Abstract
Fracture is one of the most life-threatening injuries in horses. Fracture repair is often associated with unsatisfactory outcomes and is associated with a high incidence of complications. This study aimed to evaluate the osteogenic effects of gelatin/β-tricalcium phosphate (GT) sponges loaded with different concentrations/ratios of mesenchymal stem cells (MSCs) and bone morphogenetic protein-2 (BMP-2) in an equine bone defect model. Seven thoroughbred horses were used in this study. Eight bone defects were created in the third metatarsal bones of each horse. Then, eight treatments, namely control, GT, GT/M-5, GT/M-6, GT/M-5/B-1, GT/M-5/B-3, GT/M-6/B-1, and GT/M-6/B-3 were applied to the eight different sites in a randomized manner (M-5: 2 × 105 MSCs; M-6: 2 × 106 MSCs; B-1: 1 μg of BMP-2; B-3: 3 μg of BMP-2). Repair of bone defects was assessed by radiography, quantitative computed tomography (QCT), and histopathological evaluation. Radiographic scores and CT values were significantly lower in the control group than in the other groups, while they were significantly higher in the GT/M-5/B-3 and GT/M-6/B-3 groups than in the other groups. The amount of mature compact bone filling the defects was greater in the GT/M-5/B-3 and GT/M-6/B-3 groups than in the other groups. The present study demonstrated that the GT sponge loaded with MSCs and BMP-2 promoted bone regeneration in an equine bone defect model. The GT/MSC/BMP-2 described here may be useful for treating horses with bone injuries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Musculoskeletal injuries are common causes of death or retirement among athletic horses (Johnson et al. 1994). Fractures account for the majority of fatal injuries, often in conjunction with unsatisfactory prognosis and complications such as delay or failure of bony union, fixation failure, and stress-induced laminitis (Lopez and Markel 2012). Animal welfare concerns and economic losses necessitate the development of more effective therapies for equine fracture treatment.
Tissue engineering (TE) has emerged as a promising strategy for bone regeneration. The key components of TE are cells, growth factors, and scaffolds (Koch et al. 2009). Mesenchymal stem cells (MSCs) are gaining increasing attention for bone TE because of their ability to differentiate into several cell lineages including bone cells (Koch et al. 2009; Jones and Yang 2011). Bone morphogenetic protein-2 (BMP-2), one of multiple BMPs that belong to the transforming growth factor-β superfamily, is widely known for inducing osteogenesis (Devescovi et al. 2008). BMP-2 has a very short biological half-life; therefore, specialized carriers are required to maintain their retention at the target site (Yamamoto et al. 2001; Devescovi et al. 2008). Porous gelatin sponges are widely used as scaffolds in TE because of their biodegradability, low antigenicity, and ability to deliver cells and growth factors (Yamamoto et al. 2001; Nöth et al. 2010). Beta-tricalcium phosphate (β-TCP) incorporation improves the mechanical properties of the gelatin sponge without altering the porous structure (Takahashi et al. 2005b). Previous studies demonstrated that gelatin/β-TCP (GT) sponge was useful for the delivery of MSCs and BMP-2 in bone TE (Yamamoto et al. 2001; Takahashi et al. 2005a, b; Seo et al. 2012). Furthermore, GT sponges loaded with MSCs and BMP-2 (GT/MSC/BMP-2) have been shown to facilitate new bone formation in rats (Tadokoro et al. 2011).
We have previously demonstrated that BMP-2-loaded GT sponge accelerated bone regeneration in an equine bone defect model (Tsuzuki et al. 2011). However, no studies have evaluated the osteogenic effects of GT-MSC-BMP-2 in horses. This study aimed to evaluate the osteogenic effects of GT sponges loaded with different concentrations/ratios of MSCs and BMP-2 in an equine bone defect model.
Materials and methods
Study design
Seven healthy thoroughbred horses (five females, two males), aged 5.1 ± 2.6 years and weighing 456.8 ± 85.6 kg, were used in this study. Before the experiment, their normal physical conditions were established on the basis of physical examination, complete blood cell count, blood chemistry, and radiography. For each of the seven horses, eight treatments were applied to the eight different sites in a randomized manner. Throughout the experiment, all evaluations were performed by the same veterinarians who were blinded to the treatments. This experiment was approved by the Experimental Animal Committee of Obihiro University of Agriculture and Veterinary Medicine.
