Abstract
The sorption and bioconcentration of Hg, Se, and As were measured in Schoenoplectus californicus and Typha angustifolia in a pilot constructed wetland receiving wastewater inflows containing these elements at potentially hazardous levels. Results indicated that these species bioconcentrated Hg, Se, and As at factors of up to 1,911, 10,981, and 4,927, respectively. Plant tissue concentrations decreased as Hg, Se, and As were translocated from the roots to the aerial portions of the plant. Greatest element concentrations in S. californicus were found in roots, indicating that an exclusion mechanism may be responsible for element tolerance by this plant species. Greater root:shoot transfer of Hg, Se, and As was observed with T. angustifolia than with S. californicus, suggesting that element tolerance was more likely due to an internal detoxification mechanism. To completely assess ecological risks associated with the use of constructed wetlands, contaminant bioavailability for plant uptake, translocation, and bioconcentration must be considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In an effort to decrease the amount of sulfur dioxide released into the air as regulated by the Clean Air Act of 1963 and the Clean Air Act Amendments of 1990, fossil-fueled power plants have installed flue gas desulfurization (FGD) systems, or scrubber systems. While FGD systems are effective in decreasing sulfur dioxide emissions, this process results in wastewater containing high concentrations of Hg, Se, and As (Electric Power Research Institute 1999). This wastewater must then be treated to eliminate these contaminants in order to achieve discharge limitations established under the National Pollution Discharge Elimination System (NPDES) and Clean Water Act (CWA). Constructed wetland treatment systems (CWTS) have considerable potential to remove Hg, Se, and As from FGD wastewater (Sundberg et al. 2006).
In constructed wetland treatment systems receiving wastewater inflows containing elements such as Hg, Se, and As, emergent vegetation may concentrate these elements. Macrophytes in constructed wetlands have been used in the remediation of a wide variety of contaminated wastewaters, including industrial and municipal wastewater, acid mine drainage, storm water runoff, and agricultural runoff (Moshiri 1993). Phytoremediation is a treatment technology that uses various plant species and their associated microbiological communities to remove contaminants from soil and water. Phytoextraction is the process of using a plant species to take up contaminants from the soil and water and concentrating the contaminants in the roots, shoots, and/or leaves of the plant (US Environmental Protection Agency 2000). Uptake and retention of elements such as Hg, Se, and As by macrophytes are controlled by four variables: (1) sediment geochemistry, (2) water physicochemistry, (3) plant physiology, and (4) plant genotypic differences (Outridge and Noller 1991). The first two factors control element speciation in sediments and overlying water, whereas the latter two factors control the ability of plants to concentrate bioavailable elements.
In addition to bioconcentrating contaminants, macrophytes perform important functions in constructed wetlands. Macrophytes help stabilize the sediment surface and reduce the risk of erosion, which will prevent re-suspension of precipitated elements (Brix 1997). The roots and submerged shoots of macrophytes provide a large surface area for biofilms, and may provide significant binding sites for elements. Many macrophytes are also known to release oxygen from roots into the rhizosphere, termed radial oxygen loss, which influences the biogeochemistry of Hg, Se, and As in the sediment. Radial oxygen loss may create an oxidized condition in the hydrosoil and may stimulate both aerobic decomposition of organic matter and growth of bacteria. There is evidence that at least some macrophytes actively change element bioavailability in sediments by oxidizing their root zones, either through diffusion of oxygen from root surfaces or the secretion of oxidizing compounds, resulting in the formation of a ferric oxide plaque on roots. The presence of this plaque on roots has been shown to both reduce and enhance essential and toxic element accumulation by macrophytes (Outridge and Noller 1991).
In addition to performing functional roles in wetland systems, macrophytes also present potential risks to invertebrates and other wetland inhabitants. The features that allow plants to take up nutrients and water from sediment also allow them to concentrate contaminants. There are three possible fates for elements bioconcentrated by macrophytes: (1) entry into food chain through consumption by herbivores, (2) excretion into the water column, or (3) release or re-sedimentation upon decomposition of macrophyte tissues (Outridge and Noller 1991). If macrophytes utilized in a CWTS concentrate contaminants such as Hg, Se, and As, there is concern that invertebrates and other herbivores or detritivores may be exposed to these contaminants through ingestion of plant shoots, leaves, seeds, or detritus. Although elements associated with roots would presumably remain in the sediments bound to organic matter, it has been speculated that elements in decaying above-ground plant matter could be released into the water column following senescence. Rather than being released, however, the concentrations and total mass of elements in the detritus often increase during decomposition, as a result of metal adsorption from the water column. Several studies (Giblin et al. 1980; Lacher and Smith 2002; Lindberg and Harriss 1974; Schneider and Rubio 1999; Sundberg et al. 2006; Windham et al. 2004; Zawislanski et al. 2001) have demonstrated that biomass of nonliving aquatic macrophytes, or detritus, behaves as weak cation exchange materials. During detritus decomposition, most elements are retained in the detrital structure, and their concentrations increase, often exponentially.
