Abstract
PYRIN domain (PYD) proteins have recently emerged as important signaling molecules involved in the development of innate immunity to intracellular pathogens through activation of inflammatory mediator pathways. ASC is the central adaptor protein, which links pathogen recognition by PYD-containing pathogen recognition receptors to the activation of downstream effectors, including activation of Caspase-1 and NF-κB. The cellular PYD-only protein 1 (cPOP1) can block the recruitment of ASC to activated PAN receptors and thereby functions as an endogenous inhibitor of the PYD-mediated signal transduction pathway. Here we describe the identification and characterization of a Shope Fibroma homolog to cPOP1. Like cPOP1, a Shope Fibroma virus-encoded POP (vPOP), co-localizes and directly associates with ASC and inhibits PYD-mediated signal transduction. Poxviruses are known to encode immune evasive proteins to promote host cell infection and suppression of the host immune response. Poxvirus-encoded vPOPs represent a novel class of immune evasive proteins and impair the host response by blocking Cryopyrin and ASC inflammasome-mediated activation of pro-Caspase-1 and subsequent processing of pro-interleukin (IL)-1β, and expression of vPOPs causes activation of NF-κB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
PYRIN domain (PYD) proteins are important signaling molecules involved in host defense to pathogens through activation of inflammatory mediator pathways. Humans have at least 23 proteins containing a PYD. [1–4]. Pathogens are recognized by the leucine-rich region (LRR) of PYD-containing pathogen recognition receptors, known as PYD-NLR, PAN, PYPAF, NALP, Caterpiller, and Nod proteins. Activation of these proteins is hypothesized to be achieved through displacement of the intramolecular interaction of the LRR with the NACHT domain. Activated PYD-NLR-family proteins recruit and oligomerize the PYD-containing adapter protein ASC (TMS1) [5, 6] into cytoplasmic aggregates (‘specks”), referred to as the inflammasome, where activation of downstream effectors occurs [7]. Caspase-1-mediated processing of pro-IL-1β and activation of the transcription factor NF-κB have been described as effectors of PYD-mediated signal transduction and enforced oligomerization of ASC is sufficient for activation of these downstream effectors.
Hereditary mutations in PYD-NLR-family proteins can cause deregulated recruitment of ASC, which leads to autoinflammatory disorders, such as periodic fever syndromes. Specifically, mutations in the PYD-containing protein Pyrin (Marenostrin) account for familial Mediterranean fever, whereas mutations in the PYD-NLR-family member Cryopyrin (CIAS1, PYPAF1, NALP3) have been linked to familial cold auto-inflammatory Syndrome, Muckle–Wells syndrome, and Chronic infantile neurological cutaneous and articular syndrome [8].
The PYD belongs to the family of death domain fold (DDF) domains, which also includes the caspase recruitment domain (CARD), the death domain (DD), and the death effector domain (DED). CARD and DED interactions are also regulated by CARD-only proteins (COPs) and DED-only proteins, respectively. These small proteins are just composed of a single CARD or DED and include the CARD-only proteins COP (Pseudo-ICE), Iceberg and INCA [9–12], or the DED-only proteins PED (PEA-15), FLIP-s, and viral (v) FLIP [13, 14]. In general DDF-only proteins can function as dominant-negative inhibitors for particular signaling pathways by competing for critical binding partners, thereby interrupting signal transmission to downstream effector proteins.
The recruitment of ASC to PYD-NLR-family proteins is regulated by a PYRIN-only protein 1 (POP1), which interferes with the PYD-PYD interaction between ASC and PYD-NLRs, thereby impairing host defense by blocking downstream effectors [15]. Here we report the identification and characterization of a Shope Fibroma Virus (SFV) POP homolog, which impairs the host response by blocking PYD-mediated activation of pro-Caspase-1 and subsequent processing of pro-interleukin (IL)-1β. During the preparation of this manuscript, Johnston et al. characterized a Myxoma virus-encoded POP, which also functions as an inhibitor of IL-1β secretion [16]. We continued this study and show that the Shope Fibroma virus-encoded POP functions similar to the Myxoma virus-encoded POP, and that both proteins affect also NF-κB activation.
Materials and methods
Plasmids and strains
The rabbit Myxoma virus (MV; strain Lausanne) and the rabbit Shope Fibroma virus (SFV; strain Original A) were obtained from the American Type Culture Collection. The complete open reading frame of M013L was cloned directly from the genome of MV by high fidelity PCR (PFU Ultra, Stratagene) using the primer pair 5′-CGGAATTCAAAATGGAGCACCGACCGTCATTA-3′, 5′-GCGCCTCGAGATAAAATAACAATTTGCGACATACACC-3′. The complete open reading frame of S013L was cloned directly from the genome of SFV using the primer pair 5′-CGGAATTCTACTTTAAAATGAAGGAAAATGACAT-3′, 5′-GCGCCTCGAGTTGATAAAACAAACTTTCACGATA-3′. Both PCR products were digested with EcoR1 and Xho1 restriction enzymes and cloned into pcDNA3 (Invitrogen) expression plasmids modified to contain an NH2-terminal myc or Flag epitope tag. The authenticity of S013L and M013L was confirmed by DNA sequence analysis. Expression constructs for ASC, ASC-PYD, ASC-CARD, cPOP1, Cryopyrin (R260W), pro-IL-1β, and pro-Caspase-1 were described previously [15, 17, 18].
