Abstract
We found that an L 3 resistance-breaking field isolate of Pepper mild mottle virus (PMMoV), designated PMMoV-Is, had two amino acid changes in its coat protein (CP), namely leucine to phenylalanine at position 13 (L13F) and glycine to valine at position 66 (G66V), as compared with PMMoV-J, which induces a resistance response in L 3-harboring Capsicum plants. The mutations were located to a CP domain corresponding to the outer surface of PMMoV particles in computational molecular modeling. Analyses of PMMoV CP mutants containing either or both of these amino acid changes revealed that both changes were required to efficiently overcome L 3-mediated resistance with systemic necrosis induction. Although CP mutants containing either L13F or G66V could not efficiently overcome L 3-mediated resistance, these amino acid changes had different effects on the elicitor activity of PMMoV CP. L13F caused a slight reduction in the elicitor activity, resulting in virus restriction to necrotic local lesions that were apparently larger than those induced by wild-type PMMoV, while G66V rendered wild-type PMMoV the ability to overcome L 3-mediated resistance, albeit with a lower efficiency than PMMoV with both changes. These results suggest that a cooperative effect of the L13F and G66V mutations on the elicitor activity of CP is responsible for overcoming the L 3-mediated resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant disease resistance has been shown to be activated under a gene-for-gene relationship, in which a plant disease resistance gene product recognizes a specific avirulence gene product, serving as a resistance elicitor, of pathogens, including viruses [1]. The hypersensitive response (HR) is a well-characterized active defense response involving the activation of plant defense-related pathways that lead to cell death and restriction of the pathogen to the initial site of infection [2]. In Capsicum species, four allelic genes, L 1, L 2, L 3, and L 4, are involved in resistance against Tobamovirus infection [3, 4]. This L-mediated resistance is accompanied by the induction of necrotic local lesions via the HR and increased expressions of a large number of genes, including defense-related genes, i.e., PR-protein genes such as PR1 and PR4b, and HR-related genes, such as HSR203J and HIN1 [5]. The coat protein (CP) of tobamoviruses has been shown to act as the elicitor of L-mediated resistance [6–9]. Several amino acid changes in Pepper mild mottle virus (PMMoV) CP responsible for overcoming L 3-mediated resistance have been identified by analyzing chimeric or point-mutated viruses [7, 10, 11].
One of the best-characterized interactions between a Tobamovirus CP and a plant disease resistance gene is that between Tobacco mosaic virus (TMV) CP and the N′ resistance gene from Nicotiana sylvestris [12–15]. Culver and colleagues analyzed a large set of TMV CP mutants and suggested that the quaternary structure of CP determined by the alpha-helical bundle is essential for resistance elicitation in the TMV-N′ system [16–18] and also other systems [19]. Consistent with these findings, other studies have reported that mutations at positions 43, 50 [10], and 81 [11] in the alpha-helical bundle of PMMoV CP confer the capacity to break L 3-mediated resistance (Fig. 1). In contrast, others and we have demonstrated that some mutations outside the alpha-helical bundle region at positions 7, 10 [11], and 138 [7] also affect the elicitor activity of PMMoV CP (Fig. 1), suggesting that not only the quaternary structure but also the tertiary structure of CP affect host recognition of the molecule. Since these non-alpha-helical bundle mutations are located to the outer surface region of PMMoV particles in computational molecular modeling (Fig. 1), the sites of these mutations may represent parts of the host recognition surface of the virus. Unfortunately, only a few such mutations have been available to date, and it is therefore of great importance to analyze further mutations in the non-helical bundle and surface regions of PMMoV CP to further elucidate the mechanism underlying the recognition of PMMoV by L resistance genes.
