This resulted in metal concentrations that were generally lower than the road runoff values found in the literature. This was probably due to a change in policy which increased water volume during washing, diluting the concentrations. In order to have inflow concentrations in the same range as found in the literature the two following events in April (E2 and E3) had a dilution of 1: 2 (mixture B, Table 1). In all three events the tunnel wash water was diluted with water from the stormwater channel at the RUHR station. During dry periods this channel carries a base flow of approximately 5 l s−1 originating from shallow groundwater flow and possible leakage from the water distribution network, typically with very low turbidity, less than 5 mg l−1 TSS and pH between 7 and 8.
The ratio between mixture A and B was a 1:3 in dilution, but the same 1:3 relation between the metal concentrations was not seen. This could be due to heterogeneous metal concentration in the tunnel wash water that was stored in a 1,000 l tank, but more likely the difference was caused by the water from the stormwater channel used for dilution, as this water was not analyzed separately for TSS and metal concentrations prior to mixing. For the August events no tunnel wash water was available, so stormwater from the stormwater channel at RUHR was mixed with concentrated metal solutions, and sediments from the bottom of the stormwater channel. Sediments from the channel were used to get a representative particle size distribution and composition (Mixture C, Table 1). The metal concentrates were mixed to achieve the same concentrations as in mixture B from the April events. However the resulting concentrations in mixture C were much higher, especially for total copper and lead, while the dissolved fractions were similar for the A and B mixtures. The higher total copper and lead concentrations were due to the metal concentrations in the sediment added from the RUHR stormwater channel. The concentrations of metals in the sediment were not measured prior to making mixture C.
2.5 Sampling
Samples were taken from the inflow as it entered the bioretention box and from the composite outflow tank, where the outflow from the bioretention box was collected. In addition spot samples were taken from the outflow before it entered the collection tank during peak outflow. For the April events 4, 5, and 7 samples were taken for events E1, E2, and E3 respectively. For the August events 5, 5, and 6 samples were taken for the events E1, E2, and E3 respectively. The samples were stored in 1 l bottles, and kept in a cooler until the end of the event. Prior to sampling the bottles were acid washed and rinsed at least seven times in distilled water. All samples were stored at 4°C until they were analyzed. Total suspended solids (TSS) was analyzed after each event, while metal samples were prepared and kept in sterilized 50 ml centrifugal tubes and analyzed after each season, stored for less than 7 days.
2.6 Analysis Methods
Total suspended solids were measured using Whatman GF/C 1.2 μm pore size glass microfiber filters in three replicates (Norwegian Standard (Standard Norge) 1983). The pH was measured as the samples were collected in the field, with a field pH meter (HI 991300, Hanna Instruments). The metal samples were analyzed using a High Resolution Inductively Coupled Plasma-Mass Spectrometer (HR ICP-MS). The dissolved samples were filtered using a cellulose-nitrate 0.45 μm pore size filter. The total metal samples were digested in a microwave oven with 10% HNO3, and then diluted 16 times (0.1M HNO3) prior to analysis. The HR ICP-MS detection limits for copper, zinc, and lead are 0.125, 0.2, and 0.01 μg l−1 respectively, with a relative standard deviation (rsd) less than 10%. Soil samples were dried and sieved to remove particles larger than 2 mm, and then the sample was boiled with aqua regia. Plant samples were dried and crushed, then nitric acid and hydrogen peroxide were added and digested in a microwave. Both plant and soil samples were then analyzed using an Inductively Coupled Plasma-Mass Spectrometer (ICP-AES). The CEC was determined by shaking the soil sample with 1 molar ammonium acetate and then extracted and analyzed using an ICP-AES.
A standard t-test assuming equal variances was used to determine if there was a significant difference between the April and August results. Minitab 14 statistical software was used to perform the analysis (Minitab Inc. 2004). Percentage reduction in inflow and outflow concentrations was calculated as an indication of performance. However, water retention in the system will affect the concentration, possible skewing this measurement. Therefore a better indication of performance was found by comparing the pollutant pathways through the bioretention media for the two seasons, and looking at mass of metals in the outflow.