GT sponge preparation
GT sponges (water content: 97.8 %, β-TCP content: 50 %, porosity: 95.9 %, pore size: 179.1 ± 27.8 μm, compression modulus: 1.13 ± 0.13 MPa) were prepared by previously established methods (Takahashi et al. 2005b). In brief, an aqueous solution of gelatin (isoelectric point of 9.0) and β-TCP granules (50 wt%) were mixed using a homogenizer. Then, 0.16 wt% of an aqueous glutaraldehyde solution was added and mixed. For gelatin cross-linking, the solution was cast into a polypropylene dish and allowed to set at 4 °C for 12 h. The cross-linked sponges were placed in an aqueous glycine solution to block the residual aldehyde groups of glutaraldehyde. After thorough washing with double distilled water, the sponges were freeze-dried and cut into columns (diameter: 5 mm; length: 10 mm). The GT sponges were sterilized using ethylene oxide gas.
MSCs isolation and culture
Bone marrow (BM) was aspirated from the sternum of each horse as described previously (Seo et al. 2012). The BM aspirate was then cultured (BM aspirate: medium = 100 μl: 10 ml) on culture dishes in tissue culture medium (TCM) containing Dulbecco’s modified Eagle’s medium (Sigma-Aldrich Japan, Tokyo, Japan), 15 % fetal bovine serum (Biowest, Nuaille, France), and 50 U/ml penicillin–streptomycin (Sigma-Aldrich Japan, Tokyo, Japan). TCM was changed 3 days after the initial plating and every 2 days thereafter. For seeding into scaffolds, MSCs were dissociated from culture dishes using 0.25 % trypsin/2.21 mM EDTA-4Na solution (Mediatech, Herndon, USA) and counted using a hemocytometer. Stemness of MSCs was confirmed by previously described methods (Seo et al. 2012). The MSCs showed positive staining for alkaline phosphatase, and calcium deposits were observed after osteogenic culture for 14 days. In addition, the expression of Act, Oct4, and Klf4 genes was detected by reverse transcription-PCR.
BMP-2 and MSCs loading
Different concentrations/ratios of BMP-2 and MSCs were loaded on the GT sponges (Table 1). To incorporate BMP-2, 50 μl of BMP-2 (Recombinant human BMP-2, PeproTech, New jersey, USA) solutions of two different concentrations, namely 1 μg/50 μl (B-1) and 3 μg/50 μl (B-3) were dripped onto the GT sponges, followed by incubation it at 4 °C overnight. For MSCs loading, 50 μl of cell suspensions of two different concentrations, namely 2 × 105/50 μl (M-5) and 2 × 106/50 μl (M-6) were dripped onto the GT sponges and incubated at 37 °C for 2 h before implantation.
Surgical procedures
Horses were premedicated with medetomidine hydrochloride (5 μg/kg, i.v.). Guaifenesin (5 %, 0.5–1.0 ml/kg, i.v.) was rapidly infused until the horse became ataxic. Anesthesia was induced with thiamylal sodium (4 mg/kg, i.v.) and diazepam (0.03 mg/kg, i.v.) and maintained with isoflurane in oxygen. The horses were placed in dorsal recumbency. Following aseptic preparation of the dorsal metatarsal area, four bone defects (4.5 mm in diameter, 10 mm in depth) were created on the dorsal cortex of both third metatarsal bone by surgical drilling through the stab incision. A needle and fluoroscopy were used to determine the proximal end of the third metatarsal bone. The first defect was 5-cm distal to the proximal end of the third metatarsal bone, and the other defects were distal to the first defect, with 3-cm interval (Fig. 1). All the defects were created in a dorsopalmar direction under the long digital extensor tendon. The eight treatments included control, GT, GT/M-5, GT/M-6, GT/M-5/B-1, GT/M-5/B-3, GT/M-6/B-1, and GT/M-6/B-3, and these were applied to the eight different sites in a randomized manner (n = 7 for each group). After implantation, the incisions were closed and a compression bandage was applied.
Four bone defects (4.5 mm in diameter, 10 mm in depth) were created on the dorsal cortex of both third metatarsal bone by surgical drilling through the stab incision. The first defect was 5-cm distal to the proximal end of the third metatarsal bone, and the other defects were distal to the first defect, with 3-cm interval
Postoperative care
Cefalothin sodium (20 mg/kg, i.v., BID) and phenylbutazone (4 mg/kg, p.o., SID) were administered for 4 days following surgery. The bandage was changed every day for 2 weeks, and the skin sutures were removed 10 days after surgery. During the study, all horses were maintained on stall rest, and were monitored daily for clinical signs of pain, infection, and colic.