Cell culture and transfection
HEK293N, HEK293T, and rabbit kidney (RK13) cells were obtained from the American Type Culture Collection and cultured in Dulbecco’s modified Eagle’s medium supplemented with 4 mM l-glutamine, 1.5 g/l sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, and 10% fetal bovine serum (HEK293) and Minimum essential medium (Eagle) with 2 mM L-glutamine and Earle’s BSS adjusted to contain 1.5 g/l sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, and 10% fetal bovine serum (RK13). HEK293 cells were transfected using Polyfect (Qiagen) or the Calcium precipitation method.
Viral infection
Infection of subconfluent layers of RK13 cells with the rabbit Fibroma virus (strain Original A) was carried out at a multiplicity of infection (MOI) of 10 in OPTI minimum essential medium (Invitrogen) supplemented with 2% fetal calf serum for 1 h. The inoculum was removed, the cells were washed with serum-free medium and further grown for the indicated times.
Reverse transcriptase-polymerase chain (RT-PCR) reaction
RK13 cells were infected with the rabbit SFV (strain Original A) at a MOI of 10, as described above. Sixteen hours (16 h) post-infection, RNA was isolated using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Five micrograms (5 μg) total RNA was subjected to DNase I (Invitrogen) treatment according to the manufacturer’s instructions and reverse transcribed using the Superscript II enzyme (Invitrogen) into first strand cDNA, followed by PCR amplification with GoTaq polymerase (Promega) using the S013L-specific primer pair 5′-CGGAATTCTACTTTAAAATGAAGGAAAATGACAT-3′, 5′-GCGCCTCGAGTTGATAAAACAAACTTTCACGATA-3′ (324 bp) and the βACTIN-specific primer pair 5′-GACGATGATATTGCCGCACT-3′, and 5′-GATACCACGCTTGCTCTGAG-3′ (533 bp). A control, in which the RT was omitted during the RT step, was also performed. PCR-products were excised from agarose gels and sequence verified.
Co-immunoprecipitation assay
Immunoprecipitation assays were performed following transient expression of HA-tagged ASC and either myc-tagged M013L or Myc-tagged gp013L into HEK293T cells. Thirty-six hours (36 h) post-transfection, cells were lysed in isotonic lysis buffer (150 mM NaCl, 20 mM Tris–HCl, pH 7.4, 10% glycerol, 0.2% NP-40), supplemented with protease and phosphatase inhibitors. Clarified lysates were subjected to immunoprecipitation using agarose-conjugated anti-HA antibodies (Santa Cruz Biotechnology) for 4 h at 4°C. Following extensive washing in lysis buffer, bound immune complexes were separated by SDS/PAGE and analyzed by immunoblotting using directly HRP-conjugated anti-myc antibodies (Santa Cruz Biotechnology) in conjunction with an ECL detection system (Amersham-Pharmacia). Where indicated, cell lysates (10% volume) were included along side immune complexes. Alternatively, lysates were directly analyzed by immunoblotting after normalization for total protein content.
In vitro protein interaction assay
The PYD of ASC was expressed as a GST-fusion protein in E. coli BL21 cells (Stratagene) and affinity-purified using GSH-Sepharose (Amersham-Pharmacia). GST-ASC-PYD or GST control (50 ng) immobilized on 10 μl GSH-Sepharose were blocked in 142.4 mM KCl, 5 mM MgCl2, 10 mM HEPES pH 7.4, 0.5 mM EGTA, 1 mM EDTA, 0.2% Nonidet P-40, 1 mM DTT, supplemented with protease inhibitors and 1 mg/ml BSA for 30 min at room temperature. Beads were washed twice and incubated with in vitro translated and biotinylated (Promega) vPOP proteins overnight at 4°C as above, but the buffer was supplemented with protease inhibitors and 0.5 mg/ml BSA. Bound proteins were washed extensively, separated by SDS/PAGE, immunoblotted with Streptavidin-HRP and detected with ECL (Amersham-Pharmacia).
Confocal microscopy observations
RK13 cells were seeded onto Collagen type I-coated cover slips in 6-well dishes and transiently transfected the following day with expression plasmids as indicated using Lipofectamine plus (Invitrogen). Where indicated, cells were infected the following day with SFV (MOI = 10). At 48 and 72 h post-transfection (24 and 48 h post-infection), cells were fixed in 3.7% formaldehyde, permeabilized with 0.5% Saponin (Sigma), blocked with 0.5% Saponin, 1.5% BSA, and 1.5% normal goat serum (Zymed), and immunostained with rabbit polyclonal anti-myc antibodies (1:500, Santa Cruz Biotech), mouse monoclonal anti-Flag M2 antibodies (1:3,500, Sigma) and secondary Alexa Fluor 488 and 546 conjugated antibodies (1:200, Molecular Probes) in PBS, supplemented with 0.5% Saponin, 1.5% BSA, and 1.5% normal goat serum (Zymed). The nucleus is visualized by incubation with ToPro-3 (Molecular Probes) and the actin cytoskeleton with Alexa Fluor-conjugated phalloidin. After three washes with PBS, supplemented with 0.5% Saponin, samples were mounted with Vectashield (Vectorlabs) and images were acquired by confocal laser-scanning microscopy (Zeiss LSM510).
Luciferase reporter gene assay
About 5 × 104 HEK293N cells cultured in 5% serum in 96-well plates were transfected in triplicates with the indicated expression plasmids using Polyfect transfection reagent (Qiagen) with a total of 500 ng plasmid DNA (normalized for total DNA), including 75 ng of pNF-κB-LUC (Stratagene) and 3 ng of a Renilla luciferase gene driven by a constitutive TK promoter (pRL-TK; Promega). At 36 h post-transfection, cells were treated where indicated with 20 ng/ml TNFα (Biomol) for 8 h and directly analyzed using the Dual Glow Luciferase kit (Promega) in a Genios multimode plate reader (Tecan).