Plant species capable of extracting and concentrating Hg, Se, and As have been identified. Beath et al. (1937) observed a leaf content of 14,900 ppm Se in Astragalus racemosus grown in Se-contaminated soil. In field studies by Compton et al. (2001), Potomogeton illinoiensis concentrated As nearly eight times greater than contaminated water levels (As was 8.87 mg kg−1 in roots and 4.59 mg kg−1 in shoots and leaves). Following accumulation, P. illinoiensis tissues detoxified As by forming an As–glutathione complex. Ceratophyllum demersum, a submerged, rooted aquatic species common in North America, exhibited a 20,000-fold concentration factor of As from contaminated surface waters (Reay 1972). In the same study Typha orientalis only displayed a 100-fold concentration factor. In a laboratory microcosm study, Polypogon monspeliensis accumulated higher concentrations of Se and Hg than Scirpus robustus. In both species, the translocation from root to shoot was greater for Se than Hg (de Souza et al. 1999).
Several studies have suggested that certain non-hyperaccumulating macrophytes concentrate Hg (Guthrie and Cherry 1979; Heller and Weber 1998; Weis et al. 2002), Se (Ansede et al. 1999; Estabrook et al. 1985; Shardendu et al. 2003; Wu and Guo 2002), and As (Estabrook et al. 1985; Qian et al. 1999) in varying amounts. Guthrie and Cherry (1979) reported a Hg concentration of 0.5 mg kg−1 dry weight in Typha latifolia growing in an unspecified polluted environment. Estabrook et al. (1985) investigated heavy metal concentrations in aquatic plants located 15 km from known heavy metal sources. Selenium was found in T. latifolia leaves, roots, and fruits at concentrations of 0.17, 0.14, and 0.43 mg kg−1, respectively. Arsenic was found in leaves, roots, and fruits (seeds) of T. latifolia at concentrations of 0.34, 0.45, and 0.28 mg kg−1, respectively. In addition, Typha angustifolia concentrated up to 0.30 mg kg−1 As in the roots and fruits (Estabrook et al. 1985). Shardendu et al. (2003) investigated the use of T. latifolia to phytoremediate selenium in subsurface flow constructed wetland treatment systems. Results from this study indicated that T. latifolia concentrated 2.2, 0.6, and 1.8 mg Se kg−1 dry matter in the roots, leaves and stems, respectively.
Schoenoplectus californicus and T. angustifolia were utilized in a pilot-scale constructed wetland treatment system designed to mitigate risks associated with FGD wastewater, which contained elevated Hg, Se, and As concentrations. Information on the ability of these macrophytes to take up, translocate, and bioconcentrate these wastewater constituents in a constructed wetland is limited. Therefore, the primary objective of this research was to characterize plant uptake and physical sorption of Hg, Se, and As by S. californicus and T. angustifolia harvested from the CWTS. In addition, concentrations of Hg, Se, and As in water and sediment collected from the CWTS were measured to determine plant exposure. Sediment characteristics, including particle size distribution, percent solids, cation exchange capacity (CEC), oxidation–reduction (redox) potential, percent organic matter, and pH, were determined for sediment collected from the CWTS to determine if any significant correlations between plant uptake, sorption, and sediment characteristics exist.
2 Materials and Methods
2.1 Pilot Constructed Wetland Treatment System Design
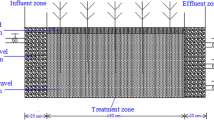
The pilot-scale CWTS was constructed at the Clemson University facility in Pendleton, South Carolina. Briefly, this system consists of a 6,800-l upstream retention basin followed by three parallel treatment systems (Fig.1). Each wetland treatment system consisted of four wetland cells in series, including two cells planted with S. californicus (#1 and #2), a gravel cell, and a final cell planted with T. angustifolia. This CWTS was used to remove Hg, Se, and As from simulated FGD wastewater. The targeted inflow concentrations of these elements in the simulated FGD wastewater ranged from 0.002 to 0.16 mg Hg l−1, 1.8 to 8.5 mg Se l−1, and 0.002 to 0.34 mg As l−1. These three constituents of FGD wastewater are recognized as toxicants of concern, and their discharge into the environment is monitored by the NPDES. Additional information about this CWTS can be found in Sundberg (2006).
2.2 Water and Sediment Collection
Water and sediment were collected from the planted wetland cells of the CWTS (Fig. 1) after 7 (water) and 17 months (sediment) of treating simulated FGD wastewater. All samples were collected and stored in high density polyethylene bottles that were pre-soaked in a 50% concentrated trace metal grade nitric acid for 24 h and rinsed thoroughly with Milli-Q (18 MΩ cm) water. Water blanks were used to ensure the bottles were not contaminated. Wetland cells from which sediment and water were collected included S. californicus #1, S. californicus #2, and T. angustifolia.
Sediment grab samples were taken from three locations in each wetland cell: the front, middle, and back of the cell in the upper 15 cm of sediment, which represents the most biologically active portion of the sediment (Burton 1991). These samples were collected after 17 months of continuously treating simulated FGD wastewater. The three grab samples were combined into one homogenized composite sample per planted wetland cell and stored wet in an air-tight plastic bag at 4°C in the dark (Burton 1991). Sediment was also collected from a local stream to serve as a control. Samples were air dried and sieved through a 2-mm sieve. Three 1.5 ± 0.1 g of dried sediment (exact weight recorded) were transferred to a 12-ml glass digesting vial. Three milliliters concentrated HNO3 and 9 ml concentrated HCl (Gleyzes et al. 2002) were added to the vial and inverted to ensure all sediment was exposed to acid. Vials were placed in a dry incubator to digest for 4 h. The digestates were diluted and brought to a known volume with Milli-Q water and filtered with a Kimwipe® (Ellington and Evans 2000) to remove any undigested material. Milli-Q water acidified with concentrated trace metal grade nitric acid to a pH < 2 was used to rinse the Kimwipe® of any residual elements. The rinsate was then added to the corresponding filtered sample and the sample brought to a known volume with acidified Milli-Q water. The sample was further filtered through a 0.45 μm pore size Millipore 25 mm syringe filter. Samples were stored at 4°C in the dark until analysis.