Structural models of PMMoV CP. (a–h) Ribbon diagrams of the alpha-carbon backbones (a–d) and electrostatic surface potentials with substituted residues shown in the space filling format (e–h) for PMMoV wild-type CP (WT), PMMoV CP with a 13F mutation (L13F), PMMoV CP with a 66V mutation (G66V) and PMMoV CP with 13F, and 66V mutations (L13F/G66V). In panels (a–d), the cyan circles and numbers indicate the amino acid residues and positions involved in the elicitor activity of the CP, respectively, according to Berzal-Herranz et al. [6; residue 138], Hamada et al. [11; residues 7, 10, and 81], Tsuda et al. [10; residues 43 and 50] and the present study (residues 13 and 66). In panels (e–h), the red, blue, and white regions in the electrostatic surface potentials indicate negatively charged, positively charged, and neutral areas, respectively. N, amino terminus; C, carboxyl terminus; LS, left-slewed helices; RS, right-slewed helices; LR, left radial helix; RR, right radial helix
Tobamoviruses are one of the most serious virus pathogens and cause tremendous damage to the quality and yield of Capsicum spp. plants, thereby leading to severe economic loss. To protect Capsicum crops from tobamovirus diseases, tobamovirus-resistant cultivars containing L resistance genes have been bred [20]. However, field cultivation of sweet pepper plants with introduced L 3 in Japan has led to an outbreak of P1,2,3 PMMoV strains that can overcome the resistance [10, 11]. Therefore, surveillance of resistance-breaking PMMoV in the field is of great importance to protect sweet pepper production from virus infections. During surveillance for L 3-mediated resistance-breaking viruses in Iwate Prefecture, Japan, we isolated PMMoV-Is, which was classified as a P1,2,3 pathotype based on its systemic infectivity on Capsicum plants homozygous for L 3.
Sequence analysis of the PMMoV-Is CP gene revealed two novel amino acid changes, as compared with the P1,2 pathotype PMMoV that cannot overcome L 3-mediated resistance. These mutations, leucine to phenylalanine at position 13 (L13F) and glycine to valine at position 66 (G66V), were located to the outer surface region of PMMoV CP. Both of these amino acid changes were required to efficiently overcome L 3-mediated resistance. Interestingly, these two amino acid changes had different effects on the elicitor activity of PMMoV CP. The present results suggest that a cooperative or additive effect of these two weak mutations reduces the elicitor activity of PMMoV CP and renders the virus the ability to systemically infect L 3 plants.
Materials and methods
Analysis of PMMoV field isolates
Total RNA was extracted from leaves with systemic necrosis from pepper plants (Capsicum annuum, L 3/L +) grown in greenhouses in commercial fields in Iwate Prefecture, Japan, after confirming PMMoV infection using a rapid immunofilter paper assay (RIPA; Tohoku Chemical Co. Ltd.). The PMMoV CP gene was amplified by reverse transcription-polymerase chain reaction (RT-PCR) using the specific primers PMF1 and PMR1 (Table 1) as described previously [11]. The RT-PCR products were purified by agarose gel electrophoresis and sequenced in an ABI 3730 DNA sequencer (Applied Biosystems Co.) to determine the nucleotide sequence of the CP gene. PMMoV-Is was obtained by three successive single-lesion transfers on Nicotiana glutinosa and then propagated in Nicotiana benthamiana. Virions were purified by differential centrifugation as described previously [21].
Construction of PMMoV CP mutant cDNA clones
The infectious cDNA clone of PMMoV-Iw (pathotype P1,2), pPIW (EMBL/GenBank/DDBJ accession no. AB254821) was constructed using the primers PIWF1/T7, and PIWR1 (Table 1) as described previously [22]. cDNA clones of the PMMoV mutations identified in PMMoV-Is were constructed using a recombinant PCR method [23] as described previously [11]. Mutated CP genes were constructed as described in Table 1. The mutated CP gene fragments were introduced into pPIW at the SnaB I (residue 5531) and Mlu I (just downstream of the PMMoV-Iw cDNA) sites. The resultant cDNA clones were digested with Mlu I, and RNA transcripts were synthesized in vitro using a RiboMAX Large Scale RNA Production System with T7 RNA polymerase (Promega). Purified PMMoV virions, namely PIW-13F, PIW-66V, PIW-13F66V, and PIW, were prepared from N. benthamiana inoculated with in vitro RNA transcripts from the cDNA clones pPIW-13F, pPIW-66V, pPIW-13F66V, and pPIW, respectively, as previously described [11] and used for infection experiments.