3 Results and Discussion
3.1 Flow and Hydraulic Loading
The lag time between start of inflow to the bioretention box and the first outflow seen at the outlet was approximately 50 min, except for the first event (E1) in April, which had a lag time of 25 min (Fig. 2). The shorter lag time for, E1 in April compared to the other events can be explained by the presence of frozen soil, −.1°C measured 20 cm below the surface, prior to the start of inflow, possibly causing preferential flow through the system. The inflow stormwater had a temperature close to the air temperature, 12°C, which heated the soil column. Between events the soil column was cooled by the surrounding soil masses, causing large fluctuations in soil temperature over the three days of the events in April.
The average peak flow reduction over the three events was 13% in April versus 26% in August. The total volume reductions were also higher in August, 25% than April, 13%. The antecedent moisture conditions in the bioretention box prior to the first events in April and August were not measured, but the initial weight measured by the strain gages was 1,086 kg in April and 1,095 kg in August. The above ground biomass in August would add a few kilograms compared to April, but the antecedent moisture condition was most likely slightly wetter prior to the first event in August compared to April. However, after the first event the box would be saturated and then left to drain for 24 h before the next event the following day, producing similar starting conditions for the second and third events, E2 and E3 for both April and August. The higher peak flow reduction in August can be attributed to a more homogenous distributed soil column with less channelization, often formed in partially frozen soils, which would increase the soil infiltration time in August, The increased volume reduction in August is most likely due to increase in the plant water consumption and evaporation rates. The reduced peak flow and volume reduction in the early spring events indicate a reduced hydraulic function during the cold months. This can be compensated with a lower hydraulic loading rate by increasing the bioretention to drainage area ratio.
3.2 Metal Concentrations
The soil medium is assumed to be the main medium for attenuation of metals in a bioretention box (USEPA 2000). Laboratory adsorption tests of metals onto sandy loam soils have shown that copper and lead have highest adsorption rates for pH 5–8, while zinc has best adsorption from pH 6–8 (Davis et al. 2001b). This indicates that a pH around 6 should be ideal for the metals of concern. In April the inflow pH varied from 6.8 to 7.8 and the outflow from 7.1–7.3. In August the inflow pH was more stable in the range 8.1–8.2, and outflow pH again 7.1–7.3.
The inflow concentrations between the events varied a great deal for the six events, but the same variation was not seen in the outflow from the bioretention box (Fig. 3). Zinc inflow concentrations were between 550–660 μg l−1 for all the events except the first event in April, with a concentration of 120 μg l−1. The zinc outflow concentrations did not show any significant difference between the outflow concentration for the mixture A and B events in April (Fig. 3). However, comparing the average outflow concentrations showed a significantly higher outflow concentration in August than April, while the percentage reduction was relatively constant between 92 to 95% (Table 2). The copper reduction in the system was significantly lower in April versus August, with 40 and 67% decrease in outflow concentration respectively (Table 2). This could be due to the five times higher inflow concentration in August compared to April, or possible change in plant uptake or organic matter complexation of copper in the soil and mulch as a function of soil and air temperature. The inflow and outflow concentrations of lead followed the same pattern as for zinc, with a fairly constant percentage reduction, but significantly higher outflow concentrations in August, possibly due to change in plant uptake or soil retention processes, however it cannot be ruled out that the higher inflow concentration in August also influenced the outflow concentration.
The changing inflow rates following the inflow hydrograph appeared to have no effect on the outflow concentrations. The August outflow copper concentrations all had an initial spike in concentration (Fig. 3), but this was not found to correspond with a peak in outflow rates (Fig. 2). This agrees with the findings by Davis et al. (2001b) investigating hydraulic loading rates using outflow ports at different depths through a bioretention box (total media depth 61 cm). It was concluded that the deeper outflow was independent of hydraulic loading rates (ranging from 2 to 8 cm h−1), while some dependency was seen in the upper outflow ports. In this study a real historical hydrograph was chosen to reproduce actual rainfall events as close as possible. The results demonstrate that the bioretention box can retain constant high levels of metals independent of rainfall intensities which indicate that it will perform over a wide range of natural precipitation events.
The overall concentration reductions of lead and copper levels were slightly lower, while zinc reductions were comparable to than that previously reported by Davis et al. (2001b). Compared to a previously reported field study involving two different sites (Davis et al. 2003) the retention of zinc was comparable to the best performing site, while lead and copper removal rates were in-between the reported values.