Water samples were collected from the wetland cell outflows by gravity flow into the sample bottles and filled to the brim. The samples were acidified with concentrated trace metal grade nitric acid to a pH < 2 and stored at 4°C. Prior to analysis, water samples were filtered though a 0.45 μm filter.
2.3 Sediment Characterization Procedures
Sediment samples were characterized within 1 week of collection. Particle size distribution, percent solids, cation exchange capacity (CEC), oxidation–reduction (redox) potential, percent organic matter, and pH were determined for sediment collected from each wetland cell. Sediment pH, which is often one of the most important factors controlling elemental speciation and equilibrium, was measured using an YSI pH meter placed into the wet sediment. Redox potential was measured using a digital millivolt meter and platinum-tipped electrodes (Faulkner et al. 1989) in situ prior to removing sediment samples from the wetland cell to avoid false measurements due to sediment disturbance. Particle size distribution analyses yielded three fraction sizes: sand, silt, and clay. The hydrometer method was used, as described by Gee and Bauder (1986). Percent solids of the sediment were measured according to Black (1986). Percent organic matter in the sediment was measured using the loss-on-ignition method, as described by Nelson and Sommers (1996). Sediment CEC is a measure of the reversibly bound cations in the sample and was determined according to methods presented by Plumb (1981).
2.4 Macrophyte Harvest and Analysis
Three plants were harvested from each planted wetland cell (front, middle, and back) of one treatment train. Uncontaminated plants were also harvested from on-site nursery wetland cells to serve as controls. Plants were rinsed with deionized water to remove sediment, and separated into roots, submerged shoots (from roots to water surface), emerged shoots, and seeds (Murray-Gulde et al. 2005). Adsorbed Hg, Se, and As were measured by separately rinsing submerged shoots and roots with 15% trace metal grade nitric acid. The rinsate was collected, brought up to 100 ml with Milli-Q water, filtered through a 0.45 μm filter, and stored at 4°C in the dark until analysis. Absorbed and concentrated Hg, Se, and As were measured by drying the rinsed plant segments at 105°C for 24–48 h, grinding the tissues into pieces <0.5 cm in size, and digesting 0.5 g in 10 ml concentrated trace metal grade nitric acid in a dry bath incubator for 3 h. Digested material was brought up to 100 ml with Milli-Q water, filtered through a 0.45 μm filter, and stored at 4°C in the dark until analysis.
2.5 Sample Analysis
Ten milliliters of water and digested detritus samples were analyzed for total Hg, Se, and As. Analyses were performed at the Laboratory for Environmental Analysis at the University of Georgia according to the standard method EPA 200.8 (USEPA 1994) using a Sciex Elan 9000 Inductively Coupled Plasma-Mass Spectrometer (ICP-MS) (Perkin-Elmer, Norwalk, CT). Three levels of standards for Hg (0.1, 0.5, and 1.0 μg l−1) and three levels for Se and As (50, 100, 250 μg l−1) were used for calibration. Quality control (QC) checks were run in 20-sample intervals, prepared from a different stock at a concentration level in the middle of the calibration standards. QC checks were considered acceptable if concentrations were within three standard deviations of the average recovery (US Environmental Protection Agency 1994). Method blanks (Milli-Q water acidified with trace metal grade nitric acid to a pH < 2) were run after calibration and before QC checks. Fifty microliters aliquots of a 10 mg l−1 solution of Rh in a 5% nitric acid solution were added as an internal standard to each 10-ml sample prior to analysis. The isotopes quantified were 202Hg, 82Se, and 75As, with method detection limits of 0.02, 0.107, and 0.05 μg l−1, respectively.
2.6 Plant Bioconcentration and Root:Shoot Transfer Factors
Bioconcentration factors (BCF) are ratios of concentrations of a contaminant in the media and dissolved in water (the presumed source of contamination) (Newman 1998). Plant tissue bioconcentration factors (BCFs) and plant surface BCFs were calculated to report the concentration of elements in plant tissues relative to the overlying water, using the following equations:
In these equations, the concentration of Hg, Se, or As in either the whole plant (sum of concentrations in all plant tissues; mg kg−1) or plant surfaces (sum of concentrations on root and submerged shoot surfaces; mg kg−1) were divided by the concentration of that element in the water (mg l−1) from the corresponding wetland cell. Plant tissue and plant surface BCF values (l kg−1) greater than 1 indicate the bioconcentration of Hg, Se, and/or As in the specified plant or plant surface.
Root:shoot transfer factors (TFs) were calculated to compare the concentrations of elements in roots versus shoots using the following equation:
In this equation, the concentration of Hg, Se, or As in the shoots and seeds (sum of the two; mg kg−1) was divided by the concentration of that element in the roots (mg kg−1). Root:shoot transfer factors (kg kg−1) greater than one indicate significant cross-membrane transport of elements from the roots to shoots in plants.