Plant growth and virus infection
C. annuum cv. Shosuke (L +/L +; L +-Ca), C. annuum cv. Verbeterde Glas (L 1/L 1), Capsicum frutescens cv. Tabasco (L 2/L 2), Capsicum chinense PI159236 (L 3/L 3; L 3-Cc), and Capsicum chacoense PI260429 (L 4/L 4) were grown in pots containing a commercial soil mixture (Sakata Seed Co.) in a glasshouse at 24°C. Purified virions (0.5 μg/ml in 10 mM phosphate buffer pH 7.2) of the field isolates, CP mutants (PIW-13F, PIW-66V and PIW-13F66V) and PIW as wild-type PMMoV (P1,2) were used as inoculums. Following mechanical inoculation, the Capsicum plants were maintained in a growth chamber at 25°C with illumination for 16 h/day, and symptom development was recorded every day until 14 or 21 days post-inoculation (dpi). Virus infection of the inoculated and uninoculated upper leaves was assessed in some experiments at 14–21 dpi using a tissue printing immunoassay and an anti-PMMoV antiserum as described previously [11].
Press blot immunoassay
Virus distribution in the inoculated pepper leaves was examined using a press blot immunoassay [24]. The right halves of leaves from L 3-Cc and L +-Ca were inoculated with purified virions (0.5 μg/ml in 10 mM phosphate buffer pH 7.2) of PIW-13F, PIW-66V, or PIW-13F66V, while the left half of each leaf was inoculated with wild-type PIW as a control. Blots were prepared at 3 and 5 dpi and PMMoV CP was detected using a specific antibody as described previously [22].
RNA gel blot analysis
L 3-Cc plants were mechanically inoculated with purified virions of PIW, PIW-13F, PIW-66V, or PIW-13F66V (2 μg/ml in 10 mM phosphate buffer pH 7.2) and maintained in a growth chamber at 25°C with illumination for 16 h/day. Total RNA was extracted from the inoculated leaves at 0, 1, 2, and 3 dpi. Aliquots (5 μg for the detection of PR1-Cc, PR4b-Cc, HSR203J-Cc or HIN1-Cc and 2 μg for the detection of viral RNA and elongation factor (EF) 1 alpha) of the total RNA samples were separated in 1.5% agarose gels under denaturing conditions, and blotted onto Hybond N+ membranes (Amersham Biosciences). The membranes were probed with digoxigenin (DIG)-labeled DNA probes for PR1-Cc, PR4b-Cc, HSR203J-Cc, or HIN1-Cc [5], which were prepared using a PCR DIG Probe Synthesis Kit (Roche). A DIG-labeled DNA probe for PMMoV viral RNA detection was synthesized with pPIW as a template and the primer pair PMF1 and PMR1. A cDNA clone of EF1 alpha (EMBL/GenBank/DDBJ accession no. AB275383) was isolated from L 3-Cc plants by PCR using the primer pair CaEF1acF3 and CaEF1acR3, and used as a template for DIG-labeled probe synthesis with the same primer pair. Hybridization was performed at 50°C for 16 h in ULTRAhyb (Ambion). The targets were detected using an alkaline phosphatase-conjugated anti-DIG antibody (Roche) and the CDP-Star Detection Reagent (Amersham Biosciences).
Computational molecular modeling
Molecular models of PMMoV CP were calculated based on the crystal structure of TMV CP and visualized using DeepView (Swiss-PdbViewer) Version 3.7a1 for OSX (http://www.expasy.org/spdbv/) as described in the user manual.
Results
Characterization of a new field isolate, PMMoV-Is
We isolated PMMoV-Is from sweet pepper plants heterozygous for L 3 (C. annuum, L 3/L +) showing systemic necrosis symptoms in commercial fields. The causal agent of the disease was identified as PMMoV based on immunodetection by the RIPA technique, induction of necrotic local lesions on inoculated leaves of N. glutinosa, which harbors a Tobamovirus resistance gene, N (data not shown), PCR amplification of the CP gene with specific primers, and the nucleotide sequence of the CP gene. The nucleotide sequence of the CP gene amplified from purified virion RNA revealed an open reading frame (ORF) of 474 nucleotides, which exhibited high identity (99.6%) to the P1,2 pathotype PMMoV-J CP gene [25]. A comparison with PMMoV-J revealed two base changes of A to T at position 42 and G to T at position 200 (Fig. 2a; EMBL/GenBank/DDBJ accession no. AB254822), each of which gives rise to an amino acid change, specifically leucine to phenylalanine at position 13 (L13F), and glycine to valine at position 66 (G66V), respectively (Fig. 2b). Since no previous PMMoV strains with these amino acid changes have been described, we designated this new field isolate PMMoV-Is. The infectivity of PMMoV-Is on Capsicum plants carrying different L genes classified PMMoV-Is as the P1,2,3 pathotype (Table 2).
