Abstract
The objective of the present study was to examine the effects of parenteral administration of iron and copper on hematological parameters, weight gain, and health of neonatal dairy calves in the period when iron and copper deficiency could be existed. Twenty-four Holstein calves were used for the experiment and randomly assigned to four different treatments. Treatments consisted of (1) control (no injections of Fe and Cu), (2) test 1 (1000 mg Fe as fe-dextran was injected to each calf at day 2 of age), (3) test 2 (160 mg Cu as methionine-copper complex was injected to each calf at day 14 of age), and (4) test 3 (Fe and Cu were injected to each calf as mentioned previously). Blood samples were collected from all of the calves within 24–48 hours after birth and at 7, 14, 21 and 28 days of age for measuring hematological parameters and within 24–48 hours after birth and at 14, 21 and 28 days of age for the determination of iron, copper, TIBC concentrations, and AST activity. Anti-coagulated blood was analyzed shortly after collection for: number of red blood cell (RBC), hemoglobin (Hb), heamatocrit (HCT), total leukocyte count (WBC), Platelet (Plt), MCH, MCV, MCHC, and differential leukocyte counts. The amounts of iron, copper, TIBC, and AST were measured in serum. Group had significant effects on the amounts of HCT, RBC, hemoglobin, MCV, neutrophil, weekly weight gain, and daily gain during each week (p < 0.05). Sampling time had significant effects on the amounts of RBC, MCV, MCH, MCHC, WBC, neutrophil, lymphocyte, monocyte, platelet, fibrinogen, copper, TIBC, AST, weight, weekly gain and, daily gain during each week (p < 0.05). significant interactions between sampling time and group were seen for HCT, RBC, hemoglobin, MCV, platelet, total protein, fibrinogen, iron, and TIBC (p < 0.05). Improved RBC parameters and MCV were seen in calves of group 4 (test 3) in comparing with control group. Total and daily gains were also significantly improved in test groups in comparing with control group (p < 0.05). No significant difference was seen for the days of treatment between groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is an essential component of hemoglobin, myoglobin and several enzymes such as catalase, peroxidase, cytochrome oxidase (Harvey 2000; NRC 2001). Iron requirement for domestic animals are influenced by age, growth rate, availability of dietary iron source, and the criteria of adequacy. The iron requirements of ruminants are not well established and most recommendations are estimate (Smith 1989). It is generally accepted, however, that the iron requirements of young animals are higher than those of mature ruminants are and thought to be about 100 ppm. Deficiencies are most likely to occur in young animals because cows’ milk is low in iron (about 10 ppm). The iron reserves of the calf, which are primarily in the liver, are generally sufficient to prevent serious anemia if calves are fed dry feeds beginning at a few weeks of age. Yet when calves are fed a milk diet exclusively for several weeks, then they may develop iron deficiency anemia, which can adversely affect growth and feed conversion (NRC 2001). Iron deficiency is associated with numerous clinical signs, including anemia, reduced growth, and increased rates of disease. Lindt and Blum (1994) showed that Fe deficiency anemia is frequent problem in veal calves but not in calves fed roughage, concentrates and mineral/vitamin supplements but Bunger et al. (1980) concluded that unrestricted feeding on concentrate rich in iron during colostral period did not prevent Fe deficiency and believed that anemia developing in the first month of life was not physiological but was due to Fe deficiency. However, numerous studies showed that the administration of iron provided an increase in hematological parameters and a better growth in calves (Mollerberg et al. 1975a; Sakozy et al. 1984; Bunger et al. 1986; Geisser et al. 1991; Gygax et al. 1993; Lindt and Blum 1993; Bostedt et al. 2000; Mohri et al. 2004).
Copper is an essential component of several enzymes such as Ceruloplasmin, Cytochrome C oxidase, lysil oxidase, Superoxid dismutase, and Tyrosinase that are required to maintain host homeostasis (Swenson and Reece 1993). Copper deficiency has been linked to a variety of clinical signs, including anemia, pale coat, spontaneous fractures, poor capillary integrity, myocardial degeneration, hypomyelinization of the spinal cord, impaired reproductive performance, and decreased resistance to infectious disease (NRC 2001).
