Abstract
We isolated a variant of Chinese pseudorabies virus from a hunting dog with symptoms similar to Aujeszky’s disease and designated the isolate MY-1 strain. The dog developed symptoms 6 days after hunting and biting a wild boar and died the day after onset. The Bam HI restriction profile of MY-1 DNA was different from those of the Japanese reference strain Yamagata-S81 and two vaccine strains, Bartha and Begonia, and resembled Bam HI-RFLP (restriction fragment length polymorphism) type IV. Complete nucleotide sequences were determined, and phylogenetic analyses revealed that MY-1 belonged to the same cluster of old Chinese strains and variant strains isolated recently in China, but most of the open reading frames of MY-1 were located on a different branch from those of these Chinese strains. Based on a gC phylogenetic analysis, MY-1 belonged to gC-genotype II composed of those Chinese strains. In mice, the 50% lethal dose (LD50) of MY-1 (103.0 TCID50) was almost the same as those of Yamagata-S81 and Bartha. The LD50 value of Begonia was 10≥4.5 TCID50. The mean survival periods of mice after infection with 104 TCID50 of MY-1, Yamagata-S81 and Bartha were 3.9 days, 2.3 days, and 8.0 days, respectively. The results suggested that the variant of Chinese PRV with slightly weaker pathogenicity than that of wild virulent viruses might be maintained in wild boars in Japan. Furthermore, we would like to propose that old Chinese strains, recent Chinese variant strains, and MY-1 should be grouped as an Asian type PRV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudorabies or Aujeszky’s disease is caused by pseudorabies virus (PRV), a double- strand DNA virus of the genus Varicellovirus, subfamily Alphaherpesvirinae, and family Herpesviridae [1]. Pigs and wild boars are the natural hosts of pseudorabies, and infected pigs show respiratory distress, fatal neurological symptoms, and abortions. In pigs that have survived acute infection, PRV is established as a latent infection in neurological tissues and lymphoid tissues. A wide range of animals including carnivores, rodents, and ungulates are susceptible to PRV infection [2]. Cats and dogs are infected by feeding on or biting PRV-infected pigs or wild boars and they show neurological signs such as pruritus and die rapidly.
In Japan, PRV was first isolated in 1981, and the isolated virus was designated as Yamagata-S81 [3, 4]. In the 1990s, PRV expanded throughout Japan except for Hokkaido, the northernmost island. Since 1991, a PRV eradication program using live-attenuated vaccines from Bartha [5] and Begonia [6] strains and the differentiation of infected from vaccinated animal (DIVA) system [7] has been successfully implemented. The number of infected pigs in Japan has gradually decreased. Since March 2018, 44 of the 47 prefectures in Japan have been free from wild-type PRV in domestic pigs, and live-attenuated vaccines have not been used in those 44 prefectures. However, PRV infection in domestic pigs remains endemic in some areas of the other three prefectures. In animals other than pigs, a dog case was first reported in 1985 [8], and then cases in cat, cattle, and other dog were reported [9,10,11,12]. These animals had lived in prefectures affected by PRV infection. It was suggested that the infectious sources of these cases might be the flesh of swine affected in the field or uncooked pork scraps and haslets. In 1997, dogs that had fed on the flesh of wild boars showed symptoms like pseudorabies and died rapidly after the onset of symptoms, and PRVs were isolated from brain tissues of the dead dogs (Kouda T. et al., In the 129th Annual Meeting of the Japanese Society of Veterinary Science, Tsukuba, 2000). Mahmoud et al. [13] reported that wild boars had antibodies against PRV. Therefore, it was suggested that wild boars might be important sources of PRV to pigs and other animals.
Restriction fragment length polymorphism (RFLP) of genomic DNA by Bam HI digestion was performed to differentiate PRV isolates, and the isolates could be grouped into four major types [14]. RFLP types I and II are found worldwide, and types III and IV had been restricted to Northern Europe and Thailand, respectively, but types III and IV have no longer been reported [15]. Currently, type I is prevalent in wild boars in Europe and type II strains have mainly been found in domestic pigs in Europe [16,17,18,19,20]. There is no information about the RFLP type of PRVs originating in wild boars in Asian countries including Japan. In another molecular analysis for comparing PRVs, based on gC phylogenetic studies, Ye et al. [21, 22] provided evidence for the existence of two genotypes, genotype II consisting of isolates from China including recent variant PRVs isolated from pigs despite vaccination with live-attenuated PRV vaccines [23,24,25] and Malaysia and genotype I consisting of other isolates from Europe, America, and Asia.