Radiographic evaluation
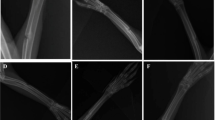
Dorsopalmar and lateromedial radiographs (70 kV, 1.6 mAs, 70-cm film-focus distance) were obtained before, at 1 day after, and at 1, 2, 3, 4, 8, 12, and 16 weeks after surgery. Bone regeneration was scored by the percentage area of the defect filled (0: none, 1: <20 %, 2: 20 %–39 %, 3: 40 %–59 %, 4: 60 %–79 %, 5:80 %–100 %). All films were scored by two veterinarians who were blinded to the treatments.
Quantitative computed tomography (QCT)
The horses were euthanatized 16 weeks after surgery. The hind limbs were severed at the tarsocrural joint. QCT was performed in sagittal sections at 0.5 mm intervals using multidetector-row CT (Asteion™ Super 4®, Toshiba, Tokyo, Japan) and a three-dimensional image processing software (Virtual Place Advance®, Aze, Tokyo, Japan). The CT instrument was calibrated using air and water phantoms according to the manufacturer’s instructions. The CT value (Hounsfiled Unit: HU) of the region of interest (4.0 mm in height , 7.0 mm in width) was obtained from the three central sagittal slices within the bone defect (Fig. 2). HU is a relative value that defines the radiodensity of air as −1000, that of distilled water as 0, and that of human compact bone as 1000. The mean CT value was used in subsequent analyses.
Histopathological evaluation
For histopathological examination, the specimens were excised using a handsaw. The specimens were fixed in 10 % neutral buffered formalin, decalcified in 20 % formic acid, embedded in paraffin and sectioned at 4-μm thickness. The sections were stained using hematoxylin-eosin (HE). The condition of the defect site was evaluated subjectively, with at least three sections evaluated per defect.
Statistical analysis
Data are expressed as mean ± standard deviation or median and range. Statistical analysis of data was performed using SPSS 12.0 software (SPSS, Illinois, USA). The results of radiography, QCT, and histopathology were compared using a nonparametric Friedman test followed by the Wilcoxon signed rank test. Statistical significance was considered at P < 0.05.
Results
Horses
All horses recovered well from anesthesia and could participate in the entire study. Horses remained sound with the described postoperative drug therapy. There were no complications associated with surgery throughout the study.
Radiographic evaluation
The results of radiographic evaluation are shown in Table 2 and Fig. 3. Radiographic scores were significantly lower in the control group than in the other groups at 4, 8, 12, and 16 weeks after surgery (P < 0.05), significantly higher in the GT/M-5/B-3 and GT/M-6/B-3 groups than in the other groups at 4, 8, and 12 weeks after surgery (P < 0.05), and significantly higher in the GT/M-5/B-3 and GT/M-6/B-3 groups than in the control, GT, GT/M-5, and GT/M-6 groups at 16 weeks after surgery (P < 0.05).
QCT
The results of QCT are listed in Table 3. CT values were significantly lower in the control group than in the other groups (P < 0.05), while they were significantly higher in the GT/M-5/B-3 and GT/M-6/B-3 groups than in the other groups (P < 0.05).
Histopathological evaluation
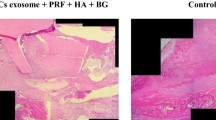
At 16 weeks, histopathology confirmed no remaining implant material and no inflammatory reactions in or near the defects. Subjectively, the amount of mature compact bone filling the defects was greater in the implanted groups than in the control group (Fig. 4). The border between the bone defect and the original bone was unclear in the GT/M-5/B-3 and GT/M-6/B-3 groups, which exhibited a greater amount of mature compact bone in the defects compared with the other groups.