Copper has an important role in the metabolism and transition of iron in the body. Microcytic hypochromic anemia is the one of the outcomes of copper deficiency. Since copper absorption reduces with rumen activity (NRC 2001), thus during the first month of life the deficiency of copper and impaired iron utilization together could be exist. Consequently, administration of copper from the time of rumen activation could be useful in iron utilization. The objective of the present study was to examine the effects of parenteral administration of iron and copper on hematological parameters, weight gain, and health of dairy calves in the period when iron and copper deficiency could be existed.
Materials and methods
The study was conducted in a dairy herd with approximately 600 calves per year at Mashhad suburb (northeast of Iran). This herd consisting of pure bred animals of Holstein breed. The herd was totally confined in free-stall housing without access to pasture. Dry cows were fed with alfalfa hay (20.08 kg), concentrate (1.25 kg) contain barley, cotton seed, bran, beet root and %1DM supplement and corn silage (11.4 kg). The ration was balanced according of NRC 2001.
Cows were dried two months before expected time of parturition and transferred to a separate stall. As the time of parturition approached, the cows were moved to straw bedded maternity pen. Prompt assistance was given to cows with dystocia. Following parturition, the umbilicus of each calf was treated with pavidone iodine and the calf was weighed and transferred to individual pen. Within first six hours of life 2.5 Kg of dam’s colostrums was fed by nipple bottle and colostrum feeding was continued every 12 hours for 48 hours. Then, herd milk was replaced for feeding twice daily (2 Kg every 12 hours) until 30 days of life. After this time calves were fed milk replacer (Table 1) twice daily (2 kg every 12 hours) until 90 days of life. Calf starter (Table 2, started from 48 hours of life) include concentrate (%90 DM) and high quality alfalfa (%10 DM) and also water offered free choice after transferring to individual pen. The calves were weaned at 90 days of life. The heifer calves were mainly used as herd replacements.
Twenty four Holstein calves were used for the experiment and randomly assigned to four different treatments. Treatments consisted of (1) control (no injections of Fe and Cu), (2) test 1 (1000 mg Fe as fe-dextran was injected to each calf at day 2 of age), (3) test 2 (160 mg Cu as methionine-copper complex was injected to each calf at day 14 of age), and (4) test 3 (Fe and Cu were injected to each calf as mentioned previously). The four groups of calves were homogeneous for parity of dams, sex, birth weight, and month of birth.
Ten milliliters of jugular blood were taken from all calves 24–48 hours after birth and at 7, 14, 21 and 28 days of age for measuring hematological parameters and within 24–48 hours after birth and at 14, 21 and 28 days of age for the determination of iron, copper, and TIBC concentrations. Two milliliters of blood was anticoagulated with EDTA for hematological analysis, and plain tubes supplied serum for analysis. The serum was separated after centrifugation at 1,800 g for 10 min and stored at −20C until analysis. Anti coagulated blood was analyzed shortly after collection for: number of red blood cell (RBC), hemoglobin (Hb), heamatocrit (HCT), total leukocyte count (WBC), Platelet (Plt), MCH, MCV and MCHC by an automatic veterinary hematology cell counter (Nihon kohden, Celltac α, Tokyo, Japan). Differential leukocyte counts were performed on routinely prepared Giemsa stained blood films using the cross-sectional technique (Jain 1986). The amounts of iron, copper, TIBC (Total Iron Binding Capacity), and aspartate amino transferase (AST) were measured by commercial kits [Pars Azmoon, Tehran, Iran for iron and TIBC (Ferene S method), and AST (L-aspartate/2-oxoglutarate as substrate); Randox, Antrim, UK for copper (3,5-Di-Br-PAESA method)] using an autoanalyser (Biotecnica, Targa 3000, Rome, Italy). Control serum (Randox control sera, Antrim, UK) was used for controlling measurement accuracy.