2.7 Statistical Analysis
Significant differences in macrophyte sorption and bioconcentration were determined by Tukey’s Multiple Comparison Test using the computer software program GraphPad Prism, Version 3.0 (GraphPad Software Inc., San Diego, CA). WINKS, Version 4.80 (TexaSoft, Dallas, TX) statistical software was used to determine correlations between macrophyte sorption and bioconcentration and sediment characteristics.
3 Results
As shown in Figs. 2 and 3, Hg and Se concentrations measured in the overlying water samples decreased in the planted wetland cells from the wastewater inflow to final outflow. Mercury concentrations ranged from 28.2 to 1.0 μg l−1, and Hg was below the detection limit of 0.02 μg l−1 in moderately hard laboratory water. Mercury in water from S. californicus #1 was significantly different (p < 0.05) from S. californicus #2 and T. angustifolia, but S. californicus #2 was not significantly different (p > 0.05) from T. angustifolia. Selenium concentrations ranged from 1,674 to 44.6 μg l−1, compared to a concentration of 8.2 μg l−1 in moderately hard laboratory water. Like Hg, Se in water from S. californicus #1 was significantly different (p < 0.05) from S. californicus #2 and T. angustifolia, but S. californicus #2 was not significantly different (p > 0.05) from T. angustifolia. Finally, As concentrations ranged from 118.2 to 12.2 μg l−1, but were not significantly different (p > 0.05) from each other (Fig. 4). The concentration of As in moderately hard laboratory water was 0.5 μg l−1. The large variance measured between water samples may be due to analytical methods.
Mercury and As concentrations measured in sediment did not vary significantly (p > 0.05) from the inflow to outflow of the CWTS (Figs. 2 and 4). The highest concentration of Se (15.6 mg kg−1) was found in sediment from the second planted wetland cell (S. californicus #2; Fig. 3). Mercury concentrations in the CWTS sediment ranged from 0.03 to 0.04 mg kg−1, Se concentrations ranged from 3.5 to 8.8 mg kg−1, and As concentrations ranged from 11.9 to 14.1 mg kg−1. Overall, sediment concentrations of Hg, Se, and As were one to two orders of magnitude less than concentrations measured in detritus from the CWTS (Sundberg et al. 2006). Sediment characteristics, including pH, CEC, organic matter, percent solids, and particle size distribution did not vary between wetland cells (Table 1).
In general, root surfaces sorbed greater amounts of Hg, Se, and As than surfaces of submerged shoots (Table 2). Sorbed element concentrations decreased from wastewater inflow to outflow (S. californicus #1 to T. angustifolia) of the treatment system. Selenium ranged from 4.8 to 0.5 mg kg−1 on root surfaces and 4.0 to 0.8 mg kg−1 on submerged shoots. Arsenic ranged from 7.6 to 0.4 mg kg−1 on roots and from 2.5 to 0.6 mg kg−1 on submerged shoots. Mercury concentrations ranged from 0.1 mg kg−1 to less than 0.01 mg kg−1 on roots and from 0.3 to less than 0.01 mg kg−1 on submerged shoots. Because of the large standard deviations among samples due to the natural variability of plants, Hg, Se, and As concentrations did not vary significantly (p > 0.05) in many cases. Concentrations of Hg adsorbed to root and submerged shoot surfaces of plants collected from the nursery cells did not exceed 0.01 mg kg−1, while Se ranged from 0.06 to 0.3 mg kg−1, and As averaged about 0.1 mg kg−1.
Concentrations of Hg, Se, and As sorbed to root and submerged shoot surfaces were in most cases greater than concentrations found in water and sediment (plant surface BCFs were in most cases greater than 1; Table 3). This suggests that the surfaces of roots and submerged shoots have a greater affinity for these constituents than the water and sediment in the system. Since Hg concentrations on roots and submerged shoots of T. angustifolia were below the detection limit, a BCF could not be calculated. Plant surface BCFs reached 5 for Hg, 29 for Se, and 111 for As. Based on these BCFs, sorption may be an important mechanism of removal of Hg, Se, and As from wastewater moving through the constructed wetland treatment system.
Mercury, Se, and As primarily concentrated in the root tissues of S. californicus and T. angustifolia (Table 4). Despite the higher concentrations of Hg, Se, and As in S. californicus in the second wetland cell (#2), minimal (<7%) translocation was observed compared to S. californicus in the first wetland cell (up to 40%). Roots consistently contained the greatest amounts of Hg, Se, and As in the individual plant tissues. Up to 64% of the total Hg, Se, and As in T. angustifolia were found in the shoots and seeds (Figs. 5, 6 through 7). In general, Hg, Se, and As content declined from the roots up to the seeds. Seeds from T. angustifolia in the fourth wetland cell contained the greatest Hg, Se, and As compared to the other wetland cells.