Histopathological examination of the defects in the control (a), GT (b), GT/M-5 (c), GT/M-6 (d), GT/M-5/B-1 (e), GT/M-5/B-3 (f), GT/M-6/B-1 (g), and GT/M-6/B-3 (h) groups at 16 weeks after surgery. Subjectively, the amount of mature bone filling the defects is higher in the implant-treated groups than in the control groups. The border between the bone defect and the original bone is unclear in the GT/M-5/B-3 and GT/M-6/B-3 groups, which also exhibit a greater amount of compact bone in their defects compared with the other groups. The yellow broken line indicates the drill-hole region. Hematoxylin and eosin staining. Scale bar: 2 mm
Discussion
The ideal scaffold for bone TE should be biodegradable, highly porous, of suitable mechanical strength, and facilitate proliferation and differentiation of bone progenitors (Bose et al. 2012). Gelatin is a denatured collagen widely used in TE and drug delivery systems because of its biodegradability and lower antigenicity compared with collagen (Nöth et al. 2010). On the other hand, β-TCP is a biodegradable ceramic commonly used in bone TE because of its similarity to the inorganic composition of bones (Bose et al. 2012). β-TCP incorporation reportedly improves the mechanical properties of gelatin scaffolds without altering the porous structure and promotes the attachment, proliferation, and osteogenic differentiation of MSCs (Takahashi et al. 2005b). GT sponge not only provides a three-dimensional (3D) structure for bone progenitors but also functions as a carrier for growth factors, including BMP-2. Controlled release of BMP-2 from GT sponge enhanced the induction activity of bone formation in a previous study (Takahashi et al. 2005a). On the basis of these findings, we decided to use the GT sponge as a scaffold for bone TE. In the present study, we confirmed that the GT sponge with or without MSCs and BMP-2 loading promoted bone regeneration without causing any complications in an equine model. Therefore, the GT sponge may be useful to treat equine bone injuries, which are usually difficult to treat.
MSCs are an attractive cell source for bone TE because of their high proliferation capacity and multipotency (Koch et al. 2009; Jones and Yang 2011). Many previous studies have shown that MSC loaded scaffolds result in greater bone regeneration compared with scaffolds alone (Kon et al. 2000; Jang et al. 2008; Khojasteh et al. 2013). However, in the present study, although horses treated with MSC loaded GT sponges showed increased radiographic scores and CT values compared with those treated with the GT sponge alone, there were no significant differences in scores between the GT/MSC groups and the GT group. This could be the result of MSC recruitment from the surrounding tissue into the GT sponge and MSC migration from the implanted site to other sites. It is well known that the scaffold can act as a temporary 3D structure for promoting osteoprogenitor cellular infiltration and proliferation for bone tissue repair (Koch et al. 2009; Cao and Kuboyama 2010; Vo et al. 2012). Furthermore, a recent study demonstrated that locally implanted osteoprogenitor cells could migrate to other sites (McDuffee et al. 2012). Massive death of MSCs after implantation is another possible reason for the insignificant effects of MSC loading. Previous studies showed that more than 60 % MSCs loaded on scaffolds die within 2 weeks after in vivo implantation (Dégano et al. 2008; Zimmermann et al. 2011).
Our previous study demonstrated that the implanted MSCs proliferate in GT sponges and that the maximum holding capacity of these sponges is approximately 106 MSCs per 5 × 5 × 5 mm3 GT sponge (Seo et al. 2012). Considering the time and cost encountered for MSC expansion, the use of small numbers of MSCs would greatly facilitate its therapeutic application. Therefore, we used two concentrations of MSCs in this study: 2 × 105/50 μl (M-5) and 2 × 106/50 μl (M-6). There were no significant differences in the results of radiographic evaluation, QCT, and histopathological examination between the M-5 and M-6 groups. These insignificant results can be attributed to MSC proliferation in the GT sponges used in the M-5 group, as well as MSC migration and death.
BMP-2 is the most commonly used growth factor for bone regeneration because it promotes migration, proliferation, and differentiation of osteoprogenitors and synthesis of extracellular matrix (Devescovi et al. 2008). Previous studies have demonstrated that the BMP-2-loaded scaffolds accelerated bone regeneration in equine bone defect models (Perrier et al. 2008; Tsuzuki et al. 2011). On the other hand, many studies have shown that the co-delivery of MSCs and BMP-2 in delivery vehicles possesses bone regenerative potential in vitro and in vivo (Kim et al. 2007, 2010; Terella et al. 2010). In a previous study, subcutaneously implanted GT/MSC/BMP-2 facilitated new bone formation in rats (Tadokoro et al. 2011). GT/MSC/BMP-2 is also expected to promote bone regeneration in horses with fractures. However, no studies have evaluated the osteogenic effects of GT/MSC/BMP-2 in horses. On the basis of these reports, we decided to evaluate the osteogenic effects of GT/MSC/BMP-2 in horses and confirmed that GT/MSC/BMP-2 promoted bone regeneration in an equine bone defect model.