In this report, we describe the first isolation of a gC-genotype II PRV other than those isolated in China and Malaysia from a hunting dog that had bitten a wild boar and died rapidly after showing pruritus resembling symptoms of pseudorabies. We also describe characterization of the pathogenicity of the isolate in a mouse model.
Materials and methods
Cell culture and virus
The ESK cell line [26], which is an established cell line derived from embryonic swine kidneys, was used. The cells were grown in Eagle’s minimum essential medium (MEM) supplemented with 10% fetal calf serum (FCS). For the maintenance medium, the serum concentration was reduced to 4%. The Yamagata-S81 strain was propagated in ESK cells and used as the reference strain of PRV in this study [3]. Two live-attenuated vaccine strains, Bartha and Begonia strains, were purchased from Kyoritsu Seiyaku Corporation, Japan and Intervet K. K., Japan, respectively, and were propagated in ESK cells.
Samples from the affected dog
Five hunting dogs had bitten a wild boar while hunting in Miyazaki Prefecture in Japan on March 14, in 2015 and all of the dogs showed pruritus compatible with pseudorabies until the 6th day after biting and died the day after the onset of pruritus. Namely, the day after biting, two dogs showed pruritus and died the day after onset. On the third day and fourth day after biting, one dog each showed the same symptoms and died the day after onset. On the 6th day after biting, the remaining dog showed pruritus and died the day after onset. Tonsils, submandibular gland, parotid gland, cerebrum, and cerebellum were collected from the last affected dog and transported to our laboratory of Rakuno Gakuen University. Ten percent homogenates of each tissue that were made with MEM supplemented with 50 μg/ml of gentamycin were centrifuged at 670×g for 10 min at 4 °C, and the supernatant fluids were used for virus isolation and DNA extraction.
Virus isolation
One hundred microliters of each of the supernatant fluids of tissue samples was inoculated onto ESK cells cultured in a 6-well plate. After adsorption at 37 °C for 30 min under a 5% CO2 atmosphere, the cells were washed twice with MEM, and maintenance medium was added. Then the cells were cultured at 37 °C under a 5% CO2 atmosphere. The cells were observed with a microscope for seven days. An isolated virus was designated MY-1.
DNA extraction
Extraction of DNAs from the supernatant fluids of tissue samples was conducted using a DNeasy Blood and Tissue kit (Qiagen K.K., Tokyo, Japan). DNA from viral nucleocapsids was extracted and purified according to a method described by Volkening and Spatz [27].
Restriction enzyme digestion
The DNA purified from viral nucleocapsids was digested to completion with Bam HI under conditions recommended by the manufacturer (Takara Bio Inc., Tokyo, Japan). Digested fragments were separated on 0.7% agarose gels as previously described [28].
Polymerase chain reaction (PCR) for detection of PRV
To detect PRV DNA in tissue samples, PCR assays targeting the gB gene were carried out using KOD-plus Neo (Toyobo Co., LTD., Osaka, Japan) and primers gB461F (5′-AGGGGATCGCCGTGCTCTTC-3′) and gB794R (5′-GTGTAGGTGTCGTTGGTGGTGTGC-3′) as described by Ma et al. [29]. The estimated amplicon size of PRV gB was 334 bp.
Sequence and phylogenetic analyses
DNA purified from viral nucleocapsids was subjected to sequencing using Illumina Miseq technology in Hokkaido System Science Co., Ltd. (Sapporo, Japan). Undetermined regions were amplified by PCR using walking primers and sequenced in the same company. Sequence analyses were conducted by DNASIS Pro (Hitachi Software Engineering Co., Ltd., Tokyo, Japan) and SnapGene (GSL Biotech LLC, Chicago, IL, U.S.A.). Phylogenetic analysis of the nucleotide sequences was conducted by using MEGA7 software with 1000 bootstrap replicates of the neighbor-joining method [30]. Evolutionary distances were estimated according to the Kimura 2-parameter method [31]. The DDBJ accession number assigned to the complete genome of MY-1 is AP018925.