For evaluation of growth and health, body weight of all of the calves was measured at birth and weekly and days of treatment for each calf were recorded at the end of the study.
Statistical analysis was conducted using SPSS for windows (release 13, SPSS Inc, Chicago, Ill). Age effect was examined using ANOVA. All analysis was corrected for repeated measurements and included age, and group as fixed factors and calves as random factor. For parameters with significant effect of group, Bonferroni p value adjusted pairwise comparision test was used. One way ANOVA with Bonferroni t-test were used to investigate significant difference between groups for total weight gain, and total daily gain. Chi-square test was also used for comparison of disease occurrence between groups. P < = 0.05 was considered as significant.
Results
Group had significant effect on the amounts of HCT, RBC, hemoglobin, MCV, neutrophil, weekly weight gain, and daily gain during each week (p < 0.05). Sampling time (age) had significant effects on the amounts of RBC, MCV, MCH, MCHC, WBC, neutrophil, lymphocyte, monocyte, platelet, fibrinogen, copper, TIBC, AST, weight, weekly gain and, daily gain during each week (p < 0.05). Significant interactions between sampling time and group were seen for HCT, RBC, hemoglobin, MCV, platelet, iron, and TIBC [(p < 0.05) (Table 3)]. Significant differences were seen between trial groups for total weight gain and total daily weight gain [(p < 0.05), (Table 4)]. The results of statistical analysis for the measured parameters with significant age (sampling time) and group interaction are showed in Table 5. Improved RBC parameters were seen in calves of group 4 (test 3) in comparing with control group (Table 3). The amounts of MCV, and MCH were higher in the test 3 group than they in the control and test 2 groups. MCH was also higher in the test 1 than it in the control group was. Total and daily gains were also significantly improved in test groups in comparing with control group (Tables 3–4). No significant difference was seen for the days of treatment between groups.
Discussion
A progressive reduction in HCT, RBC, and Hb occurs over the first weeks of life (Mohri et al. 2004; Swenson and Reece 1993; Cole et al. 1997). Bomba et al. (1986) reported the changes in RBC parameters and serum iron in calves fed milk replacer, hay, and grain mix. They showed that 50% of calves had subnormal iron values at 2 weeks of age, 10% at 5 weeks, and 20% at weaning and believed that the most critical period of anemia was 3–5 weeks of age. In the study of Reece and Hotchkiss (1987) on blood parameters among calves reared by different methods, calves represented practice common to the rearing for dairy herd replacement had significantly higher values of RBC parameters, iron, and saturation of transferrin and significantly lower TIBC level than other trial groups.
Various studies reported that administration of iron provided an increase in RBC parameters in calves (Bostedt et al. 2000; Bunger et al. 1986; Geisser et al. 1991; Sakozy et al. 1984; Mohri et al. 2004; Rajora et al. 1995; Lindt and Blum 1994). In the present study, administration of iron in the test 1 group similarly provided an increase in RBC parameters although the difference with control group was not significant. In accordance to the study performed by Gengelbach et al. (1994), RBC parameters were not affected by the administration of copper. As shown in Table 5, RBC parameters in second, third, and fourth weeks of age were higher in the test 3 group than those in the control were. The interesting subject is that there were no significant differences between test 1 and test 3 with test 2 for RBC parameters (Tables 3, 5). Two reasons could be caused these results: 1) copper deficiency of calves in the herd and 2) better utilization of dietary iron. It seems the second one probably better explained the results because copper deficiency in calves at first few weeks of life attributed to deficiency of copper in dams diet in dry period. Although we did not measured the amount of copper in dams diet but the ration was prepared according to NRC 2001 for dairy cows. Therefore, our results showed that by administration of iron and thereafter copper, greater increase in RBC parameters were resulted. In accordance to our results, Hart et al. (2001) suggested that iron and copper supply in anemic rats revealed better outcomes than iron alone.