Despite the relatively low concentrations of Hg, Se, and As on the surfaces of roots and submerged shoots, the calculated BCFs for the plant surfaces were all greater than 1 (Table 3). The highest plant surface BCFs were for As, ranging from 78 to 111 l kg−1. Plant surface BCFs for Se increased from wastewater inflow (4 l kg−1) to outflow (29 l kg−1) of the treatment system, and plant surface BCFs for Hg did not exceed 5 l kg−1 in S. californicus. Root:shoot TFs for Hg, Se, and As in S. californicus did not exceed 1, indicating that these plants were not efficiently translocating these elements to the aerial portions of the plant. T. angustifolia, on the other hand, was more efficient in translocating Hg, Se, and As to its shoots, as evident by TFs averaging 2 kg kg−1. Plant tissue BCFs ranged from 170 to 1911 l kg−1 for Hg, 483 to 10,981 l kg−1 for Se, and 766 to 4,927 l kg−1 for As.
A simple correlation matrix was used for the correlation analysis of Hg, Se, and As. There were no significant correlations (p < 0.05) between macrophyte sorption, bioconcentration, and root:shoot transfer with water and sediment concentrations or sediment characteristics.
4 Discussion
Water concentrations of Hg, Se, and As decreased as the wastewater moved through the CWTS. The final outflow concentrations of 1.0 μg l−1 Hg and 45 μg l−1 Se were below NPDES limits of 1 μg l−1 Hg and 400 μg l−1 Se for the site for which this system was designed (permits for As were not available at the time this study was completed). Sediment concentrations were greater than those measured in water but were one to two orders of magnitude less than concentrations measured in detritus (Sundberg et al. 2006). Sediment from S. californicus #2 contained the highest Hg and Se, while As was greatest in sediment collected from T. angustifolia.
High levels of elements in sediment can be phytotoxic. Poor plant growth and sediment cover caused by element toxicity can lead to remobilization of Hg, Se, and As in the CWTS, which could potentially lead to noncompliance with discharge regulations. It is well known that metals are associated with several sediment fractions: (1) in sediment solution, as free metal ions and soluble metal complexes, (2) adsorbed to inorganic sediment constituents at ion exchange sites, (3) bound to sediment organic matter, (4) precipitated as sulfides, oxides, hydroxides, or carbonates, and (5) embedded in the structure of the silicate minerals (Tessier et al. 1979). Contaminants must be in bioavailable forms in sediment for plant uptake to occur. Bioavailability depends on element solubility in the sediment solution; only elements associated with the sediment solution (free metal ions and soluble metal complexes) and adsorbed to inorganic sediment constituents at ion exchange sites are potentially available for plant uptake (Filgueiras et al. 2002). Based on sequential extraction procedures, which are commonly employed to isolate and quantify metals associated with the different fractions, approximately 80% of the total Hg, 35–60% of the total Se, and less than 5% of the total As in the CWTS sediment was available for plant uptake (Sundberg 2006).
Previous attempts have been made to determine correlations between metal uptake by aquatic plants and sediment concentrations (Cardwell et al. 2002). However, these studies have generally shown only poor correlations. Total sediment metal concentrations do not distinguish between different metal fractions, including those which are bioavailable for plant uptake and those which are not. No significant correlations were observed in this study between element uptake and sediment concentrations.
In general, sorption to sediment particles reduces the activity of metals in the system. Thus, the higher the CEC, the greater the sorption and immobilization of cationic elements (which includes some forms of Hg, but not Se or As). Sandy sediments, such as sediments found in this CWTS (only 2% organic matter), generally had low CECs ranging from 5 to 7 meq 100 g−1. In comparison, organic soils generally have a CEC of 50–100 meq 100 g−1. Since sediment characteristics, with the exception of redox, didn’t vary in this CWTS, no generalizations of the effect of the characteristics on macrophyte sorption and concentration can be made. No significant correlations (p > 0.05) between element uptake and sediment characteristics were observed in this study.
Sorption of element ions to plant surfaces is known to occur through a specific ion exchange mechanism, involving the replacement of protons, alkali, alkaline earth, or other cations by element ions (Schneider et al. 2001). Researchers have provided evidence that for each element ion sorbing, an equivalent of protons and/or other element ions appear in solution (Crist et al. 1991; Schneider et al. 1999). An alternative sorption mechanism is the surface precipitation or condensation of element hydroxides onto the biosurfaces of aquatic plants (Schneider et al. 2001). However, it was not possible to differentiate between the two mechanisms based on sorption data alone in the present study.
Mercury, Se, and As primarily concentrated in the root tissues of S. californicus and T. angustifolia (Fig. 3 and Table 3). Differences in uptake and root:shoot transfer were measured in plants from the first and second wetland cells (S. californicus #1 and #2). Approximately 40% of the total Hg and 30% of the total Se in plant tissues of S. californicus #1 translocated to the shoots and seeds. Interestingly, less than 10% of the total Hg and Se in plant tissues of S. californicus #2 translocated to shoots and seeds. Although root:shoot TFs for all three elements in S. californicus were less than 1, the TFs were greater in the first wetland cell than the second despite the much greater total tissue concentrations in S. californicus #2. Root:shoot transfer of these elements is therefore not dependent on total plant tissue concentrations. It has been shown that of the total amount of ions associated with the root, only a part is absorbed into cells resulting in root:shoot transfer (Estabrook et al. 1985; Shardendu et al. 2003). A significant ion fraction in roots may be physically adsorbed at the extracellular negatively charged sites of the root cell walls. This cell wall-bound fraction cannot be translocated to the shoots. This may explain the decrease in root:shoot transfer in S. californicus #2 despite higher metal concentrations. This difference in root:shoot transfer was not measured for As.