IL-1β secretion assay
HEK293N cells were transiently transfected with the indicated expression plasmids in 24-well plates using the Polyfect transfection reagent (Qiagen). Twenty-four hours (24 h) post-transfection, the culture medium was replenished with 0.5 ml of fresh culture medium. Thirty-six hours (36 h) post-transfection, IL-1β secreted into the culture supernatants was measured by ELISA using a commercial kit (BD Pharmingen), normalizing data for cell number, as determined by crystal violet assay, and precise culture volume, and performing assays in triplicates.
Results
Poxviruses encode PYD-only proteins
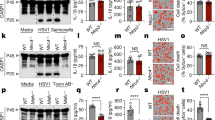
Viruses are known to interfere with host immune and apoptosis pathways. Since the PYD emerged recently as a key domain in innate immunity, we performed database mining to identify potential virus-encoded PYD-containing proteins. Using this approach, we identified open reading frames in several poxviruses that encode potential viral PYD-only proteins (vPOPs) (Fig. 1A). Clustal W alignment of the deduced amino acid sequences of the rabbit Myxoma virus (MV) POP (M013L) [20], the Shope Fibroma virus (SFV) POP (gene: S013L, protein gp013L) [21], the Swinepox virus (SPV) POP (SPV14L) [22], the Yaba-like disease virus (YLDV) POP (18L) [23], and the Mule deer poxvirus (DpV) POP (DPV83gp024) [24] show significant homologies to cPOP1 and the PYD of ASC (Fig. 1B). The M013L and gp013L proteins are only 59.5% identical (65% conserved) to each other. To verify that S013L is also transcribed in cells infected with poxviruses, we performed RT-PCR analysis of S013L, due to the lack of specific antibodies. Total RNA was isolated from RK13 cells and RK13 cells infected with the SFV for 16 h, and transcribed into first strand cDNAs. Subsequent PCR analysis using S013L-specific primers demonstrated the presence of vPOP-specific transcripts only in SFV-infected cells, which show the typical cytopathic effects (Fig. 1C, lane 3). Primer specificity for vPOP versus cPOP is shown by the absence of PCR product in non-infected cells (Fig. 1C, lane 2). Viral genomic DNA-contamination was excluded by omitting cDNA from the reverse transcription in the PCR reaction, which did not amplify a PCR product (Fig. 1C, lane 4).
The Shope Fibroma virus encodes a PYD-only protein. Comparison of viral and cellular PYD-only proteins. (A) Schematic representation of Myxoma virus (MV) POP (M013L), the Shope Fibroma virus (SFV) POP (gp013L), the Swinepox virus (SPV) POP (SPV14L), the Yaba-like disease virus (YLDV) POP (18L), the Mule deer poxvirus (DpV) POP (DPV83gp024), cPOP1, and ASC. (B) Clustal W alignment of M013L, gp013L, SPV14L, 18L, DPV83gp024, cPOP1 and the PYD of ASC. Black and gray boxes indicate identical and similar (conserved) amino-acid residues, respectively. The α-helices, as determined for the PYD of ASC are marked on top [19]. (C) The presence of S013L-specific transcripts was determined in RK13 cells before and 16 h post-infection with the rabbit Fibroma virus (SFV) (MOI = 10) by RT-PCR. RT-PCR was performed with S013L-specific primers and primers specific for β-actin. RT: reverse transcriptase. A representative phase contrast image of uninfected RK13 cells (top) and RK13 cells infected with SFV (bottom) is shown. (D) Myc-tagged expression constructs for cPOP1, SFV gp013L, and MV M013L were transiently transfected into HEK293T cells. Thirty-six hours (36 h) post-transfection, normalized cell lysates were separated by SDS/PAGE and analyzed by immunoblot for expression of myc-tagged proteins
For further studies we amplified the ORF of S013L and M013L by PCR directly from virus particles, cloned it into mammalian expression vectors in frame with a myc-epitope tag and sequence verified these vPOPs. Transient transfection of S013L and M013L in HEK293 cells resulted in expression of vPOPs of the predicted molecular weight (Fig. 1D). Myc-tagged cPOP1 was used as a control.
gp013L co-localizes with the central PYD-containing adaptor protein ASC
The central adaptor protein ASC binds to activated PYD-NLR-family proteins and mediates activation of downstream effectors by PYD-PYD interactions. ASC frequently localizes to characteristic aggregates, referred to as specks [6], where ASC interacting proteins such as cPOP1 are frequently recruited [15]. To investigate whether SFV gp013L co-localizes with ASC similar to cPOP1 and MV M013L, we performed immunofluorescence analysis of cellular and viral POPs in the absence or presence of ASC. Myc-tagged SFV gp013L (Fig. 2A-a), or myc-tagged MV M013L (Fig. 2B-a) alone localized to diffusively throughout the cytoplasm and the nucleus at 48 h post-transfection. At 72 h post-transfection, SFV gp013L (Fig. 2A-b) and MV M013L (Fig. 2B-b) localized to punctuate, vesicular structures in the cytoplasm. To investigate whether virus infection impacts the localization of vPOPs, we transiently transfected SFV gp013L into RK13 cells, followed by infection with SFV. Twenty-four hours (24 h) post-infection SFV gp013L started to aggregate in the cytoplasm of infected cells (Fig. 2A-c), with stronger aggregation visible at 48 h post-infection (Fig. 2A-d).