Experimental infection of mice
All animal experiments were conducted according to the Guidelines for the Proper Conduct of Animal Experiments of the Science Council of Japan. All procedures involving animals and their care were approved by the Animal Care Committee of Rakuno Gakuen University in accordance with the regulations for animal experiments outlined by Rakuno Gakuen University.
Ninety-six 6-week-old specific pathogen-free BALB/c mice were purchased from CLEA, Tokyo, Japan and randomly divided into 24 groups of four mice each. After inhalation anesthesia with isoflurane, groups 1–20 were each inoculated intra-nasally with 10 μl of different doses (1, 10, 102, 103, and 104 TCID50) of MY-1, Yamagata-S81, Bartha or Begonia, and groups 21–24 were inoculated with MEM serving as an uninfected control. Food and water were supplied ad libitum. Following inoculation, clinical signs were monitored twice a day, and the 50% lethal dose (LD50) was calculated. At 14 days post-inoculation, all surviving mice were euthanatized.
Results
Virus isolation and identification
PRV infection was strongly suspected from the clinical symptoms observed in five hunting dogs [2]. CPE were observed in ESK cells 2 days after inoculation of cerebellum and submandibular gland samples but not in tonsil, parotid gland, and cerebrum samples from the last case of the dogs with onset of symptoms. CPE were characterized by rounded cells (data not shown). The isolate from the cerebellum was designated MY-1 strain. By PCR amplification of the partial gB gene of PRV, identical products with the same estimated size, approximately 330 bp, were detected from DNAs of the cerebellum, cerebrum, submandibular gland, and parotid gland but not from those of the tonsil (data not shown). Bam HI restriction profiles revealed that MY-1 showed a different pattern from those of Yamagata-S81, Bartha, and Begonia (Fig. 1). Yamagata-S81 was RFLP-type II [32], and Bartha and Begonia were RFLP-type I [14]. The pattern of MY-1 was almost the same as that for the previously reported variant Chinese isolate HeN1 in the literature [21]. Based on complete nucleotide sequences of old Chinese strains Fa (GenBank accession No.: KM189913) and Ea (KX423960) and recent Chinese variant strains HNX (KM189912) and HeN1 (KP098534), Bam HI restriction profiles of these strains and MY-1 (DDBJ accession No.: AP018925 described below) determined by computer analysis using the software SnapGene (GSL Biotech LLC, USA) were almost the same (Fig. 2). Furthermore, the patterns of MY-1 and Chinese strains resembled the pattern for previously reported RFLP-type lV [14].
Bam HI restriction profile image on 0.7% agalose gel electrophoresis of DNAs of MY-1 strain, two old Chinese strains (Fa and Ea) and two Chinese variant strains (HNX and HeN1) by computer analysis using the software SnapGene. Lane 1: molecular mass marker, lambda DNA Hind III digest, Lane 2: MY-1 (DDBJ accession No.: AP018925), Lane 3: Fa (GenBank accession No.: KM189913), Lane 4: Ea (KX423960), Lane 5: HNX (KM189912), Lane 6: HeN1 (KP098534)
Genetic analysis of the isolate MY-1
The genome DNA of the MY-1 strain was completely sequenced and found to be 143,277 bps in length with an average G+C content of 73.58%. The MY-1 genome includes 69 open reading frames (ORFs). The complete genomic sequence of MY-1 was submitted to DDBJ under the accession number AP018925.
The complete genomic sequences of MY-1 were compared to those of three European-American strains, Kaplan (KJ717942), Bartha (JF797217), and Becker (JF797219), one old Chinese strain, Fa, and two Chinese variant strains, HNX and HeN1 (Table 1). MY-1 shared 94.2, 91.6, 93.6, 96.6, 96.7, and 96.8% identities with Kaplan, Bartha, Becker, Fa, HNX, and HeN1 strains, respectively. MY-1 showed higher identities (96.6% to 96.8%) to those of the three Chinese strains.
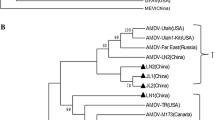
Phylogenetic comparisons of the full-length genome of MY-1 and those six PRV strains and one European strain, Kolchis (KT983811), revealed that MY-1 was closely related to the Chinese strains Fa, HNX, and HeN1. MY-1 and European-American strains belong to different phylogenetic groups (Fig. 3).