To investigate if there was a change in metal solubility through the system, dissolved metal fractions were measured for all the samples. For the inflow mixed from the tunnel wash water used in the April events, the dissolved metal fractions in the inflow were generally lower than the August inflow which was mixed from stormwater and added metals (Fig. 4). The average dissolved zinc retention in the system was 70% in concentration and 79% in mass. The retention varied little for all the events. The dissolved lead fraction appeared to increase through the column in April (−88%) but decreases through the column in August (19%), however the dissolved fraction was small compared to the total lead for all the events. The dissolved lead inflow and outflow values for April were 0.05 and 0.026 μg l−1 (±0.1 μg l−1) respectively. The values from August range from 1.4 to 1.1 μg l−1 (±0.3 μg l−1). The dissolved copper concentrations increased for all the events both in April and August, with less than 10 μg l−1 dissolved copper in the inflow for all the events and as high as 44 μg l−1 in the outflow. While the April events showed only a minor increase from inflow to outflow that August events had a large increase.
These results could suggest leaching from the soil, in order to investigate this further a laboratory scale soil extraction test was conducted using 600 ml soil in a glass jar with a tap at the bottom and a small pump adding deionized water to the column at 120 ml h−1 for 6 h. The soil medium was divided into three layers; top (0–20 cm), middle (20–40 cm) and bottom (40–60 cm) and each layer was tested separately, measuring only what was leached for that layer. The pH of the deionized waster ranged from 5.7 to 6.2, while the outflow from the soil layers had a higher pH, ranging from 7.7 to 8.0. The extraction showed some leaching of all the metals. There was evidence of leached lead in the top soil layer and low leaching values from the two lower layers. The movement of leached lead downward in the soil column could mean higher leaching of lead as the concentration of lead in the bottom layer increases. The leached copper (avg. 19.9 μg l−1 over all three layers) was the same concentration as observed in the August outflow (avg. 19.49 μg l−1). No leaching of zinc was observed in the bioretention box, however in the laboratory extraction some leaching (avg. 22.99 μg l−1 over all three layers) occurred, and some leaching of lead (avg. 3,4 μg l−1 over all three layers) was measured.
A study investigating the extractability and mobility of zinc and copper in sandy soils reported a sharp change in mobility above 100 mg kg−1 concentrations for copper and 60 mg kg−1 for zinc. The initial soil metal concentrations were more important than pH alone. With a high soil concentration, the leaching became sensitive to changes in pH, and increased exponentially for pH values less than 7 (Zhang et al. 2006). The pH in the outflow from the bioretention box was stable at 7.1–7.3 for all events. The soil metal concentrations in the bioretention box were also well below 100 mg kg−1. A study investigating the solubility of metals in contaminated soils found that the soluble zinc and lead contents were more correlated with pH and total metal content in the soil than soluble copper, and did not tend to complex strongly with soluble OM like copper. Furthermore a stronger correlation was found between free metal copper Cu2+ and OM than with total soluble copper and OM, this suggests that it is the free metal activity that is directly controlled by sorption (McBride et al. 1997). The soluble copper is influenced by soluble OM, inorganic ligand concentrations and reactivity and dispersed colloidal particles too small to be separated by filtration. This makes it complex to identify the individual factors responsible for the total soluble copper content. However this suggests that the OM and especially the dissolved OM in the soil media is responsible for the copper leaching and is more important than in which form the copper enters the soil media (particulate or dissolved) (McBride et al. 1997). Another study investigating the effect of dissolved organic carbon on leaching of Zn and Cu from swine manure compost (Hsu and Lo 2001), found that the leachability of the metals were independent of the total metal content, but rather a function of dissolution of organic carbon as a result of pH changes. An increase in dissolved organic carbon substantially modified the copper solubility, while having only negligible effect on the dissolved zinc content. The dissolution of OM and subsequent formation of organometallic complexes indicated that copper is primarily bound to OM, while the zinc sorption to OM is low. The organic matter content in the bioretention box was 8.7% and in addition the top mulch layer consisted of shredded bark decaying over time. Based on the literature findings the mulch and also the soil OM is most likely responsible for the copper leaching from the media. A thorough evaluation of the sampling procedures, materials in the bioretention filter, and laboratory analysis methods were conducted and no indication of sample contamination or copper source in the materials could be identified. Controlling the pH in the soil and mulch can reduce this leaching. This can be done by adding lime, using a mulch sorption layer with a naturally higher pH to minimize the dissolution of OM.