SFV gp013L co-localizes with ASC. Localization of epitope-tagged proteins was analyzed following transient transfection into RK13 cells. (A) Localization of SFV gp013L. Myc-tagged SFV gp013L was immunostained with a rabbit polyclonal anti-myc antibody and visualized with an Alexa Fluor 488-conjugated anti-rabbit antibody 48 h post-transfection (panel a) and 72 h post-transfection (panel b). Actin was visualized with Alexa Fluor 546-conjugated phalloidin and the nucleus was stained with ToPro-3. Shown is from left to right: Myc-tagged SFV gp013L (green), nucleus (blue), actin (red), and a merged image. Twenty-four hours (24 h) post-transfection cells were also infected with SFV (MOI = 10) and cells were immunostained as described above at 24 (panel c) and 48 h (panel d) post-infection (48 and 72 h post-transfection). Myc-tagged SFV gp013L and Flag-tagged ASC were immunostained 72 h post-transfection with rabbit polyclonal anti-myc and mouse monoclonal anti-Flag antibodies and visualized with Alexa Fluor 488 and 546-conjugated anti-rabbit and mouse antibodies, respectively. The nucleus was stained with ToPro-3. Shown is from left to right: Myc-tagged SFV gp013L (green), Flag-tagged ASC (red), nucleus (blue), and a merged image (panel e). Twenty-four hours (24 h) post-transfection cells were also infected with SFV (MOI = 10) and cells were immunostained as described above at 48 h post-infection (72 h post-transfection) (panel f). (B) Localization of MV M013L. Myc-tagged MV M013L was immunostained with a rabbit polyclonal anti-myc antibody and visualized with an Alexa Fluor 488-conjugated anti-rabbit antibody 48 h post-transfection (panel a) and 72 h post-transfection (panel b). Actin was visualized with Alexa Fluor 546-conjugated phalloidin and the nucleus was stained with ToPro-3. Shown is from left to right: Myc-tagged MV M013L (green), nucleus (blue), actin (red), and a merged image. Myc-tagged MV M013L and Flag-tagged ASC were immunostained 72 h post-transfection with rabbit polyclonal anti-myc and mouse monoclonal anti-Flag antibodies and visualized with Alexa Fluor 488 and 546-conjugated anti-rabbit and mouse antibodies, respectively. The nucleus was stained with ToPro-3. Shown is from left to right: Myc-tagged MV M013L (green), Flag-tagged ASC (red), nucleus (blue), and a merged image (panel c)
Also cPOP1 showed cytoplasmic and nuclear localization, and co-expression of cPOP1 and ASC leads to recruitment of cPOP1 into ASC-formed specks [15]. Therefore, localization of vPOPs to these structures was investigated. Co-expression of Flag-tagged ASC resulted in co-localization of SFV gp013L (Fig. 2A-e) and MV M013L (Fig. 2B-c) proteins with ASC in the characteristic specks. Infection of RK13 cells with SFV did not impact the localization of ASC and SFV gp013L, likely because these proteins already localize to specks (Fig. 2A-f).
gp013L interacts with the central PYD-containing adaptor protein ASC
cPOP1 co-localizes with ASC into ASC-formed specks and has been identified as an ASC-binding protein. Since SFV gp013L, and MV M013L co-localized with ASC, their capability to interact with each other was investigated. Myc-tagged SFV gp013L or MV M013L were co-expressed in HEK293T cells with HA-tagged ASC by transient co-transfection. Co-immunoprecipitation experiments of cleared protein lysates with anti-HA sepharose demonstrated the presence of myc-tagged SFV gp013L or MV M013L in these immune complexes, further indicating that SFV gp013L and MV M013L form complexes with ASC in vivo, reminiscent to cPOP1 (Fig. 3A).
SFV gp013L associates with ASC. (A) In vivo binding between ASC and SFV gp013L. HEK293T cells were transiently transfected with HA-tagged ASC, myc-tagged SFV gp013L, and myc-tagged MV M013L, as indicated. Thirty-six hours (36 h) post-transfection, clarified and normalized cell lysates were subjected to co-immunoprecipitation using immobilized anti-HA antibodies (Santa Cruz Biotechnology). Immune complexes were separated by SDS/PAGE transferred onto PVDF membranes and probed with anti-myc antibodies directly conjugated to HRP. Membranes were stripped and reprobed with anti-HA-HRP antibodies. Ten percent (10%) of the total lysate was run alongside the immunoprecipitation (IP). WB: western blot. (B) In vitro binding between ASC and SFV gp013L. SFV gp013L and MV M013L were in vitro translated, labeled with biotin, and subjected to in vitro GST-pull down assays using GST-ASC-CARD, GST-ASC-PYD, and GST immobilized to GSH sepharose, as indicated. Protein complexes were separated by SDS/PAGE, transferred onto PVDF membranes and bound proteins were visualized by immunoblotting with streptavidin-HRP and ECL-Plus (Amersham Pharmacia Biotech) detection. A membrane is stained with coomassie blue to visualize the GST fusion proteins. An asterisk denotes two degradation products, which is present in the ASC-PYD GST fusion protein purification, but which is not affecting this assay. A molecular weight standard is indicated to the right
PYD-containing proteins usually associate by PYD-PYD interaction. POPs only contain the PYD, but ASC consists of a PYD and a CARD [5, 6]. To map the interaction domain of ASC and SFV gp013L or MV M013L, we performed in vitro GST-pull down assays. Recombinant proteins of the PYD or the CARD of ASC were expressed as GST fusion proteins in E. coli BL21 and affinity purified, whereas SFV gp013L and MV M013L were generated by in vitro translation. These in vitro interaction studies further confirmed that SFV gp013L and MV M013L interact with the PYD of ASC, while no interaction was observed with the CARD of ASC or the GST control (Fig. 3B). These results demonstrated that SFV gp013L and MV M013L directly interact with ASC by PYD-PYD interaction.