Contributions of L13F and G66V to the pathogenicity of PMMoV-Is
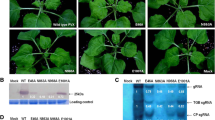
As described above, two amino acid changes, L13F and G66V, were detected between PMMoV-Is CP and P1,2 pathotype PMMoV CP. To investigate whether either or both of these amino acid changes were responsible for the phenotypes (L 3 resistance-breaking, symptomatology etc.), PMMoV CP mutants, designated PIW-13F, PIW-66V, and PIW-13F66V, were constructed. These CP mutants and PIW as a control for P1,2 phenotype PMMoV were tested for pathogenicity in L 3-Cc plants (Table 3). Local and/or systemic infection was observed in these experiments and direct sequencing of the RT-PCR products obtained from locally and systemically infected leaves of the inoculated plants confirmed that the progeny viruses maintained the original sequence in the CP ORF (data not shown). PIW-13F66V, which carried the identical mutations to PMMoV-Is, induced systemic necrosis symptoms in L 3-Cc plants, which were similar to those induced by PMMoV-Is (compare Fig. 3g and h with Fig. 3b and c), indicating that these two mutations were sufficient for breaking L 3 resistance. Similar to the parental PIW, PIW-13F induced necrotic local lesions on inoculated leaves, and did not spread systemically in L 3-Cc (Fig. 3a and d). Although the timing of the lesion appearance and the forms of the lesions induced by PIW-13F were similar to those caused by PIW, the sizes of the lesions induced by PIW-13F in the later phase of infection were larger than those induced by PIW (Fig. 3a and d). PIW-66V induced necrosis along the leaf veins in inoculated leaves, which first appeared at 4–5 dpi and then systemically infected uninoculated upper leaves associated with systemic necrosis, which was observed around 10 dpi and thereafter (Fig. 3e and f). The necrosis induced by PIW-66V in inoculated leaves was delayed in comparison with the necrotic local lesions accompanying the HR induced by PIW, and also differed in shape (compare Fig. 3a and e). However, some L 3-Cc plants inoculated with PIW-66V showed restricted systemic virus infection. To compare the systemic infectivity of PIW-66V and PIW-13F66V in L 3-Cc plants, five L 3-Cc plants were inoculated with each virus, respectively, and observed until 14 dpi. The experiments were repeated five times (Table 3). Systemic PIW-66V infection was only observed in 20–60% of the inoculated L 3-Cc plants in the five independent experiments. Most of the PIW-66V-inoculated plants defoliated the inoculated leaves, which exhibited necrosis, by 7 dpi, whereas plants inoculated with PIW-13F66V hardly did so until at least 14 dpi. Leaf abscission was also observed in plants inoculated with PIW, which induced the HR (data not shown). These results suggest that the introduction of either L13F or G66V into PIW CP is insufficient to efficiently overcome the L 3-mediated resistance, and demonstrate that the combination of both L13F and G66V in CP is responsible for the infectivity and symptomatology of PMMoV-Is in L 3-Cc plants.