Identities of the nucleotide sequences of all ORFs of MY-1 against those of the European-American strains Kaplan, Bartha, and Becker and Chinese strains Fa, HNX, and HeN1 were 91.1% to 99.7% (mean 97.1%), 91.4% to 99.7% (mean 97.0%), 90.5% to 100.0% (mean 97.0%), 86.1% to 100.0% (mean 99.1%), 90.5% to 100.0% (mean 99.2%), and 91.8% to 100.0% (mean 99.3%), respectively (Table S1, available in the online version of this article). Identities of the amino acid sequences of all ORFs of MY-1 against those of Kaplan, Bartha, Becker, Fa, HNX and HeN1 were 87.3% to 100.0% (mean 95.9%), 87.9% to 99.7% (mean 95.8%), 87.0% to 100.0% (mean 95.9%), 85.3% to 100.0% (mean 98.8%), 88.0% to 100.0% (mean 98.7%), and 90.6% to 100.0% (mean 98.9%), respectively (Table S1). These results indicated that the ORFs of MY-1 had higher identities to those of the three Chinese strains. Among the seven PRV strains, highly conserved amino acid sequences of ORFs that showed more than 99.0% identities were six ORFs, UL18 (VP23, capsid protein), UL19 (VP5, major capsid protein), UL22 (gH), UL23 (thymidine kinase), UL30 (DNA polymerase) and UL38 (VP19c, capsid protein). One hundred percent amino acid sequence identities between MY-1 and three Chinese strains were found in 13 ORFs, UL4 (infected-cell protein), UL18 (VP23, capsid protein), UL23 (thymidine kinase), UL24 (nuclear protein), UL35 (VP26, capsid protein), UL38 (VP19c, capsid protein), UL40 (ribonucleotide reductase subunit 2), UL41 (vhs, host-cell shut-off), UL48 (VP16, tegument), UL53 (gK), US2 (membrane protein), US3 (protein kinase), and US9 (membrane protein). However, 100% amino acid sequence identities of ORFs between MY-1 and the three European-American strains were not found. The nucleotide and amino acid sequences of MY-1 UL36 (VP1/2, tegument) showed the lowest identities to those of the three European-American strains, 90.5% to 91.4% and 87.0% to 87.9%, respectively. The nucleotide and amino acid sequences of MY-1 US1 (ICP22, gene regulation) showed the most variable identities to those of the other six strains, 86.1% to 94.9% and 85.3% to 94.6%, respectively.
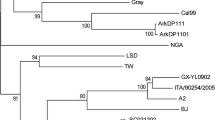
Phylogenetic analysis based on nucleotide sequences of all ORFs was carried out. Phylogenetic trees of the variable glycoprotein C (gC) (UL44), a non-essential component of the virion envelope to act as an attachment protein that was used in various studies to classify PRVs, are shown in Fig. 4. Phylogenetic trees of envelope glycoproteins gB (UL27), gD (US6), gE (US8), gH (UL22), gI (US7), gK (UL53), gL (UL1), gM (UL10), and gN (UL49.5), which play essential roles in attachment, penetration, cell-to-cell spread, neurological spread, and immunogenicity, are shown in Fig. 5. Phylogenetic trees of the other ORFs are shown in the supplementary file as Fig. S1. In the phylogenetic tree of partial nucleotide sequences of gC (Fig. 4a), two genotypes, I and II, were observed [21, 22]. MY-1 belonged to gC-genotype II composed of various Chinese strains. However, MY-1 was located in a different branch from those Chinese strains. The same results were also obtained for the phylogenetic tree of complete nucleotide sequences of gC (Fig. 4b). The phylogenetic trees of gD, gE, gI, gM, and gH showed almost the same pattern as that of gC (Fig. 5). In the phylogenetic trees of gK, gL, and gN, MY-1 is located in the same cluster as that of Chinese strains. The other phylogenetic trees in Fig. S1 showed results similar to the results described above.