gp013L interferes with PYD-mediated activation of Caspase-1
The best-characterized effector protein downstream of the PYD signal transduction pathway is Caspase-1. Interaction of cPOP1 with ASC impairs PYD-mediated activation of downstream effectors by preventing association with upstream PYD-NLR-family proteins [15]. Since vPOPs share significant homology with cPOP1, we investigated the effect of SFV gp013L or MV M013L interaction with ASC on PYD-mediated activation of Caspase-1. The PYD signal transduction pathway was reconstituted in HEK293N cells, which are deficient in endogenous components of this pathway, including ASC. Co-expression of a constitutively active Cryopyrin R260W (a PYD-NLR-family member), ASC, pro-Caspase-1, and pro-IL-1β leads to activation of the PYD-mediated signal transduction pathway, and can be measured by secretion of bioactive IL-1β. Co-expression of the disease-associated Cryopyrin mutant R260W interacts with ASC even in the absence of a ligand, promotes ASC oligomerization, activation of pro-Caspase-1, and subsequently IL-1β secretion [25]. Co-expression of either SFV gp013L (Fig. 4A) or MV M013L (Fig. 4B) in this system impaired ASC-mediated activation of Caspase-1 in a dose-dependent manner and reduced activity of Caspase-1 to baseline, as measured by secretion of bioactive IL-1β, which is processed from pro-IL-1β into bioactive IL-1β by Caspase-1. Expression of all proteins, except IL-1β, which we measured by ELISA, was verified by immunoblot (Fig. 4C).
SFV gp013L inhibits Caspase-1-mediated processing of pro-IL-1β. HEK293N cells were transiently transfected in triplicates with expression constructs for pro-Caspase-1, murine pro-IL-1β, Cryopyrin (R260W), ASC, and SFV gp013L (A) or MV M013L (B), as indicated. Thirty-six hours (36 h) post-transfection, secreted IL-1β was measured by ELISA (BD Pharmingen) from normalized culture supernatants using a standard curve generated with a recombinant IL-1β. Data are presented as picograms per milliliter of secreted IL-1β (mean SD; n = 3). (C) HEK293N cells were transfected with the indicated expression constructs using the concentrations as used for the IL-1β assay (A, B), and cleared protein lysates were separated by SDS/PAGE and immunoblotted with antibodies to detect expression of pro-caspase-1, Cryopyrin (R260W), ASC, SFV gp013L, and MV M013L. (+ and ++ indicate the two different expression levels of viral POPs)
gp013L promotes activation of the transcription factor NF-κB
Recruitment of ASC to activated PYD-NLR-family members, such as Cryopyrin, has also been implicated in the activation of the transcription factor NF-κB, though this finding is not yet supported by animal studies [17, 26]. cPOP1 can interfere with the interaction of ASC and Cryopyrin, thereby impairing oligomerization of ASC and subsequently activation of NF-κB [15]. Therefore, the effect of SFV gp013L and MV M013L on NF-κB activation was investigated. Expression of SFV gp013L and MV M013L alone potently activated NF-κB, in contrast to cPOP1 (Fig. 5A). In contrast to the inhibitory effect of cPOP1 on NF-κB activation, neither SFV gp013L (Fig. 5B) nor SFV M013L (Fig. 5C) could impair Cryopyrin (R260W) and ASC-mediated NF-κB activation, but rather enhanced activation of NF-κB in a dose-dependent manner. SFV gp013L and MV M013L also enhanced TNFα-mediated activation of NF-κB (Fig 5D), further confirming the positive impact of vPOPs on the NF-κB activation pathway.
SFV gp013L activates the transcription factor NF-κB. HEK293N cells were transiently transfected in 96-well plates in triplicates with the indicated expression constructs, including pNF-κB-LUC (Stratagene) and pRL-TK (Promega), keeping the total amount of DNA constant. (A) Cells were transfected with either a control plasmid or SFV gp013L or MV M013L expression plasmids, as indicated. (B) Cells were transfected with a control plasmid or ASC and Cryopyrin (R260W) in the presence or absence of increasing amounts of SFV gp013L, or cPOP1. (C) Cells were transfected with a control plasmid or ASC and Cryopyrin (R260W) in the presence or absence of increasing amounts of MV M013L, or cPOP1. (D) Cells were transfected with a control plasmid or SFV gp013L or MV M013L. Where indicated, 36 h post-transfection, cells were treated for 8 h with 20 ng/ml recombinant human TNFα. Samples were analyzed using the Dual Glow Luciferase kit (Promega) in a Genios multimode plate reader (Tecan). Results are presented as fold induction of NF-κB relative to control transfected cells not induced with TNFα, normalized to tymidine kinase (TK) reporter gene activity (mean SD; n = 3)
Discussion
PYD-containing proteins are important mediators of inflammatory responses during host defense. In humans, 16 potential PYD-containing pathogen recognition receptors (PYD-NLR-family members) have been recognized, including Cryopyrin. It is hypothesized that these proteins are activated by pathogen-associated molecular patterns (PAMPs) [1–4], and some examples of bacterial and recently viral ligands for PYD-NLRs have been specifically identified [27–31]. Activation of PYD-NLR-family proteins results in oligomerization and recruitment of the adaptor protein ASC via PYD-PYD interactions [25, 32]. Subsequently, ASC links pathogen recognition to downstream effector activation, including Caspase-1-mediated processing and secretion of IL-1β, IL-18, and possibly IL-33 [27, 33, 34]. NF-κB activation has been proposed as another effector of PYD signal transduction. The PYD-mediated signal transduction pathway can be modulated by the cPOP1, which impairs the interaction of PYD-NLR-family members and ASC and subsequent activation of downstream effectors [15].