Symptoms induced by PMMoV-Is, PIW, PIW-13F, PIW-66V, and PIW-13F66V infection of C. chinense PI159236 (L 3/L 3). (b, c) PMMoV-Is induces chlorosis on inoculated leaves (b) and vein necrosis on upper uninoculated leaves (c). (a, d) PIW (a) and PIW-13F (d) induce necrotic local lesions on inoculated leaves. (e, f) PIW-66V induces necrosis on inoculated leaves (e) and uninoculated upper leaves (f). (g, h) PIW-13F66V induces chlorosis on inoculated leaves (g) and vein necrosis on uninoculated upper leaves (h). The photographs shown were taken at 14 (a–d, f–h) and 7 (e) days post-inoculation
Spread of CP mutants in inoculated leaves of L 3-Cc plants
PMMoV-Is and its derivatives, PIW-13F and PIW-66V, exhibited interesting infection patterns as described above. We previously reported that the L 3-mediated elicitor activity of PMMoV CP is correlated with the virus spread in inoculated leaves [5]. To investigate the effects of L13F and G66V on the virus spread in inoculated L 3-Cc plant leaves, the virus distributions in pepper leaves inoculated with PMMoV CP mutants or PIW were examined by press blot immunoassays using an antibody specific for PMMoV CP. In L +-Ca plants, all the CP mutants showed similar distribution patterns to that of PIW within the inoculated leaves, albeit with slightly delayed spread (Fig. 4c, d, g, h, k, and l), indicating that none of the amino acid changes in the CP mutants significantly affected cell-to-cell movement of the virus in susceptible L +/L + plants. In L 3-Cc leaves, the immunostained spots of PIW-13F were similar in size to those of PIW at 3 dpi (Fig. 4a), but became larger than those of PIW at 5 dpi (Fig. 4b). These results are consistent with the observation of local necrosis symptoms described above, and suggest that although L13F reduces the elicitor activity of CP, its effect is insufficient to overcome L 3-mediated resistance. The PIW-66V and PIW-13F66V spots were larger than those of PIW at 3 dpi (Fig. 4e and i), and continued to increase in size up to at least 5 dpi (Fig. 4f and j). These results suggest that the G66V mutation in CP is sufficient to overcome the L 3-mediated restriction of virus spread in inoculated leaves.
Distributions of PIW, PIW-13F, PIW-66V, and PIW-13F66V in C. chinense PI159236 (L 3/L 3) and C. annuum cv. Shosuke (L +/L +). (a–l) The left half of the each leaf was inoculated with purified virions (0.5 μg/ml) of PIW, while the right half of each leaf was inoculated with purified virions (0.5 μg/ml) of PIW-13F (a–d), PIW-66V (e–h), or PIW-13F66V (i–l). The inoculated leaves were analyzed by press blot immunoassays. Signals for CP were detected at 3 (a, c, e, g, i, k) and 5 (b, d, f, h, j, l) days post-inoculation using an antibody raised against PMMoV
Induction of defense-related and HR-related genes
Tissue blot analyses revealed that PIW-66V spread as efficiently as PIW-13F66V in inoculated L 3-Cc leaves (Fig. 4f and j), although some plants inoculated with PIW-66V showed restricted systemic infection of the virus (Table 3). To clarify the cause of these differences, we analyzed the expression kinetics of PR1-Cc, PR4b-Cc, HSR203J-Cc, and HIN1-Cc following virus infection, since we previously found that the elicitor activity of PMMoV is correlated with the timing and extent of defense-related, and HR-related gene expressions [5]. L 3-Cc plant leaves were inoculated with PIW, PIW-13F, PIW-66V, or PIW-13F66V, or with phosphate buffer (10 mM; pH 7.2) as a mock inoculation. Total RNAs extracted from the inoculated leaves at 0, 1, 2, and 3 dpi were analyzed by northern blotting (Fig. 5). Detection of EF1 alpha and ethidium bromide staining of ribosomal RNA served as a loading control. The transcripts of PR1-Cc, PR4b-Cc, HSR203J-Cc, and HIN1-Cc accumulated at 1 dpi and thereafter in leaves inoculated with PIW or PIW-13F. The kinetics of the defense gene transcripts in PIW-13F infection was similar to those in PIW infection with slight differences. Specifically, high levels of PR4b-Cc and HSR203J-Cc transcript accumulation were observed at 1 dpi in PIW infection, and then exhibited a slight elevation and apparent decrease, respectively, while in PIW-13F infection, both transcripts gradually increased up to 3 dpi. These slight differences would be correlated with the differences in the spread of these viruses in the inoculated leaves (Fig. 4a and b) and the sizes of the necrotic local lesions (Fig. 3c and d), suggesting that L13F in CP reduces the elicitor activity. In contrast, induction of these four transcripts in PIW-66V-inoculated leaves was observed at later time points with lower accumulation levels. It should be noted that these transcripts were strongly induced in PIW-66V infection at 3 dpi with similar accumulation levels to those in PIW-inoculated leaves at 2 dpi. No apparent induction of these transcripts was observed after infection with PIW-13F66V or the mock inoculation. These results suggest that the G66V mutation decreases the elicitor activity of PMMoV CP, resulting in a drastic delay of the defense responses, and that the combination of G66V with L13F totally cancels the induction of the defense response that blocks the virus spread. The detection of viral RNAs of PIW, PIW-13F, PIW-66V, and PIW-13F66V again showed slight attenuation of the CP mutants. The RNA accumulation levels of PIW at 1 dpi were slightly greater than those of PIW-13F, PIW-66V, and PIW-13F66V (Fig. 5), suggesting that the amino acid mutations in CP affect not only elicitor activity but also the replication and/or spread of PMMoV. However, viral RNA accumulation levels of these CP mutants thereafter increased more than those of PIW (Fig. 5, 3 dpi). Furthermore, tissue blot analyses indicated that, in L 3-Cc plants, PIW-13F, PIW-66V, and PIW-13F66V multiplied much more than wild-type PIW (Fig. 4). These results collectively suggest that attenuation has a marginal effect on the overall ability of CP mutant viruses to infected pepper plants, and that the reduced, or delayed induction of HR- and defense-related genes in CP mutants-infected plants is a reflection of reduced elicitor activity of their CP rather than the result of reduced virus replication and/or spread.