Phylogenetic trees based on complete nucleotide sequences of gB (UL27) (a), gD (US6) (b), gE (US8) (c), gG (US4) (d), gH (UL22) (e), gI (US7) (f), gK (UL53) (g), gL (UL1) (h), gM (UL10) (i), and gN (UL49.5) (j) genes of MY-1 and other strains available from GenBank. MY-1 is indicated by a closed circle. Bootstrap values less than 50% are not shown on the corresponding nodes
Pathogenicity of MY-1 in mice
The pathogenicity of MY-1 was compared to those of virulent Yamagata-S81 and two vaccine strains, Bartha and Begonia, in a mouse model (Table 2). The LD50 values of MY-1, Yamagata-S81, Bartha, and Begonia were 103.0 TCID50, 102.5 TCID50, 103.0 TCID50, and 10≥4.5 TCID50, respectively. The mean survival periods of mice after infection with 104 TCID50 of MY-1, Yamagata-s81, and Bartha were 3.9 days, 2.3 days and 8.0 days, respectively. Begonia did not show any clinical signs in mice.
Discussion
In this study, we isolated a PRV designated MY-1 from a hunting dog that had bitten a wild boar in Miyazaki Prefecture, where PRV infection of domestic pigs was eradicated, in Japan. Bam HI-RFLP analysis of MY-1 showed a type IV pattern based on Herrmann’s classification in the literature [14] and suggested that MY-1 might belong to the same group of old Chinese strains and recent Chinese variant strains. Japanese PRV isolates since 1981 belonged to RFLP-type I or II, not to type III or IV [10, 32,33,34]. Therefore, we determined the whole genomic sequences of MY-1 to characterize the virus at the molecular level in detail. Phylogenetic analysis of MY-1 revealed that MY-1 belonged to the same cluster as that of Chinese isolates, not to classical isolates of Europe and U.S.A., and MY-1 should be classified as a variant of Chinese strains.
Identities of nucleotide sequences and amino acid sequences of all ORFs of MY-1 against those of Chinese strains Fa, HNX and HeN1 were higher than those of MY-1 against European-American strains Kaplan, Bartha and Becker. The PRV US1 protein showed the most variable identities between MY-1 and other isolates (85.3% to 94.6%) (Table S1). In the second half of the US1 amino acid sequences, aspartic acid and glutamic acid (ED)-rich regions were observed. The variability was due to these regions. N-terminal 207 amino acid sequences, the first half of US1 of MY-1 without ED-rich regions, showed 98.9% to 99.5% identities against those of 6 strains used in this study (data not shown).
The results of phylogenetic analysis of each ORF were almost the same. A phylogenetic tree of partial gC (UL44) sequences has been used for molecular characterization of PRV [21, 22]. Figure 4 shows that MY-1 belonged to gC-genotype II with some of the old Chinese isolates and all of the new variant Chinese strains. However, MY-1 was located in a different branch from those of recent Chinese isolates and old Chinese isolates. MY-1 has a relatively large branch length compared to those of Chinese isolates. The phylogenetic trees of other ORFs showed almost the same results. It has been suggested that variant Chinese strains that have recently emerged might have evolved independently from old Chinese isolates such as Fa and Ea strains [35, 36]. MY-1 might therefore have evolved from a strain similar to old Chinese strains maintained in wild boars in Japan and might not have been transmitted directly from China as a new emergent virus.
It was shown that the virulence of PRV for pigs was correlated to that for mice [37]. Furthermore, mice are thought to be more susceptible than pigs to PRV infection, because mice infected with a gE/gI deletion mutant had high rates of morbidity and mortality, whereas infected pigs remained clinically normal [38]. Therefore, mice may be more suitable for evaluating the pathogenicity of PRV [39]. Intranasal inoculation of mice with MY-1 and Yamagata-S81 resulted in almost the same LD50 values. However, the mean survival periods of mice inoculated with 104 TCID50 of MY-1 (3.9 days) was longer than that of mice inoculated with 104 TCID50 of Yamagata-S81 (2.3 days). Recent Chinese variant PRV strains show high pathogenicity in mice compared to that of old Chinese PRV strains [40]. The low pathogenicity of Bartha compared to those of virulent PRV strains in this study coincide with previous reports [41, 42]. Begonia (gE-, gI-, and TK-) derived from 2.4-N3A strain (gE- and gI-) originating from NIA-3 [6] did not show any clinical symptoms in mice inoculated with the virus. These results coincide with results for a gE, gI and TK deleted mutant [38]. From these observations, MY-1 might have slightly weaker pathogenicity than that of Japanese standard PRV and Chinese variant PRV strains. Therefore, it is thought that PRV vaccine strains used in Japan might protect pigs from MY-1 infection. However, direct experimental infection in pigs to evaluate the effectiveness of the vaccines will be necessary.