Newborns of domestic animals have large erythrocytes at the birth, and then over the first weeks of life, the volume of erythrocytes reduces and results in reduction in MCV (Jain 1993). Miltenburg et al. (1991) reported that MCV was not affected by the administration of iron. In the present study, MCV decreased over the 4 weeks in all groups, demonstrating that calves developed microcytosis and probably it is a physiological event. MCH and MCHC also decreased throughout the study. But decreases in the control and test 2 groups were higher than them in the test 1 and test 3 groups. These changes could be attributed to the iron levels. The amounts of MCV, and MCH were higher in the test 3 group than they in the control and test 2 groups were. MCH was also higher in the test 1 than it in the control group was. These results suggested that iron and copper supplementation together had better consequences on hematology of dairy calves.
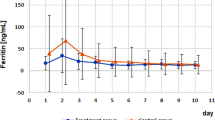
Scheidegger (1973), Bostedt and Schramel (1982), and Bostedt et al. (1990) reported that a progressive reduction in serum Fe concentration occurs over the first few days of life. Various studies reported that the administration of iron provided an increase in serum iron concentration in calves (Mollerberg et al. 1975b; Bomba et al. 1986; Bunger et al. 1986; Rajora et al. 1995; Bostedt et al. 2000; Steinhardt and Thielscher 2003; Mohri et al. 2004). In the present study, the administration of iron provided no significant changes in serum Fe concentration between trial groups because there was a 12 to 13 days interval between iron injection and the first measurement of the concentrations in serum. Probably, in this time gap iron was transferred to tissues such as liver or bone marrow. In the present study similar to the study performed by Rabiansky et al. (1999) serum iron concentration was not affected by the administration of copper. In the test 3 group similar to the test 1 group, an increase in serum iron concentration was observed throughout the experiment and was higher in this group than it in the control and test 2 groups, but the difference was not significant. Iron deficiency is associated with higher iron binding capacity. In the present study, significant lower concentrations of TIBC were observed in Test 1 and Test 3 in week 4 and week 2 than control group was, respectively.
Mollerberg et al. (1975a) and Bostedt et al. (2000) reported that there were no difference in the body weight between the iron supplemented and control groups of calves. But, Geisser et al. (1991) and Sakozy et al. (1984) reported that, by the administration of iron, a better weight gain resulted. In the study performed by Gengelbach et al. (1998), by the administration of copper, daily weight gain was higher in the copper supplemented group than it in the control group. On the other hand, Rabiansky et al. (1999) reported that body weight was not affected by the administration of copper. In the present study, better performance was resulted in supplemented groups than control group was. Best performance was seen for Test 3 group than others.
In the present study, the haematological findings indicate that untreated calves were at risk from iron deficiency whereas the growth rate studies suggest that calves were at risk from both iron and copper deficiency. However, in cattle it is unlikely that calves will be born with depleted liver copper reserves unless the dams were severely copper deficient during pregnancy. Priority is given to the foetus to ensure adequate liver copper reserves at birth (Gooneratne and Christensen 1989). Thus, it is probable that copper supplementation caused better utilization of iron content of diet in calves of test 2 and 3 groups.
Iron is a nutrient related to health and immunity. Bunger et al. (1986) reported that the prevalence of pneumonia and diarrhea and the frequency of treatments for these diseases were highest in the group of calves not supplemented with iron, and lowest in the group given oral iron supplementation. But Kadis et al. (1984) reported that the prevalence and severity of diarrhea were higher in the iron supplemented pigs than them in the control group. In the present study, administration of iron had no effect on the incidence of neonatal diseases and the frequency of treatments for them.
In vitro studies have revealed that copper deficiency can affect various cells functions in the immune system. Gengelbach et al. (1997) reported that the antimicrobial activity of neutrophils from copper deficient calves decreased compared with neutrophils from copper supplemented calves. The activity of antioxidant enzyme cu,zn-superoxidedismotase in neutrophils from cattle and sheep and in peritoneal macrophages of rats decreased due to copper deficiency. Ward et al. (1997) reported that control calves had higher secondary antibody response to pig erythrocytes than copper, molybdenum and iron supplemented calves. Muehlenbein et al. (2001) reported that administration of copper had no effect on incidence of neonatal diseases. In the present study, no significant differences were detected in incidence of neonatal diseases and days of treatment due to administration of iron and copper.