In this study, we characterized a poxviral-encoded PYD-containing protein, which is the product of S013L in the SFV genome (gp013L). As a control the product of M013L in the MV genome (M013L) was used. MV M013L has been recently shown to inhibit IL-1β secretion [16]. The SFV gp013L and MV M013L proteins are only 59.5% identical (65% conserved), but nevertheless function identically with respect to Caspase-1 and NF-κB activation. Poxviruses are large dsDNA containing viruses, which are well known to encode immune evasive proteins to impair the host response upon infection [35, 36]. These immunomodulatory genes usually lack conservation between poxvirus family members, which may reflect differences in host tropism. Poxviruses are known to hijack cellular proteins that are then employed to evade immune recognition and to impair apoptosis of infected host cells. Many crucial pathways of the cellular and humoral host defense can be targeted [35, 36] and the fact that poxviruses also target PYD proteins further emphasizes the significance of this signal transduction pathway for host defense. SFV and MV encode inhibitors of the PYD-mediated signal transduction pathway called PYD-only proteins, SFV gp013L and MV M013L, respectively. Additional members were identified in the genome of SPV, YLDV, and DpV, suggesting that these genes encode a novel class of common immune evasive proteins. To discriminate the viral and cellular POPs, we propose to rename POP1 to cPOP1. Like the cellular homolog, vPOPs interact with ASC, the central adaptor protein of the PYD-signal transduction pathway. The interaction is mediated by PYD-PYD, but not PYD-CARD interaction, as shown by in vitro GST pull down assays, and it appears that the binding of SFV gp013L to ASC is slightly stronger than that of MV M013L to ASC.
ASC frequently localizes to characteristic structures in the cytoplasm or forms characteristic cytoplasmic aggregates, known as specks, to which ASC-interacting proteins are frequently recruited. However, the physiological function of specks is not known. Initially vPOPs localize diffusively throughout the cell, and are found inside vesicular cytoplasmic structures 72 h post-transfection. Interestingly, infection of RK13 cells with SFV caused aggregation of SFV gp013L in a time-dependent manner. Co-expression with ASC caused recruitment of vPOPs into ASC-formed specks, reminiscent of cPOP1 [15]. cPOP1 localizes to the cytoplasm and the nucleus, and co-expression with ASC causes redistribution of cPOP1 into ASC formed specks [15]. Since SFV gp013L already co-localized with ASC into specks, infection of RK13 cells with SFV did not alter their localization.
The best-characterized PYD-activated downstream effector is Caspase-1. Activation of Caspase-1 is required for the processing, activation and subsequent secretion of the proinflammatory cytokines pro-IL-1β, pro-IL-18, and pro-IL-33 [37, 38]. Activation of Caspase-1 requires prior activation of PYD-NLR-family proteins by their cognate ligands, subsequent recruitment of ASC, and formation of inflammasomes. Both SFV gp013L and MV M013L impaired Cryopyrin and ASC-mediated activation of Caspase-1, which might be explained by the impaired formation of an inflammasome containing ASC and Cryopyrin in the presence of vPOPs. The ability of vPOPs to impair Caspase-1 activation in response to other than Cryopyrin containing inflammasomes will need further investigation. Apart from vPOPs, poxviruses are known to encode other proteins that interfere with the Caspase-1-IL-1β pathway. Several poxviruses encode soluble IL-1 receptors, which bind only to IL-1β. The cellular receptor, however, binds IL-1α, IL-1β and IL-1RA, suggesting that specific inhibition of IL-1β is crucial during poxvirus infection [35, 36]. MV and SFV encode Serp2, which shows similarity to the cowpox virus crmA, and also inhibits Caspase-1 [35, 36]. Poxviruses acquired mechanisms to impair the generation and function of IL-1β at three different levels: (1) at the inflammasome formation and signal transduction leading to Caspase-1 activation, (2) directly at Caspase-1 activation, and (3) at the binding of IL-1β to its receptor. Blocking three key steps by three distinct poxviral-encoded proteins emphasizes the importance of this cytokine for host defense. Poxviruses also encode proteins, such as IL-18BPs, to interfere with IL-18, a cytokine that also requires Caspase-1 for processing [35, 36].