Kinetics of PR1-Cc, PR4b-Cc, HSR203J-Cc, and HIN1-Cc transcript and viral RNA accumulation in C. chinense PI159236 (L 3/L 3) after virus inoculation. Total RNA samples (5 μg for PR1-Cc, PR4b-Cc, HSR203J-Cc, HIN1-Cc, and rRNA; 2 μg for viral RNA and EF1 alpha) extracted from leaves inoculated with purified virions (2 μg/ml) of PIW, PIW-13F, PIW-66V, or PIW-13F66V, or with phosphate buffer (10 mM; pH 7.2) as a mock inoculation (Mock), at 0, 1, 2, and 3 days post-inoculation (dpi) were analyzed by northern blotting. EF1 alpha and ethidium bromide staining of rRNA are shown as a loading control
Molecular modeling of PMMoV CP carrying L13F and/or G66V
As mentioned in the Introduction, the structure of the alpha-helical bundle is one of the major determinants of the elicitor activity of Tobamovirus CP. To investigate whether or not the L13F and G66V mutations in PMMoV-Is affect the structure of the alpha-helical bundle, we examined the locations of these mutations in a molecular model depicted on the basis of the crystal structure of TMV CP. Both amino acid residues were located to the outer surface region of the CP (Fig. 1, upper panel, WT). Next, we examined the effects of these mutations on the local and overall structures of the CP molecule. The molecular models suggested that the mutations affect the local structure of the outer surface domain but do not alter the overall structure or electrostatic surface potentials of PMMoV CP (Fig. 1, lower panels). Therefore, these mutations are most unlikely to cause changes in the quaternary structure of PMMoV CP.
Discussion
In the present study, we identified two mutations, L13F and G66V, in the CP of a newly isolated P1,2,3 pathotype, designated PMMoV-Is, which were involved in the ability of the virus to break L 3-mediated resistance. Importantly, and unlike previously reported mutations that were characterized to affect elicitor activity in the TMV-N′ resistance gene system [16–18], the mutations in PMMoV-Is CP are not located in the alpha-helical bundle region, but in the region corresponding to the outer surface of the virus particles. Furthermore, dissection of the mutations revealed that both these mutations in PMMoV CP are required for the virus to efficiently overcome L 3-mediated resistance. Although introduction of either L13F or G66V into PIW CP decreased the elicitor activity, neither was sufficient to efficiently overcome L 3-mediated resistance alone. These results indicate that the two weak mutations in PMMoV-Is CP cooperatively or additively affect the activation of L 3-mediated resistance and enable the virus to overcome the resistance.