In conclusion parenterally supplementation of iron and copper could improved RBC parameters (in combination) and weight gain (alone or in combination) during the first month of life in neonatal dairy calves but further studies with higher number of calves and also longer duration of study may be needed.
References
Bomba A, Sevcik A, Poldauf M. Changes in erythrocytes, serum iron and serum copper in calves and after weaning, with reference to anaemia. Veterinarstvi. 1986; 36: 227–229.
Bostedt H, Schramel P. Dynamics of blood concentrations of calcium, magnesium and the trace elements iron, copper, and zinc in the newborn calf. Tierarztliche Umschau. 1982; 37: 471–472, 475–476.
Bostedt H, Jekel E, Schramel P. Development of iron and copper concentration in blood plasma of calves during the first few days and weeks after birth, also a finding of covert neonatal iron deficiency aneamia. Deutsche Tierarztliche Wochenschrift. 1990; 97: 400–403.
Bostedt H, Hospes R, Wehrend A, Schramel P. Effects of the parenteral administration of iron preparations in the early development of calves. Tierarztliche Umschau. 2000; 55: 305.
Bunger U, Kaphangst P, Fiebig U, Schonfelder E, Jentsch D, Ponge J. Anaemia in male calves during rearing. 4. Relations between birth weight, duration of trial and body weight gain while the calves were fed on colostrums, and the blood picture during weaning. Archiv fur Tierernahrung. 1980; 30: 611–631.
Bunger U, Schmoldt P, Ponge J. Oral and parentral control of iron deficiency in relation to the course diseases in milk fed calves originating from different farms. Monatshefte fur Veterinarmedizin. 1986; 41: 302–306.
Cole DJ, Roussel AJ, Whitney MS. interpreting a bovine CBC. Journal of Veterinary Medicine. 1997; 92: 460–468.
Geisser P, Hole H, Baer M, Heim H, Fischer W. Investigation on the dosage/efficacy relationship of iron dextran in veal calves. Arzneimittel Forschung. 1991; 41: 32–37.
Gengelbach GP, Spears JW. Effects of dietary copper and molybdenum on copper status, cytokine production and humoral immune responses of calves. Journal of Dairy Science. 1998; 81: 3286–3292.
Gengelbach GP, Ward JD, Spears JW. Effect of Dietary copper, iron and molybdenum on growth and copper status of beef cows and calves. Journal of Animal Science. 1994; 72: 2722–2727.
Gengelbach GP, Ward JD, Spears JW, Brown TT. Effects of copper deficiency and copper deficiency coupled with high dietary iron or molybdenum on phagocytic cell function and response of calves to respiratory disease challenge. Journal of Animal Science. 1997; 75: 1112–1118.
Gooneratne SR, Christensen DA. A survey of maternal copper status and fetal tissue copper concentrations in Saskatchewan bovine. Canadian Journal of Animal Science. 1989; 69: 141–150
Gygax M, Hirni H, Zwahlen R . Immune functions of veal calves fed low amount of iron. Journal of Veterinary Medicine (series A). 1993; 40: 345–358.
Hart EB, Steenbock H,Waddell J, Elvehjem CA,Van Donk E, Riising BM. Iron in Nutrition: VII. Copper as a Supplement to Iron for Hemoglobin Building in the Rat. Journal of Trace Elements in Experimental Medicine. 2001; 14:195–206
Harvey JW. Microcytic anemia. In: Feldman BF, Zinkl JG, Jain NC (eds) Schalm’s veterinary hematology, 5th edn. Lippincott, Williams and Wilkins, Philadelphia, 2000; pp 201–204
Jain NC. Schalms Veterinary Hematology, (Lea and Febiger, Philadelphia), 1986; PP: 66–67
Jain NC. Essentials of Veterinary Hematology. Lea and Febiger, Philadelphia, 1993
Kadis S, Udeze FA, Polanko J, Dreesen DW. Relationship of iron administration to susceptibility of newborn pigs to enterotoxic colibacillosis. American Journal of Veterinary Research. 1984; 45: 255–259.