Water concentrations of Hg, Se, and As declined from S. californicus #1 to S. californicus #2, while sediment concentrations increased. This suggests that S. californicus root uptake may be dependent on sediment concentrations. In constructed wetlands, elements may be bound to insoluble fractions in anoxic sediments. However, plants can oxidize the sediment immediately adjacent to the roots through the movement of oxygen downwards through aerenchyma tissue (radial oxygen loss). This oxidation can potentially remobilize the element contaminants, thus increasing the otherwise low availability of elements in anoxic wetland sediments (Weis and Weis 2004).
Significant root:shoot transfer of Hg, Se, and As was measured for T. angustifolia in the last wetland cell. Over 60% of the total Hg, Se, and As in T. angustifolia tissues translocated to the shoots and seeds, resulting in root:shoot transfer factors of ≥1.50 (Fig. 3 and Table 3). However, T. angustifolia contained the least amount of Hg, Se, and As in tissues compared to all S. californicus in the system. Therefore, the greater root:shoot transfer did not depend on tissue metal concentrations. Physiological differences between S. californicus and T. angustifolia may account for the differences in root:shoot transfer of metals. S. californicus may have developed specific mechanisms to prevent or exclude the movement of metal ions into shoots, or the root cell walls may absorb much greater amounts of metals than those of T. angustifolia. The low total concentrations of Hg, Se, and As in tissues of T. angustifolia may be due to the low water concentrations in the wetland cell. Another possible explanation for the low amounts of Hg, Se, and As taken up by roots of T. angustifolia may be that the bioavailabilities of Hg, Se, and As in the sediment decreased, resulting in less root uptake. However, according to sediment sequential extraction data (Sundberg 2006), the fractions of Hg, Se, and As available for plant uptake in the last wetland cell did not significantly vary from the first two wetland cells. Sediment characteristics were similar as well, except for the increase in redox potential.
Plant tissue BCFs were calculated for both S. californicus and T. angustifolia (Table 3), and did not only indicate the importance of plant uptake in a constructed wetland treatment system as a removal mechanism of Hg, Se, and As, but also indicate the potential hazard to herbivores and detritivores. As stated previously, above-water plant tissues fall back into the water column during senescence. The resulting detritus would be expected to not only retain the element concentrations in the tissues, but also absorb additional elements from the water column, increasing the total element concentration significantly. In a previous study (Sundberg et al. 2006), element concentrations were measured in detritus from S. californicus #1, S. californicus #2, and T. angustifolia in this CWTS. Concentrations of Hg, Se, and As found in detritus of these plants were one to two orders of magnitude greater than the concentrations found in shoots in the present study. These results suggest that although macrophytes in this CWTS bioconcentrate Hg, Se, and As, the emerged shoot concentrations contribute little to the final detritus concentrations. Since the system may not have been at equilibrium at the time samples were collected, the distribution of Hg, Se, and As throughout the CWTS, including within the plants, may vary with time. However, the amounts of Hg, Se, and As bioconcentrated in above-water plant tissues, including seeds, may still be potentially hazardous to wetland inhabitants through ingestion.
Selenium toxicity had been reported to be a cause of death and deformities of embryos and chicks within the Kesterson Reservoir National Wildlife Refuge in the Central Valley of California. Selenosis was caused by high concentrations of Se in the run off, which had bioaccumulated in the bird’s food chain by plants, invertebrates, and fish (Spallholz and Hoffman 2002). In recent studies, the emerged macrophytes Typha domingensis (cattails) and Scirpus maritima (alkali bulrush) were harvested from the Kesterson area and analyzed to determine Se concentrations within plant tissues. These plants contained Se concentrations at 17–160 mg kg−1 in leaves, 89–320 mg kg−1 in rhizomes, and 2–34 mg kg−1 in seeds (Wu 2004). By comparison, plants from the pilot CWTS for FGD wastewater treatment contained Se concentrations at 66–136 mg kg−1 in shoots, 170–4,224 mg kg−1 in roots, and 19–113 mg kg−1 in seeds. Therefore, it may be necessary to use plant species with seeds that are unfavorable to wildlife, or to use genetically engineered plants that do not produce seeds.
There are two mechanisms proposed to explain the tolerance of plants to metals (Kupper et al. 1999). Some plant species are able to cope with elevated concentrations of metals inside of their tissues through production of metal-binding compounds, cellular and subcellular compartmentilization, or alternations of metabolism. Plants that use this strategy are often referred to as hyperaccumulators. The second proposed mechanism is exclusion, by which uptake and root:shoot transport of metals in plants are restricted. Results from this research indicate that S. californicus contained elevated concentrations of metals in root tissues, but not in the plant tissues. Therefore, the first mechanism does not explain the tolerance of this plant species to metals. It is evident that the exclusion mechanism is likely responsible for the tolerance of S. californicus to elements in the constructed wetland treatment system, whereas the tolerance of T. angustifolia may be explained by the first mechanism.