Our previous study demonstrated that GP sponges loaded with 3 μg of BMP-2 accelerated bone regeneration in an equine bone defect model (Tsuzuki et al. 2011). However, the cost of BMP-2 is a major obstacle to its clinical application, and therefore, the use of a smaller dose of BMP-2 would resolve this issue. Accordingly, in the present study, we used two concentrations of BMP-2: 1 μg/50 μl (B-1) and 3 μg/50 μl (B-3). GT/M-5/B-3 and GT/M-6/B-3 implanted into the bone defects resulted in significantly higher radiographic scores and CT values compared with the other materials. In addition, the amount of mature compact bone was greater in the defects of these groups than in the defects of the other groups. These results indicate that GT/MSC/BMP-2 promotes bone regeneration in equine bone defect models. However, there were no significant differences in the results of radiographic evaluation, QCT, and histopathological examination between the GT/MSC/B-1 groups and GT/MSC groups, indicating that the bone regenerative effects of GT/MSC/BMP-2 is dependent on the BMP-2 dose used. Previous studies have also shown that BMP-2 stimulates bone formation in a dose-dependent manner (Schmökel et al. 2004; Wang et al. 2010). On the other hand, excessive bone formation or decreased bone formation has been reported after the use of a high dose of BMP-2 (Linde and Hedner 1995; Yamaji et al. 2007). The optimal dose of BMP-2 is dependent on several factors such as species, implant location, and age (Groeneveld and Burger 2000; Yamaji et al. 2007). Further studies are required to determine the most effective dose of BMP-2 in horses.
In conclusion, the present study demonstrated that the GT sponge loaded with MSCs and BMP-2 promoted bone regeneration in an equine bone defect model. Our results suggest that the GT/MSC/BMP-2 described herein may be useful for treating horses with bone injuries.
References
Bose S, Roy M, Bandyopadhyay A (2012) Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 30:546–554
Cao H, Kuboyama N (2010) A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering. Bone 46:386–395
Dégano IR, Vilalta M, Bagó JR, Matthies AM, Hubbell JA, Dimitriou H, Bianco P, Rubio N, Blanco J (2008) Bioluminescence imaging of calvarial bone repair using bone marrow and adipose tissue-derived mesenchymal stem cells. Biomaterials 29:427–437
Devescovi V, Leonardi E, Ciapetti G, Cenni E (2008) Growth factors in bone repair. Chir Organi Mov 92:161–168
Groeneveld EH, Burger EH (2000) Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol 142:9–21
Jang BJ, Byeon YE, Lim JH, Ryu HH, Kim WH, Koyama Y, Kikuchi M, Kang KS, Kweon OK (2008) Implantation of canine umbilical cord blood-derived mesenchymal stem cells mixed with beta-tricalcium phosphate enhances osteogenesis in bone defect model dogs. J Vet Sci 9:387–393
Johnson BJ, Stover SM, Daft BM, Kinde H, Read DH, Barr BC, Anderson M, Moore J, Woods L, Stoltz J (1994) Causes of death in racehorses over a 2 year period. Equine Vet J 26:327–330
Jones E, Yang X (2011) Mesenchymal stem cells and bone regeneration: current status. Injury 42:562–568
Khojasteh A, Behnia H, Hosseini FS, Dehghan MM, Abbasnia P, Abbas FM (2013) The effect of PCL-TCP scaffold loaded with mesenchymal stem cells on vertical bone augmentation in dog mandible: a preliminary report. J Biomed Mater Res B Appl Biomater 101:848–854
Kim J, Kim IS, Cho TH, Kim HC, Yoon SJ, Choi J, Park Y, Sun K, Hwang SJ (2010) In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering. J Biomed Mater Res A 95:673–681
Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, Noh I, Lee SH, Park Y, Sun K (2007) Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials 28:1830–1837
Koch TG, Berg LC, Betts DH (2009) Current and future regenerative medicine - principles, concepts, and therapeutic use of stem cell therapy and tissue engineering in equine medicine. Can Vet J 50:155–165
Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giardino R, Cancedda R, Quarto R (2000) Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res 49:328–337
Linde A, Hedner E (1995) Recombinant bone morphogenetic protein-2 enhances bone healing, guided by osteopromotive e-PTFE membranes: an experimental study in rats. Calcif Tissue Int 56:549–553
Lopez MJ, Markel MD (2012) Bone biology and fracture healing. In: Auer JA, Stick JA (eds) Equine surgery, 4th edn. Saunders, Philadelphia, pp 1025–1040
McDuffee LA, Pack L, Lores M, Wright GM, Esparza-Gonzalez B, Masaoud E (2012) Osteoprogenitor cell therapy in an equine fracture model. Vet Surg 41:773–783
Nöth U, Rackwitz L, Steinert AF, Tuan RS (2010) Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev 62:765–783
Perrier M, Lu Y, Nemke B, Kobayashi H, Peterson A, Markel M (2008) Acceleration of second and fourth metatarsal fracture healing with recombinant human bone morphogenetic protein-2/calcium phosphate cement in horses. Vet Surg 37:648–655
Schmökel HG, Weber FE, Seiler G, von Rechenberg B, Schense JC, Schawalder P, Hubbell J (2004) Treatment of nonunions with nonglycosylated recombinant human bone morphogenetic protein-2 delivered from a fibrin matrix. Vet Surg 33:112–118
Seo JP, Tsuzuki N, Haneda S, Yamada K, Furuoka H, Tabata Y, Sasaki N (2012) Proliferation of equine bone marrow-derived mesenchymal stem cells in gelatin/β-tricalcium phosphate sponges. Res Vet Sci 93:1481–1486
Tadokoro M, Matsushima A, Kotobuki N, Hirose M, Kimura Y, Tabata Y, Hattori K, Ohgushi H (2011) Bone morphogenetic protein-2 in biodegradable gelatin and β-tricalcium phosphate sponges enhances the in vivo bone-forming capability of bone marrow mesenchymal stem cells. J Tissue Eng Regen Med 6:253–260
Takahashi Y, Yamamoto M, Tabata Y (2005a) Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials 26:4856–4865
Takahashi Y, Yamamoto M, Tabata Y (2005b) Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and beta-tricalcium phosphate. Biomaterials 26:3587–3596
Terella A, Mariner P, Brown N, Anseth K, Streubel SO (2010) Repair of a calvarial defect with biofactor and stem cell-embedded polyethylene glycol scaffold. Arch Facial Plast Surg 12:166–171
Tsuzuki N, Otsuka K, Seo J, Yamada K, Haneda S, Furuoka H, Tabata Y, Sasaki N (2011) In vivo osteoinductivity of gelatin β-tri-calcium phosphate sponge and bone morphogenetic protein-2 on an equine third metacarpal bone defect. Res Vet Sci 93:1021–1025
Vo TN, Kasper FK, Mikos AG (2012) Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev 64:1292–1309
Wang L, Huang Y, Pan K, Jiang X, Liu C (2010) Osteogenic responses to different concentrations/ratios of BMP-2 and bFGF in bone formation. Ann Biomed Eng 38:77–87
Yamaji K, Kawanami M, Matsumoto A, Odajima T, Nishitani Y, Iwasaka K, Yoshimitsu K, Yoshiyama M (2007) Effects of dose of recombinant human BMP-2 on bone formation at palatal sites in young and old rats. Dent Mater J 26:481–486
Yamamoto M, Ikada Y, Tabata Y (2001) Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed 12:77–88
Zimmermann CE, Gierloff M, Hedderich J, Açil Y, Wiltfang J, Terheyden H (2011) Survival of transplanted rat bone marrow-derived osteogenic stem cells in vivo. Tissue Eng A 17:1147–1156
Acknowledgments
The authors thank Takashi Yamaga, Takafumi Tanabe, Hiroki Uchiyama, and Yoshinori Kambayashi for their assistance in bone marrow aspiration and postoperative care.
Conflict of interest
The authors declare no financial or personal conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seo, Jp., Tsuzuki, N., Haneda, S. et al. Osteoinductivity of gelatin/β-tricalcium phosphate sponges loaded with different concentrations of mesenchymal stem cells and bone morphogenetic protein-2 in an equine bone defect model. Vet Res Commun 38, 73–80 (2014). https://doi.org/10.1007/s11259-013-9587-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-013-9587-5