Tsuda et al. reported another case in which two amino acid changes are required to overcome L 3-mediated resistance [10]. Specifically, they described that both T43K and D50G mutations in PMMoV-Ij CP were required for overcoming the resistance. However, in their case, both the amino acid residues are located within an alpha-helix of the helical bundle region, and more importantly, CP mutants carrying either of these amino acid changes show deficient systemic infectivity in L 3-harboring plants, although neither mutation induces the HR in the inoculated leaves [10]. The authors therefore suggested that PMMoV-Ij CP mutants carrying either T43K or D50G would have defects in virion assembly or stability [10]. We would like to emphasize the difference between PMMoV-Ij and PMMoV-Is regarding the requirement for a combination of two particular mutations for overcoming L 3-mediated resistance. Unlike PMMoV-Ij, PMMoV-Is acquires the ability to overcome L 3-mediated resistance by combining two mutations that are insufficient to efficiently overcome L 3-mediated resistance alone, thereby providing us with unique evidence that cooperation of mutations with weak effects on the elicitor activity could give rise to the generation of resistance-breaking virus strains.
The three-dimensional structure model of PMMoV CP suggested that L13F and G66V are located in an exposed position of the CP and have no effects on the structure of the virus particles. Interestingly, L13F and G66V are located to positions close to amino acid changes A7S, L10I and M138N, which were reported to have different effects on the elicitor activity in L 3-mediated resistance [7, 11]. This domain of CP seems to be exposed on the outer surface of the virus particles, suggesting that it has important roles, such as acting as a direct recognition target of the L 3 gene product itself, or a protein complex including that product. On the contrary, S81A, and T43K/D50G involved in overcoming the resistance are located in the right radial helix and right-slewed helix of CP, respectively (Fig. 1) [10, 11]. Structural maintenance of the alpha-helical bundle of CP is reportedly essential for HR elicitation by tobamoviruses in L 1-pepper, N′-tobacco, and eggplant plants [19]. Collectively, our results suggest that, in addition to the stability of the CP quaternary structure determined by the alpha-helical bundle, the structure of the CP domain exposed on the surface of virus particles is important for the elicitor activity of CP in L 3-mediated resistance.
PIW-66V induced different symptoms from those induced by PIW-13F66V. In inoculated leaves, PIW-66V infection induced vein necrosis, but not necrotic local lesions accompanied by the HR, whereas PIW-13F66V infection induced symptoms of chlorosis, but not necrosis, on the inoculated leaves. Induction of necrosis symptoms on uninoculated upper leaves following PIW-66V infection was observed at an earlier stage than the chlorosis induced by PIW-13F66V infection. These differences are consistent with their different induction patterns of defense- and HR-related transcripts, since PIW-66V induced all four defense- and HR-related genes examined in this study within 3 days of infection, whereas PIW-13F66V hardly did so. If the vein necrosis in PIW-66V-inoculated leaves is regarded as a delayed resistance response, the results support our previous findings that the elicitor activity of PMMoV is correlated with the timing and extent of defense-related and HR-related gene expressions [5].
Another interesting finding from the dissection of the PMMoV-Is mutations is that PIW-66V causes frequent abscission of inoculated leaves, although this remains to be examined statistically. Frequent inoculated leaf abscission has often been observed in leaves of pepper plants with the HR. These observations suggest that leaf abscission forms part of the processes activated during disease resistance responses in pepper plants. Ethylene is known to be induced in leaves infected with TMV in resistant hosts [26] and reportedly promotes leaf abscission and fruit ripening in pepper plants [27]. PIW-66V did not induce the HR but did cause vein necrosis. These observations give rise to the hypothesis that ethylene production induced by cell death, including both HR and the delayed response, is a key molecular event connecting leaf abscission, and disease resistance in pepper plants.
We also isolated a PMMoV strain with an S81A mutation, in addition to PMMoV-Is carrying both L13F and G66V, from sweet pepper plants of the same cultivar showing systemic necrosis symptoms in the same field. These PMMoVs and pathotype P1,2 PMMoV-J [25] share high levels of homology in the nucleotide sequence of the CP gene. Large populations of PMMoV-J were detected in the field soil by ELISA and RT-PCR (our unpublished observations). These results suggest that the pathotype P1,2,3 PMMoV may have been generated during cycles of infection of sweet pepper plants by P1,2 PMMoV maintained in the soil. It is of great importance to analyze the viral population in the field soil not only for controlling the viral disease but also for understanding the pathway(s) that the viruses have evolved to acquire the ability to overcome the plant disease resistance mechanism.