5 Conclusions
Results from this research provided information on the sorption and bioconcentration of Hg, Se, and As by macrophytes in a pilot constructed wetland. The importance of macrophytes in element cycling in a constructed wetland treatment system depends on the fate of elements contained within the tissues. Surface sorption and, to a more significant extent, root uptake appeared to be important mechanisms of removal for these constituents, as apparent by water concentrations. The element content in the above-water portions of the plant constitutes an indicator of the bioavailability of the element of concern in the host sediment. The extent of uptake and how elements are distributed within plants can have important effects on the residence time of metals in plants and in constructed wetlands, and the potential release of metals. For example, a majority of emerged shoots become dormant and fall back into the water during senescence. This potentially releases any elements that may have been contained within the tissues, and may also introduce the potentially toxic elements to the detrital food chain. In addition, if elements are accumulated in above-water plant tissues, particularly in the seeds, they may potentially be passed on to herbivores.
As industries such as coal-fired power plants attempt to comply with the Clean Water Act and NPDES permits, cost-effective and ecologically sound wastewater treatment systems such as constructed wetlands are being utilized across the nation. This pilot-scale CWTS reduced aqueous concentrations of targeted constituents, primarily Hg, Se, and As, in wastewater. To completely assess ecological risks associated with the use of CWTSs, contaminant bioavailability for plant uptake, translocation, and bioconcentration must be considered
References
Ansede, J. H., Pellechia, P. J., & Yoch, D. C. (1999). Selenium biotransformation by the salt marsh cordgrass Spartina alterniflora: Evidence for dimethylselenoniopropionate formation. Environmental Science and Technology, 33, 2064–2069.
Beath, O. A., Eppsom, H. F., & Gilbert, C. S. (1937). Selenium distribution in and seasonal variation of vegetation type occurring on seleniferous soils. Journal of American Pharmacology Association, 26, 394–405.
Black, W. C. (1986). Methods of soil analysis part 1 (2nd ed.). Wisconsin: American Society of Agronomy.
Brix, H. (1997). Do macrophytes play a role in constructed treatment wetlands? Water Science and Technology, 35(5), 11–17.
Burton, G. A. (1991). Assessing the toxicity of freshwater sediments. Environmental Toxicology and Chemistry, 10, 1585–1627.
Cardwell, A. J., Hawker, D. W., & Greenway, M. (2002). Metal accumulation in aquatic macrophytes from southeast Queensland, Australia. Chemosphere, 48, 653–663.
Compton, A., Faust, R. D., & Salt, D. (2001). Arsenic accumulation in Potomogeton ellinoiensis in Montezuma Well, Arizona. Meeting of the American Chemical Society Abstracts, 221(1–2), ENR22.
Crist, R. H., Martin, J. R., & Crist, D. R. (1991). Interactions of metals and protons with algae. Equilibrium constants and ionic mechanisms for heavy metal removal as sulfides and hydroxides. In R. W. Smith & M. Misra (Eds.), Mineral bioprocessing (pp. 275–287). Warrendale, PA: TMS.
de Souza, M. P., Huang, C. P. A., Chee, N., & Terry, N. (1999). Rhizosphere bacteria enhance the accumulation of selenium and mercury in wetland plants. Planta, 209(2), 259–263.
Electric Power Research Institute (EPRI) (1999). Flue gas desulfurization by-products: Composition, storage, use, and health and environmental information. An issue report from EPRI. Available at http://www.epri.com.
Ellington, J. J., & Evans, J. J. (2000). Determination of perchorate at parts-per-billion levels in plants by ion chromatography. Journal of Chromatography A, 898, 193–199.
Estabrook, G. F., Burk, D. W., Inman, D. R., Kaufman, P. B., Wells, J. R., & Jones, J. D. et al. (1985). Comparison of heavy metals in aquatic plants on Charity Island, Saginaw Bay, Lake Huron, U.S.A., with plants along the shoreline of Saginaw Bay. American Journal of Botany, 72(2), 209–216.
Faulkner, S. P., Patrick, W. H., & Gambrell, R. P. (1989). Field techniques for measuring wetland soil parameters. Soil Science Society of America Journal, 53, 883–890.
Filgueiras, A. V., Lavilla, I., & Bendicho, C. (2002). Chemical sequential extraction for metal partitioning in environmental samples. Journal of Environmental Monitoring, 4, 823–857.
Gee, G. W., & Bauder, J. W. (1986). Particle-size analysis. In Methods of soil analysis part 1 (2nd ed., pp. 383–410). Wisconsin: American Society of Agronomy.
Giblin, A. E., Bourg, A., Valiela, I., & Teal, J. M. (1980). Uptake and losses of heavy metals in sewage sludge by a New England salt marsh. American Journal of Botany, 67(7), 1059–1068.
Gleyzes, C., Tellier, S., & Astruc, M. (2002). Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. Trends in Analytical Chemistry, 21(6), 451–467.
Guthrie, R. K., & Cherry, D. S. (1979). Trophic level accumulation of heavy metals in a cool ash basin drainage system. Water Research Bulletin, 15, 244–248.
Heller, A. A., & Weber, J. H. (1998). Seasonal study of speciation of mercury (II) and monomethylmercury in Spartina alterniflora from the Great Bay Estuary, NH. Science of the Total Environment, 221, 181–188.
Kupper, H., Zhao, F. J., & McGrath, S. P. (1999). Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiology, 119, 305–311.
Lacher, C., & Smith, R. (2002). Sorption of Hg(II) by Potamogeton natans dead biomass. Mineral Engineering, 15, 187–191.