Lindt F, Blum JW. Physical performance of veal calves during chronic iron deficiency aneamia and after acute iron overload. Journal of Veterinary Medicine (series A). 1993; 41: 237–246.
Lindt F, Blum JW. Occurrence of iron deficiency in growing cattle. Journal of Veterinary Medicine (series A). 1994; 41: 237–246.
Miltenburg GAJ, Wensing T, Van Vliet JPM, Schuijt G, Van de Broek J, Breukink HJ. Blood hematology, plasma iron and tissue iron in dams in late gestation, at calving, and in veal calves at delivery and later. Journal of Dairy science. 1991; 74: 3086–3094.
Mohri M, Sarrafzadeh F, Seifi HA, Farzaneh N. Effects of oral iron supplementation on some haematological parameters and iron biochemistry in neonatal dairy calves. Comparative Clinical Pathology 2004; 13: 39–42
Mollerberg L, Ehlers T, Jacobsson SO. et al. The effect of parenteral iron supply on hematology, health, growth and meat classification in veal calves. Acta Veterinaria Scandinavica. 1975a; 16: 197–204
Mollerberg L, Ekman L, Jacobsson SO. Ferrokinetic studies in normal and iron deficiency aneamia calves. Acta Veterinaria Scandinavica. 1975b; 16: 205–217
Muehlenbein EL, Brink DR, Deutscher GH, Carlson MP, Johnson AB. Effect of inorganic and organic copper supplemented to first-calf cows on cow reproduction and calf health and performance. Journal of Animal Science. 2001; 79: 1650–1659.
National Research Council. Nutrient requirements of dairy cattle. Natl. Acad. Sci Washington DC 2001
Rabiansky PA, Mc Dowell LR,Velasquez J, Wilkinson NS, Percival SS, Martin FG, Bates DB, Johnson AB, Barta TR, Salgado Madriz E. Evaluating copper lysine and copper sulfate sources for heifers. Journal of Dairy Science. 1999; 82: 2642–2650
Rajora VS, Pachauri S, Gupta GC. et al. Use of iron preparation in aneamia associated with anorexia in dairy cattle. Indian Journal of veterinary medicine. 1995; 15: 1–4.
Reece WO, Hotchkiss DK. Blood studies and performance among calves reared by different methods. Journal of Dairy Science 1987; 70: 1601–1611.
Sakozy P, Misley A, Simon F. Iron deficiency in calves. Megyar Allator Vosok Lapja. 1984; 39: 485–491.
Scheidegger HA. Variations in the red blood picture and serum iron concentration in Simmental calves. Schweizer Archive fur Tierhcilkundle. 1973; 115: 483–497.
Smith JE. Iron Metabolism and Its Diseases. In: Kaneko jj (Ed), Clinical Biochemistry of Domestic Animals. Academic Press, San Diego, 1989; pp, 262.
Steinhardt M, Thielscher HH. Effects of a single oral iron application on growth and on hemoglobin variables, hemoglobin derivatives and blood gas content in group housed feeder-fed dairy calves. Archive fur Tierzucht. 2003; 464: 321–330.
Swenson MJ, Reece WO. Duckes physiology of Domestic Animal. 11th ed. Cornell University Press, 1993.
Ward JD, Gengelbach GP, Spears JW. The effect of copper deficiency with or without high dietary iron or molybdenum on immune function of cattle. Journal of Animal Science. 1997; 75: 1400–1408.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heidarpour Bami, M., Mohri, M., Seifi, H.A. et al. Effects of parenteral supply of iron and copper on hematology, weight gain, and health in neonatal dairy calves. Vet Res Commun 32, 553–561 (2008). https://doi.org/10.1007/s11259-008-9058-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-008-9058-6