References
B. Baker, P. Zambryski, B. Staskawicz, S.P. Dinesh-Kumar, Science 276, 726–733 (1997)
M.C. Heath, Plant Mol. Biol. 44, 321–334 (2000)
I.W. Boukema, Euphytica 29, 433–439 (1980)
I.W. Boukema, Capsicum Newsl 3, 47–48 (1984)
H. Hamada, S. Takeuchi, A. Kiba, S. Tsuda, K. Suzuki, Y. Hikichi, T. Okuno, J. Gen. Plant Pathol. 71, 90–94 (2005)
A. Berzal-Herranz, A. de la Cruz, F. Tenllado, J.R. Diaz-Ruiz, L. Lopez, A.I. Sanz, C. Vaquero, M.T. Serra, I. Garcia-Luque, Virology 209, 498–505 (1995)
A. de la Cruz, L. Lopez, F. Tenllado, J.R. Diaz-Ruiz, A.I. Sanz, C. Vaquero, M.T. Serra, I. Garcia-Luque, Mol. Plant Microbe. Interact. 10, 107–113 (1997)
P. Gilardi, I. Garcia-Luque, M.T. Serra, Mol. Plant Microbe. Interact. 11, 1253–1257 (1998)
P. Gilardi, I. Garcia-Luque, M.T. Serra, J. Gen. Virol. 85, 2077–2085 (2004)
S. Tsuda, M. Kirita, Y. Watanabe, Mol. Plant Microbe. Interact. 11, 327–331 (1998)
H. Hamada, S. Takeuchi, A. Kiba, S. Tsuda, Y. Hikichi, T. Okuno, J. Gen. Plant Pathol. 68, 155–162 (2002)
J.N. Culver, Annu. Rev. Phytopathol. 40, 287–308 (2002)
J.N. Culver, W.O. Dawson, Mol. Plant Microbe. Interact. 2, 209–213 (1989)
D.A. Knorr, W.O. Dawson, Proc. Natl. Acad. Sci. USA 85, 170–174 (1988)
T. Saito, K. Yamanaka, Y. Watanabe, N. Takamatsu, T. Meshi, Y. Okada, Virol. 173, 11–20 (1989)
J.N. Culver, G. Stubbs, W.O. Dawson, J. Mol. Biol. 242, 130–138 (1994)
Z.F. Taraporewala, J.N. Culver, Plant Cell 8, 169–178 (1996)
Z.F. Taraporewala, J.N. Culver, Mol. Plant Microbe. Interact. 10, 597–604 (1997)
C.D. Dardick, Z. Taraporewala, B. Lu, J.N. Culver, Mol. Plant Microbe. Interact. 12, 247–251 (1999)
A. Barta, H.A.T. Niemi, P. Salamon, Capsicum Newsl. 4, 49 (1985)
S. Takeuchi, Y. Hikichi, Y. Kawada, T. Okuno, Ann. Phytopathol. Soc. Jpn. 65, 189–191 (1999)
H. Hamada, S. Takeuchi, Y. Morita, H. Sawada, A. Kiba, Y. Hikichi, J. Gen. Plant Pathol. 69, 199–204 (2003)
R. Higuchi, B. Krummel, R.K. Saiki, Nucleic Acids Res. 16, 7351–7367 (1988)
I. Srinivasan, S.A. Tolin, Phytopathology 82, 721 (1992)
M. Kirita, K. Akutsu, Y. Watanabe, S. Tsuda, Ann Phytopathol Soc Jpn 63, 373–376 (1997)
A.M.M. de Laat, L.C. Van Loon, Plant Physiol. 69, 240–245 (1982)
L. Ferrarese, L. Trainotti, P. Moretto, P. Polverino de Laureto, N. Rascio, G. Casadoro, Plant Mol. Biol. 29, 735–747 (1995)
Acknowledgements
We thank Mrs. Harumi Takahashi and Ms. Nozomi Takahashi for plant growth and maintenance. We also thank the Iwate Plant Protection Office and Mizusawa Agricultural Extension Services in Iwate Prefecture for sampling PMMoV-infected plants. This study was supported by the prefectural government of Iwate Prefecture, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamada, H., Tomita, R., Iwadate, Y. et al. Cooperative effect of two amino acid mutations in the coat protein of Pepper mild mottle virus overcomes L 3-mediated resistance in Capsicum plants. Virus Genes 34, 205–214 (2007). https://doi.org/10.1007/s11262-006-0049-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-006-0049-9