Expression of vPOPs strongly promoted activation of NF-κB, which is in contrast to cPOP1 [15]. POPs most likely impair the PYD-PYD association between ASC and PYD-NLR-family proteins, thereby disrupting the pathway leading to the activation of these effectors, which occurs in the inflammasome. It is therefore surprising that NF-κB activation is not impaired by vPOPs, suggesting selectivity for Caspase-1 activation. We recently demonstrated that ASC can compete with Cardiak (Rip2) for pro-Caspase-1 binding [18], and a recent study suggested that ASC interaction with Caspase-1 prevents the interaction of Caspase-1 with Cardiak, and that this interaction is required for the activation of NF-κB [39]. According to this model, the disruption of the PYD-NLR-ASC interaction by vPOPs might also impair the subsequent interaction of ASC with pro-Caspase-1. As a result, pro-Caspase-1 would be available to associate with Cardiak to activate NF-κB. Upon infection, poxviruses, including the MV, potentially block the NF-κB activation pathway using virus-encoded conserved ankyrin-containing proteins, such as the critical virulence factor M150R (MNF), which function analogous to cellular IκBs [40]. PYD proteins link to the NF-κB activation pathway at the level of the IKK complex, and also Caspase-8 has been implicated in this pathway [17, 26, 41, 42]. However, many viruses activate NF-κB, which establishes a more efficient infection, which promotes viral replication and prevents virus-induced apoptosis of infected cells [43]. High and constitutive activation of NF-κB has also been linked to tumorigenesis, and MV and SFV are tumorigenic in their natural hosts, and activation of NF-κB by virulence factors could support tumor development by promoting proliferation and impairing the apoptotic host response [44].
Cryopyrin, one of the PYD-NLR-family proteins for which ligands have been identified, senses several bacterial-derived PAMPs [27–30]. A very recent report also provides evidence that Cryopyrin is required for Caspase-1 activation in response to viral infections and recognizes viral-derived PAMPs, such as dsRNA [31]. The observation that poxviruses encode immune evasive proteins that target the PYD-signal transduction pathway, further support that viral PAMPs might function as ligands for some PYD-NLRs. In summary, our data provide evidence for the existence of a SFV-encoded gp013L, which in spite of the rather low sequence similarity compared to the MV-encoded M013L, functions identically by inhibiting activation of Caspase-1 and activating NF-κB. Virus-encoded PYD proteins represent a novel class of conserved immune evasive proteins, which target the host inflammasome. This observation further emphasizes the importance of PYD proteins for host defense and support that viral-derived PAMPs are recognized by the inflammasome.
References
C. Stehlik, J.C. Reed, J. Exp. Med. 200, 551–558 (2004)
J. Tschopp, F. Martinon, K. Burns, Nat. Rev. Mol. Cell Biol. 4, 95–104 (2003)
J.P. Ting, B.K. Davis, Annu. Rev. Immunol. 23, 387–414 (2004)
N. Inohara, G. Nunez, Nat. Rev. Immunol. 3, 371–381 (2003)
K.E. Conway, B.B. McConnell, C.E. Bowring, C.D. Donald, S.T. Warren, P.M. Vertino, Cancer Res. 60, 6236–6242 (2000)
J. Masumoto, S. Taniguchi, K. Ayukawa, H. Sarvotham, T. Kishino, N. Niikawa, E. Hidaka, T. Katsuyama, T. Higuchi, J. Sagara, J. Biol. Chem. 274, 33835–33838 (1999)
F. Martinon, K. Burns, J. Tschopp, Mol. Cell. 10, 417–426 (2002)
S. Brydges, D.L. Kastner, Curr. Top. Microbiol. Immunol. 305, 127–160 (2006)
S.-H. Lee, C. Stehlik, J.C. Reed, J. Biol. Chem. 276, 34495–34500 (2001)
E.W. Humke, S.K. Shriver, M.A. Starovasnik, W.J. Fairbrother, V.M. Dixit, Cell 103, 99–111 (2000)
A. Druilhe, S.M. Srinivasula, M. Razmara, M. Ahmad, E.S. Alnemri, Cell Death Differ. 8, 649–657 (2001)
M. Lamkanfi, G. Denecker, M. Kalai, K. D’Hondt, A. Meeus, W. Declercq, X. Saelens, P. Vandenabeele, J. Biol. Chem. 279, 51729–51738 (2004)
G. Condorelli, G. Vigliotta, A. Cafieri, A. Trencia, P. Andalo, F. Oriente, C. Miele, M. Caruso, P. Formisano, F. Beguinot, Oncogene 18, 4409–4415 (1999)
A. Krueger, I. Schmitz, S. Baumann, P.H. Krammer, S. Kirchhoff, J. Biol. Chem. 276, 20633–20640 (2001)
C. Stehlik, M. Krajewska, K. Welsh, S. Krajewski, A. Godzik, J.C. Reed, Biochem. J. 373, 101–113 (2003)
J.B. Johnston, J.W. Barrett, S.H. Nazarian, M. Goodwin, D. Ricuttio, G. Wang, G. McFadden, Immunity 23, 587–598 (2005)