In summary, we isolated a variant of Chinese PRVs from a hunting dog that had bitten a wild boar in Japan. The isolated virus belonged to gC-genotype II and Bam HI-RFLP- type IV with some of old Chinese PRVs and new variant Chinese strains. Therefore, we would like to propose that some of the old Chinese strains, recent Chinese variant strains and MY-1 should be grouped as an Asian type PRV. Wild boars are distributed throughout Japan except for Hokkaido, the northernmost island. Despite successful elimination of PRV from domestic pigs in several parts of the world, this virus seems to be circulating globally in populations of non-domestic swine [18]. Since the origin of MY-1 was a wild boar, the potential risk of PRV infections via infected wild boars has become a growing matter of concern, especially in areas where domestic pig husbandry overlaps with wild boars in Japan.
References
Mettenleiter TC (2000) Aujeszky’s disease (pseudorabies) virus: the virus and molecular pathogenesis–state of the art, June 1999. Vet Res 31:99–115
Pensaert MB, Kluge P (1989) Pseudorabies virus (Aujeszky’s disease). In: Pensaert MB (ed) Virus infections of porcines. Elsevier, New York, pp 39–64
Fukusho A, Shimizu M, Kubo M, Nanba K, Shimizu Y, Kono S, Suzuki K, Otaki T (1981) The first outbreak of Aujeszky’s disease in swine in Japan. II. Virus isolation. Bull Natl Inst Anim Health 82:5–11
Onodera M, Tsuruta M, Sudo S, Niizeki H, Saito F, Taneichi A (1981) The first outbreak of Aujeszky’s disease in swine in Japan. I. Clinical observation. Bull Natl Inst Anim Health 82:13–18
Bartha A (1961) Experimental reduction of virulence of Aujeszky’s disease virus. Magy Allatorv Lapja 16:42–45 (in Hungarian)
Visser N, Lütticken D (1989) Experiences with a gI-/TK modified live pseudorabies virus vaccine: strain Begonia. In: Van Oirschot JT (ed) Proceedings of the CEC seminar on vaccination and control of Aujeszky’s disease. Kluwer Academic, Dordrecht, pp 37–44
Freuling CM, Müller TF, Mettenleiter TC (2017) Vaccines against pseudorabies virus (PrV). Vet Microbiol 206:3–9. https://doi.org/10.1016/j.vetmic.2016.11.019
Hara M, Shimizu T, Fukuyama M, Nomura Y, Shirota K, Une Y, Hirota A, Yago K, Yamada H, Ishihara M (1987) Natural case of Aujeszky’s disease in the dog in Japan. Jpn J Vet Sci 49:645–649
Hara M, Shimizu T, Fukuyama M, Ikeda T, Kiuchi A, Tabuchi K, Nomura Y, Shirota K, Une Y, Ishizaki R (1991) A natural case of Aujeszky’s disease in the cat in Japan. J Vet Med Sci 53:947–949
Hara M, Shimizu T, Nemoto S, Fukuyama M, Ikeda T, Kiuchi A, Tabuchi K, Nomura Y, Shirota K, Une Y, Ishizaki R (1991) The genome type of Aujeszky’s disease virus isolated from a cat in Japan. J Vet Med Sci 53:1087–1089
Matsuoka T, Iijima Y, Sakurai K, Kurihara T, Kounosu Y, Tamiya K, Oki M, Haritani M, Imada T (1987) Outbreak of Aujeszky’s disease in cattle in Japan. Jpn J Vet Sci 49:507–510
Kano Y (1992) A clinical case of Aujeszky’s disease in the dog. J Jpn Vet Med Assoc 5:414–417 (in Japanese)
Mahmoud HY, Suzuki K, Tsuji T, Yokoyama M, Shimojima M, Maeda K (2011) Pseudorabies virus infection in wild boars in Japan. J Vet Med Sci 73:1535–1537
Herrmann S, Heppner B, Ludwig H (1984) Pseudorabies viruses from clinical outbreak and latent infections grouped into four major genotypes. In: Wittmann G, Gaskell RM, Rziha HJ (eds) Latent herpesvirus infections in veterinary medicine. Martinus Nijhoff Publishers, Boston, pp 387–401
Christensen LS (1995) The population biology of suid herpesvirus 1. APMIS Suppl 48:1–48