Lindberg, E., & Harriss, C. (1974). Mercury enrichment in estuarine plant detritus. Marine Pollution Bulletin, 5, 93–95.
Moshiri, G. A. (Ed.). (1993). Construction wetlands for water quality improvement. Michigan: Lewis.
Murray-Gulde, C. L., Huddleson, G. M., Garber, K. V., & Rodgers, J. H. (2005). Contributions of Schoenoplectus californicus in a constructed wetland system receiving copper contaminated wastewater. Water, Air, and Soil Pollution, 163, 355–378.
Nelson, D. W., & Sommers, L. E. (1996). Organic matter. In Methods of soil analysis part 3 (p. 1004). Wisconsin: Soil Science Society of America.
Newman, M. C. (1998). Fundamentals of ecotoxicology. Michigan: Ann Arbor Press.
Outridge, P. M., & Noller, B. N. (1991). Accumulation of toxic trace elements by freshwater vascular plants. In Reveiws of environmental contamination and toxicology. New York: Springer.
Plumb Jr., R. H. (1981). Procedures for handling and chemical analysis of sediment and water samples. Technical Report. EPA/CE-18-1.
Qian, J. H., Zayed, A., Zhu, Y. L., Yu, M., & Terry, N. (1999). Phytoaccumulation of trace elements by wetland plants: III. Uptake and accumulation of ten trace elements by twelve plant species. Journal of Environmental Quality, 28, 1448–1455.
Reay, P. F. (1972). The accumulation of arsenic from arsenic-rich natural waters by aquatic plants. Journal of Applied Ecology, 9, 557–565.
Schneider, I. A. H., & Rubio, J. (1999). Sorption of heavy metal ions by the nonliving biomass of freshwater macrophytes. Environmental Science and Technology, 33, 2213–2217.
Schneider, I. A. H., Rubio, J., & Smith, R. W. (2001). Biosorption of metals onto plant biomass: exchange adsorption or surface precipitation? International Journal of Mineral Processing, 62, 111–120.
Schneider, I. A. H., Smith, R. W., & Rubio, J. (1999). Effect of some mining chemicals on biosorption of Cu(II) by the non living biomass of the freshwater macrophyte Potomogenten lucens. Mineral Engineering, 12, 255–260.
Shardendu, Salhani, N., Boulyga, S. F., & Stengel, E. (2003). Phytoremediation of selenium by two helophyte species in subsurface flow constructed wetland. Chemosphere, 50, 967–973.
Spallholz, J. E., & Hoffman, D. J. (2002). Selenium toxicity: Cause and effect in aquatic birds. Aquatic Toxicology, 57, 27–37.
Sundberg, S. E. (2006). Partitioning and toxicity of mercury, selenium, and arsenic in a constructed wetland for flue gas desulfurization wastewater treatment. Dissertation, Clemson University.
Sundberg, S. E., Hassan, S. M., & Rodgers Jr., J. H. (2006). Enrichment of elements in destritus from a constructed wetland and consequent toxicity to Hyalella azteca. Ecotoxicology and Environmental Safety, 64, 264–272.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
US Environmental Protection Agency (USEPA) (1994). EPA Method 200.8, Determination of trace elements in water and wastes by inductively coupled plasma-mass spectrometry, Revision 5.4, methods for the determination of metals in environmental samples. Supplement 1, EPA/600/R-94-111.
US Environmental Protection Agency (USEPA) (2000). Introduction to Phytoremediation. EPA/600/R-99/107.
Weis, J. S., & Weis, P. (2004). Metal uptake, transport, and release by wetland plants: Implications for phytoremediation and restoration. Environment International, 30, 685–700.
Weis, P., Windham, L., Burke, D. J., & Weis, J. S. (2002). Release into the environment of metals by two vascular salt marsh plants. Marine Environmental Research, 54, 325–329.
Windham, L., Weis, J. S., & Weis, P. (2004). Metal dynamics of plant litter of Spartina alterniflora and Phragmites australis in metal-contaminated salt marshes. Part 1: Patterns of decomposition and metal uptake. Environmental Toxicology and Chemistry, 23(6), 1520–1528.
Wu, L. (2004). Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotoxicology and Environmental Safety, 57(3), 257–269.
Wu, L., & Guo, X. (2002). Selenium accumulation in submerged aquatic macrophytes Potamogeton pectinatus L. and Ruppia maritima L. from water with elevated chloride and sulfate salinity. Ecotoxicology and Environmental Safety, 51, 22–27.
Zawislanski, P. T., Chau, S., Mountford, H., Wong, H. C., & Sears, T. C. (2001). Accumulation of selenium and trace metals on plant litter in a tidal marsh. Estuary and Coastal Shelf Science, 52, 589–603.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer
The research described in this dissertation has been funded in part by the United States Environmental Protection Agency (EPA) under the Greater Research Opportunities (GRO) Graduate Program. EPA has not officially endorsed this publication and the views expressed herein may not reflect the views of the EPA.
Rights and permissions
About this article
Cite this article
Sundberg-Jones, S.E., Hassan, S.M. Macrophyte Sorption and Bioconcentration of Elements in a Pilot Constructed Wetland for Flue Gas Desulfurization Wastewater Treatment. Water Air Soil Pollut 183, 187–200 (2007). https://doi.org/10.1007/s11270-007-9368-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9368-2