3.3 Metal Mass Balance
To investigate the flow, sinks, and transfer of metals in the system, a mass balance based on the measured concentration and volume characteristics was performed. The total inflow of zinc into the bioretention box over all the events was 597 mg, and only 63 mg left the bioretention box in the outflow. This is a 90% mass retention of zinc in the system. The retention for lead was 82 and 72% for copper. Comparing the concentrations, the dissolved copper leaving the bioretention box had increased, however looking at the mass balance the April events show a slight increase of 1 mg, and a doubling from 6 to 12 mg in August. The increase in dissolved copper concentration for the August events was almost 200% (from 6.7 to 18.4 μg l−1) raise while the actual mass increase was a 100% from 6 to 12 mg (Table 3). The reduction in water volume leaving the bioretention box increased the actual concentration of dissolved copper, which was most likely just transported through the system, and some copper was added to the soluble phase as well. The mass balance comparison between April and August events indicates no difference in mass removal of zinc and lead between the seasons, while copper had a significantly higher mass removal in August (75%) versus April (69%) (Table 3).
Composite soil and plant tissue samples were collected before the runoff experiments (natural) and after both sets of runs in August. An intermediate mulch and top soil sample was also taken after the April events. A clear accumulation of metals in the top soil and mulch layers were seen with a higher accumulation in the mulch (Table 4). This corresponds to results from previously reported laboratory studies by (Davis et al. 2001b), where the mulch was found to have absorbed most of the retained metals. The plant tissue samples showed relative high accumulation of zinc and copper in the Vinca minor, while the Hippophaë rhamnoides showed an increase in the zinc and copper concentrations in the twigs and leaves, and a decrease in the roots, which could indicate a transport of zinc and copper from the roots into the twigs and leaves, but no further uptake of zinc and copper in the plant from the soil water interface. The lead concentrations showed some accumulation in the V. minor, while no detectable difference could be found in the H. rhamnoides.
A study from 2003 investigating metal accumulation in submerged, free floating, emergent, and terrestrial plants found that zinc and cadmium had a concentration factor (concentration in plants related to concentration in surrounding soil) above 1 in terrestrial and emergent plants (Fritioff and Greger 2003). The plants used in the study were different than the plants used in the bioretention box, however the V. minor plant tissue showed a 1.14 concentration factor for zinc compared to the surrounding top soil (Table 4). However the V. minor is relative sensitive to standing water and prone to drowning under prolonged wetting. A well drained bioretention media with minimum standing water would be required for this plant, however with suitable conditions it has shown promising metal accumulation results for use in cold climate bioretention.
To identify the major metal sinks in the system an estimation of plant biomass was performed. This was done for the combined plant biomass in the bioretention box on a dry weight basis. The combined plant metal uptake was estimated to be 3% copper and 7% zinc, and 2% lead. This is in the same range as was reported by (Davis et al. 2001b), though with different plant species. The mulch retained 50% of the copper, 20% of the zinc, and 17% of the lead and the remaining 47% of copper, 73% of zinc and 81% of lead was retained in the soil column. The mechanisms responsible for the removal in the mulch and soil can be expected to be a combination of mechanical filtration and adsorption processes. Metal particles in road runoff have been found associated with the smallest particles sizes in urban runoff. Westerlund and Viklander (2006) found the strongest correlation between particles 4–6 μm and Cu, Zn, and Pb. Urban highway runoff was found to have a particle size distribution from 0 to 9,500 μm but the majority of the Zn, Cu, and Pb concentrations were found bound to particles less than 100 μm (Sansalone and Buchberger 1997). Based on these findings some of the removed metals can be assumed to be too small to settle out by mechanical filtration, leaving specific and non specific sorption as the most dominant processes. The mulch layer was 5 cm thick compared to the 55 cm thick soil column, making the mulch layer the most efficient metal sink in the system; in addition the mulch serves as weed control and aesthetic value in combination with the plants. The mulch layer is also the easiest part of the system to regularly exchange, as it only covers the top few centimeters of the system. Despite the minor metal uptake the plants had other functions, making them an essential part of the system. The roots help improve root zone development in the soil which maintains infiltration. Furthermore, plants are a key part of nutrient removal, and they have a high aesthetical value for use in stormwater treatment systems in urban areas. In cold climates with snowmelt leaving the topsoil and mulch layer covered with a thick layer of gray sediments and pollutants they also serve as a regenerator of the bioretention areas in the spring, turning the gray layer of muddy sediments into a green vegetation plot. The metal accumulation in more local plant species should be investigated to find the most area specific suitable bioretention box plants.