C. Stehlik, L. Fiorentino, A. Dorfleutner, J.M. Bruey, E.M. Ariza, J. Sagara, J.C. Reed, J. Exp. Med. 196, 1605–1615 (2002)
C. Stehlik, S.H. Lee, A. Dorfleutner, A. Stassinopoulos, J. Sagara, J.C. Reed, J. Immunol. 171, 6154–6163 (2003)
E. Liepinsh, R. Barbals, E. Dahl, A. Sharipo, E. Staub, G. Otting, J. Mol. Biol. 332, 1155–1163 (2003)
C. Cameron, S. Hota-Mitchell, L. Chen, J. Barrett, J.X. Cao, C. Macaulay, D. Willer, D. Evans, G. McFadden, Virology 264, 298–318 (1999)
D.O. Willer, G. McFadden, D.H. Evans, Virology 264, 319–343 (1999)
C.L. Afonso, E.R. Tulman, Z. Lu, L. Zsak, F.A. Osorio, C. Balinsky, G.F. Kutish, D.L. Rock, J. Virol. 76, 783–790 (2002)
H.J. Lee, K. Essani, G.L. Smith, Virology 281, 170–192 (2001)
C.L. Afonso, G. Delhon, E.R. Tulman, Z. Lu, A. Zsak, V.M. Becerra, L. Zsak, G.F. Kutish, D.L. Rock, J. Virol. 79, 966–977 (2005)
T.A. Dowds, J. Masumoto, L. Zhu, N. Inohara, G. Nunez, J. Biol. Chem. 279, 21924–21928 (2004)
G.A. Manji, L. Wang, B.J. Geddes, M. Brown, S. Merriam, A. Al-Garawi, S. Mak, J.M. Lora, M. Briskin, M. Jurman, J. Cao, P.S. DiStefano, J. Bertin, J. Biol. Chem. 277, 11570–11575 (2002)
F.S. Sutterwala, Y. Ogura, M. Szczepanik, M. Lara-Tejero, G.S. Lichtenberger, E.P. Grant, J. Bertin, A.J. Coyle, J.E. Galan, P.W. Askenase, R.A. Flavell, Immunity 24, 317–327 (2006)
T.D. Kanneganti, N. Ozoren, M. Body-Malapel, A. Amer, J.H. Park, L. Franchi, J. Whitfield, W. Barchet, M. Colonna, P. Vandenabeele, J. Bertin, A. Coyle, E.P. Grant, S. Akira, G. Nunez, Nature 440, 232–236 (2006)
F. Martinon, V. Petrilli, A. Mayor, A. Tardivel, J. Tschopp, Nature 440, 237–241 (2006)
S. Mariathasan, D.S. Weiss, K. Newton, J. McBride, K. O’Rourke, M. Roose-Girma, W.P. Lee, Y. Weinrauch, D.M. Monack, V.M. Dixit, Nature 440, 228–232 (2006)
T.D. Kanneganti, M. Body-Malapel, A. Amer, J.H. Park, J. Whitfield, Z.F. Taraporewala, D. Miller, J.T. Patton, N. Inohara, G. Nunez, J. Biol. Chem.281, 36560–36568 (2006)
J.W. Yu, J. Wu, Z. Zhang, P. Datta, I. Ibrahimi, S. Taniguchi, J. Sagara, T. Fernandes-Alnemri, E.S. Alnemri, Cell Death Differ. 13, 236–249 (2006)
S. Mariathasan, K. Newton, D.M. Monack, D. Vucic, D.M. French, W.P. Lee, M. Roose-Girma, S. Erickson, V.M. Dixit, Nature 430, 213–218 (2004)
M. Yamamoto, K. Yaginuma, H. Tsutsui, J. Sagara, X. Guan, E. Seki, K. Yasuda, M. Yamamoto, S. Akira, K. Nakanishi, T. Noda, S. Taniguchi, Genes Cells 9, 1055–1067 (2004)
B.T. Seet, J.B. Johnston, C.R. Brunetti, J.W. Barrett, H. Everett, C. Cameron, J. Sypula, S.H. Nazarian, A. Lucas, G. McFadden, Annu. Rev. Immunol. 21, 377–423 (2003)
B. Moss, J.L. Shisler, Semin. Immunol. 13, 59–66 (2001)
G. Fantuzzi, C.A. Dinarello, J. Clin. Immunol. 19, 1–11 (1999)
J. Schmitz, A. Owyang, E. Oldham, Y. Song, E. Murphy, T.K. McClanahan, G. Zurawski, M. Moshrefi, J. Qin, X. Li, D.M. Gorman, J.F. Bazan, R.A. Kastelein, Immunity 23, 479–490 (2005)
A. Sarkar, M. Duncan, J. Hart, E. Hertlein, D.C. Guttridge, M.D. Wewers, J. Immunol. 176, 4979–4986 (2006)
C. Camus-Bouclainville, L. Fiette, S. Bouchiha, B. Pignolet, D. Counor, C. Filipe, J. Gelfi, F. Messud-Petit, J. Virol. 78, 2510–2516 (2004)
L. Wang, G.A. Manji, J.M. Grenier, A. Al-Garawi, S. Merriam, J.M. Lora, B.J. Geddes, M. Briskin, P.S. DiStefano, J. Bertin, J. Biol. Chem. 277, 29874–29880 (2002)
M. Hasegawa, R. Imamura, T. Kinoshita, N. Matsumoto, J. Masumoto, N. Inohara, T. Suda, J. Biol. Chem. 280, 15122–15130 (2005)
J. Hiscott, H. Kwon, P. Genin, J. Clin. Invest. 107, 143–151 (2001)
M. Karin, Y. Cao, F.R. Greten, Z.-W. Li, Nat. Rev. Cancer 2, 301–310 (2002)
Acknowledgments
The project described was supported by Grant Number 5P20-RR-016440 (CS, DCF) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and Grant Numbers 1R21-AI-067680 (CS), 1R03-AI-067806 (CS), and R 01-AI-56324 (JCR) from NIAID/NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. We are also grateful to the support from the Alexander Bland Osborn Cancer Center Endowment (CS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Andrea Dorfleutner and Siera J. Talbott contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dorfleutner, A., Talbott, S.J., Bryan, N.B. et al. A Shope Fibroma virus PYRIN-only protein modulates the host immune response. Virus Genes 35, 685–694 (2007). https://doi.org/10.1007/s11262-007-0141-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-007-0141-9