Capua I, Casaccia C, Calzetta G, Caporale V (1997) Characterisation of Aujeszky’s disease viruses isolated from domestic animals and from a wild boar (Sus scrofa) in Italy between 1972 and 1995. Vet Microbiol 57:143–149
Müller T, Klupp BG, Freuling C, Hoffmann B, Mojcicz M, Capua I, Palfi V, Toma B, Lutz W, Ruiz-Fon F, Gortárzar C, Hlinak A, Schaarschmidt U, Zimmer K, Conraths FJ, Hahn EC, Mettenleiter TC (2010) Characterization of pseudorabies virus of wild boar origin from Europe. Epidemiol Infect 138:1590–1600. https://doi.org/10.1017/S0950268810000361
Müller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, Freuling C (2011) Pseudorabies virus in wild swine: a global perspective. Arch Virol 156:1691–1705. https://doi.org/10.1007/s00705-011-1080-2
Steinrigl A, Revilla-Fernández S, Kolodziejek J, Wodak E, Bagó Z, Nowotny N, Schmoll F, Köfer J (2012) Detection and molecular characterization of Suid herpesvirus type 1 in Austrian wild boar and hunting dogs. Vet Microbiol 157:276–284. https://doi.org/10.1016/j.vetmic.2011.12.033
Verpoest S, Cay AB, De Regge N (2014) Molecular characterization of Belgian pseudorabies virus isolates from domestic swine and wild boar. Vet Microbiol 172:72–77. https://doi.org/10.1016/j.vetmic.2014.05.001
Ye C, Zhang QZ, Tian ZJ, Zheng H, Zhao K, Liu F, Guo JC, Tong W, Jiang CG, Wang SJ, Shi M, Chang XB, Jiang YF, Peng JM, Zhou YJ, Tang YD, Sun MX, Cai XH, An TQ, Tong GZ (2015) Genomic characterization of emergent pseudorabies virus in China reveals marked sequence divergence: evidence for the existence of two major genotypes. Virology 483:32–43. https://doi.org/10.1016/j.virol.2015.04.013. (Erratum. In: Virology 2015. 485:473-474)
Ye C, Guo JC, Gao JC, Wang TY, Zhao K, Chang XB, Wang Q, Peng JM, Tian ZJ, Cai XH, Tong GZ, An TQ (2016) Genomic analyses reveal that partial sequence of an earlier pseudorabies virus in China is originated from a Bartha-vaccine-like strain. Virology 491:56–63. https://doi.org/10.1016/j.virol.2016.01.016
Wu R, Bai C, Sun J, Chang S, Zhang X (2013) Emergence of virulent pseudorabies virus infection in northern China. J Vet Sci 14:363–365. https://doi.org/10.4142/jvs.2013.14.3.363
An TQ, Peng JM, Tian ZJ, Zhao HY, Li N, Liu YM, Chen JZ, Leng CL, Sun Y, Chang D, Tong GZ (2013) Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg Infect Dis 19:1749–1755. https://doi.org/10.3201/eid1911.130177
Sun Y, Luo Y, Wang CH, Yuan J, Li N, Song K, Qiu HJ (2016) Control of swine pseudorabies in China: opportunities and limitations. Vet Microbiol 183:119–124. https://doi.org/10.1016/j.vetmic.2015.12.008
Sugimori T, Morimoto T, Miura Y, Sazawa H (1969) Hemagglutinin of Japanese encephalitis virus produced in embryonic swine kidney cell cultures. Natl Inst Anim Health Q 9:55–64
Volkening JD, Spatz SJ (2009) Purification of DNA from the cell-associated herpesvirus Marek’s disease virus for 454 pyrosequencing using micrococcal nuclease digestion and polyethylene glycol precipitation. J Virol Methods 157:55–61. https://doi.org/10.1016/j.jviromet.2008.11.017
Kirisawa R, Ohmori H, Iwai H, Kawakami Y (1993) The genomic diversity among equine herpesvirus-1 strains isolated in Japan. Arch Virol 129:11–22
Ma W, Lager KM, Richt JA, Stoffregen WC, Zhou F, Yoon KJ (2008) Development of real-time polymerase chain reaction assays for rapid detection and differentiation of wild-type pseudorabies and gene-deleted vaccine viruses. J Vet Diagn Investig 20:440–447