Estimating the soil metal sorption capacity based on the average annual loadings suggest that soil metal accumulation will not be the limiting factor in the system. It will take 10–20 years before the soil would need to be exchanged, while the mulch would need more frequent replacement about every 4–5 years. The top mulch layer made of bark will gradually decompose and need to be refilled as a regular maintenance activity. Harvesting the plant biomass will prolong the lifespan and remove metals from the system. Several studies have identified vegetation that can hyperaccumulate metals (Hammer et al. 2003; Hasan et al. 2007; Yanqun et al. 2004). The use of hyperaccumulating plants to increase metal accumulation in plant biomass could make it a significant sink that can be easily harvested to regenerate the system.
4 Conclusions
The variable hydraulic loading rates from the three different inflow hydrographs did not have an effect on the metal retention in the bioretention box within the tested hydraulic loading rates. The peak flow and total volume reduction was halved in April compared to August due to partially frozen soil and less above ground biomass in April. The mass removal of zinc was constant at 90% for both seasons. Mass removal of lead was slightly higher in August compared to April (83 versus 89%). Copper was the only metal showing a significantly lower removal in April (60%) versus 75% in August. The mulch layer and the soil were identified as the major sinks for the removed metals, while the plants played a minor role for metal removal (2–7%), but an important role in root zone development and adding aesthetical value to the system with above ground biomass.
References
Alloway, B. J. (Ed.) (1995). Heavy metals in soils. London: Blackie.
Boller, M. (1997). Tracking heavy metals reveals sustainability deficits of urban drainage systems. Water Science and Technology, 35, 77–87.
Clar, M. L., Barfield, B. J., & O’Connor, T. P. (2004). Stormwater best management practice design guide, volume 1 vegetative biofilters. Washington, D.C.: United States Environmental Protection Agency.
Crabtree, B., Moy, F. & Whitehead, M. (2005). Pollutants in highway runoff. In 10th International Conference on Urban Drainage, Copenhagen.
Davis, A. P., Sholouhian, M., & Ni, S. (2001a). Loadings of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere, 44(5), 997–1009.
Davis, A. P., Shokouhian, M., Sharma, H., & Minami, C. (2001b). Laboratory study of biological retention for urban stormwater management. Water Environment Research, 73(1), 5–14.
Davis, A. P., Shokouhian, M., Sharma, H., Minami, C., & Winogradoff, D. (2003). Water quality improvement through bioretention: Lead, copper, and zinc removal. Water Environment Research, 75(1), 73–82.
Dietz, M. E. (2005) Rain garden design and function: A field monitoring and computer modeling approach. Dissertation, University of Connecticut.
Dietz, M. E., & Clausen, J. C. (2005). A field evaluation of rain garden flow and pollutant treatment. Water, Air, and Soil Pollution, 167, 123–138.
Ellis, J. B., Revitt, D. M., Harrop, D. O., & Beckwith, P. R. (1987). The contribution of highway surfaces to urban stormwater sediments and metal loadings. Science of the Total Environment, 59, 339–349.
Ellis, J. B., Revitt, D. M., Shutes, R. B. E., & Langley, J. M. (1994). The performance of vegetated biofilters for highway runoff control. Science of the Total Environment, 146/147, 543–550.
Fritioff, Å., & Greger, M. (2003). Aquatic and terrestrial plant species with potential to remove heavy metals from stormwater. International Journal of Phytoremediation, 5(3), 211–224.
Fritioff Å., Kautsky L., & Greger, M. (2004). Influence of temperature and salinity on heavy metal uptake by submersed plants. Environmental Pollution, 133, 265–274.
Gregory, P. J. (2006). Roots, rhizosphere and soil: The route to a better understanding of soil science? European Journal of Soil Science, 57(1), 2–12.
Hammer, D., Kayser, A., & Keller, C. (2003). Phytoextraction of Cd and Zn with Salix viminalis. Soil Use and Management, 19(3), 187–192.
Hasan, S. H., Talat, M., & Rai, R. (2007). Sorption of cadmium and zinc from aqueous solutions by water hyacinth (Eichchornia crassipes). Bioresources Technology, 98(4), 918–928.
Hsu, J.-H., & Lo, S.-L. (2001). Effect of composting on characterization and leaching of copper, manganese, and zinc from swine manure. Environmental Pollution, 114(1), 119–127.
Makepeace, D. K., Smith, D. W., & Stanley, S. J. (1995). Urban stormwater quality: Summary of contaminant data. Environmental Science & Technology, 25(2), 93–139.
Marsalek, J., Rochfort, Q., Brownlee, B., Mayer, T., & Servos, M. (1999). An exploratory study of urban runoff toxicity. Water Science and Technology, 39(12), 33–39.
McBride, M., Sauvé, S., & Hendershot, W. (1997). Solubility control of Cu, Zn, Cd and Pb in contaminated soils. European Journal of Soil Science, 48(2), 337–346.
Minitab Inc. (2004). Minitab Statistical Software version 14.0
Norwegian Standard (Standard Norge) (1983). Norwegian standard for suspended particulate matter (NS4733E).
Petterson, T. J. R., German, J., & Svensson, G. (1999). Pollutant removal efficiency in two stormwater ponds in Sweden. In 8th International Conference on Urban Storm Drainage, Sydney, Australia.
Prince George’s County (1993). Design manual for use of bioretention in stormwater management. Prince George’s County (MD) Government, Department of Environmental Protection. Watershed Protection Branch, Landover.
Roseth, R., Amundsen, C. E., Snilsberg, P., Langseter, A. M., & Hartnik, T. (2003). Wash water from road tunnels – Content of pollutants and treatment options. In Proceedings of 1st International Conference on Urban Drainage and Highway Runoff in Cold Climate, Riksgränsen, Sweden.
Sansalone, J. J., & Buchberger, S. G. (1997). Characterization of solid and metal element distributions in urban highway stormwater. Water Science and Technology, 36(8–9), 155–160.
Stähli, M., Jansson, P.-E., & Lundin, L.-C. (1999). Soil moisture redistribution and infiltration in frozen sandy soils. Water Resources Research, 35(1), 95–103.
Stoecker, J. H., & Weitzman, S. (1960). Infiltration rates in frozen soil in Northern Minnesota. Soil Science of America Journal, 24(2): 137–139.
Thorolfsson, S. T., Matheussen, B. V., Frisvold, H., Nilsen, O., Kristiansen, V., & Pettersen-Øverleir, A. (2003). Urban hydrological data collection in cold climate. In 1st International Conference on Urban Drainage and Highway Runoff in Cold Climate, Riksgränsen, Sweden.
Tuccillo, M. E. (2006). Size fractionation of metals in runoff from residential and highway storm sewers. Science of the Total Environment, 355(1–3), 288–300.
USEPA (2000). Low impact development (LID). A literature review. Washington, D.C.: Office of Water.
Westerlund, C. (2005). Seasonal variations of road runoff in cold climate. Licentiate thesis, Luleå University of Technology.
Westerlund, C., & Viklander, M. (2006). Particles and associated metals in road runoff during snowmelt and rainfall. Science of the Total Environment, 362, 143–156.
Yanqun, Z., Yuan, L., Schvartz, C., Langlade, L., & Fan, L. (2004). Accumulation of Pb, Cd, Cu and Zn in plants and hyperaccumulator choice in Lanping lead–zinc mine area, China. Environment International, 30(4), 567–576.
Zhang, M.-K., He, Z.-L., Calvert, D. V., & Stoffella, P. J. (2006). Extractability and mobility of copper and zinc accumulated in sandy soils. Pedosphere, 16(1), 19–43.
Acknowledgments
This study was supported by a PhD grant from the Norwegian University of Science and Technology NTNU, Trondheim, Norway.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthanna, T.M., Viklander, M., Gjesdahl, N. et al. Heavy Metal Removal in Cold Climate Bioretention. Water Air Soil Pollut 183, 391–402 (2007). https://doi.org/10.1007/s11270-007-9387-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9387-z