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Nishimori T, Imada T, Sakurai M, Kitabayashi T, Kawamura H, Nakajima H (1987) Restriction endonuclease analysis of Aujeszky’s disease viruses isolated in Japan. Jpn J Vet Sci 49:365–367
Shibata I, Asai T, Akashi H, Inaba Y (1992) Characterization of Japanese isolates of Aujeszky’s disease virus by restriction endonuclease cleavage patterns, virulence in mice and thymidine kinase activity. J Vet Med Sci 54:523–528
Yamada S, Nishimori T, Shimizu M (1992) Characterization of pseudorabies viruses recently isolated in Japan by restriction endonuclease assay. J Vet Med Sci 54:541–549
Wang X, Wu CX, Song XR, Chen HC, Liu ZF (2017) Comparison of pseudorabies virus China reference strain with emerging variants reveals independent virus evolution within specific geographic regions. Virology 506:92–98. https://doi.org/10.1016/j.virol.2017.03.013
Yu T, Chen F, Ku X, Fan J, Zhu Y, Ma H, Li S, Wu B, He Q (2016) Growth characteristics and complete genomic sequence analysis of a novel pseudorabies virus in China. Virus Genes 52:474–483. https://doi.org/10.1007/s11262-016-1324-z
Jestin A, Blanchard P, Garbar-Chenon A, Vannier P, Nicolas JC (1990) Restriction fragment pattern analysis of genomes from French isolates of suis herpes virus 1 (Aujeszky’s disease virus). Arch Virol 112:149–167
Cong X, Lei JL, Xia SL, Wang YM, Li Y, Li S, Luo Y, Sun Y, Qiu HJ (2016) Pathogenicity and immunogenicity of a gE/gI/TK gene-deleted pseudorabies virus variant in susceptible animals. Vet Microbiol 182:170–177. https://doi.org/10.1016/j.vetmic.2015.11.022
Tang YD, Liu JT, Wang TY, Sun MX, Tian ZJ, Cai XH (2017) Comparison of pathogenicity-related genes in the current pseudorabies virus outbreak in China. Sci Rep 7:7783. https://doi.org/10.1038/s41598-017-08269-3
Luo Y, Li N, Cong X, Wang CH, Du M, Li L, Zhao B, Yuan J, Liu DD, Li S, Li Y, Sun Y, Qiu HJ (2014) Pathogenicity and genomic characterization of a pseudorabies virus variant isolated from Bartha-K61-vaccinated swine population in China. Vet Microbiol 174:107–115. https://doi.org/10.1016/j.vetmic.2014.09.003
Brittle EE, Reynolds AE, Enquist LW (2004) Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol 78:12951–12963
Klopfleisch R, Klupp BG, Fuchs W, Kopp M, Teifke JP, Mettenleiter TC (2006) Influence of pseudorabies virus proteins on neuroinvasion and neurovirulence in mice. J Virol 80:5571–5576
Author information
Authors and Affiliations
Contributions
RK performed a virus isolation. KM and RK performed sequences. SK provided samples. KS conducted animal experiments. KM and RK wrote the manuscript. RK finalized the draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experiments were conducted according to the Guidelines for the Proper Conduct of Animal Experiments of the Science Council of Japan. All procedures involving animals and their care were approved by the Animal Care Committee of Rakuno Gakuen University. The animal ethics approved number was VH16A4.
Additional information
Edited by Juergen A Richt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The DDBJ accession number for the complete genomic sequences of MY-1 is AP018925.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Minamiguchi, K., Kojima, S., Sakumoto, K. et al. Isolation and molecular characterization of a variant of Chinese gC-genotype II pseudorabies virus from a hunting dog infected by biting a wild boar in Japan and its pathogenicity in a mouse model. Virus Genes 55, 322–331 (2019). https://doi.org/10.1007/s11262-019-01659-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-019-01659-x