Abstract
Plants usually respond to the changes of growth irradiance by a combination of the physiological modifications in their preexisting leaves and the production of new leaves. However, those with a determinate growth habit produce certain number of leaves in a growing season and cannot produce new leaves when light condition changes. We used an epiphytic orchid with only one leaf produced every growing season to examine whether and how determinate growth species adapt to changing environments after their preexisting leaves mature. Leaf photosynthesis and anatomy of Pleione aurita were investigated at full expansion and at 40 days after the fully expanded leaves were transferred from high to low light or from low to high light. Leaves show large physiological and morphological plasticity to light gradients at full expansion and the transferred leaves exhibited multiple physiological modifications, including reallocation of nitrogen between light harvesting and carbon fixation, and enhancement of thermal dissipation in their new environments, to optimize carbon assimilation and avoid photoinhibition. Irrespective of the various changes either to shade or sun, the sole preexisting leaf could not fully acclimate to new light environments due to the mesophyll thickness constraint. This leads to the consequence that only plants exposed to high light throughout the experiment had a positive annual biomass gain. Our results highlighted the importance of new leaf production in the carbon accumulation during photosynthetic light acclimation, and contribute new insights of epiphytes physiological responses to their highly dynamic arboreal habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dynamic acclimation of the photosynthetic apparatus in response to changes in light levels is a widely observed and important phenomenon which helps maintain optimal photosynthetic efficiency (Anderson et al. 1995). Plants often acclimate to new irradiance levels through complex physiological changes in their preexisting leaves. For example, leaves may have modifications in their photosynthetic enzymes, pigments, and chloroplast arrangements in response to an altered growth irradiance (Anderson et al. 1995; Oguchi et al. 2003, 2006; Sims and Pearcy 1992; Walters 2005). Due to the morphological constraint, fully expanded leaves have less capacity than immature leaves to cope with such changes in light intensity (Brooks et al. 1994; Ishii and Ohsugi 2011; Oguchi et al. 2003, 2005; Sims and Pearcy 1992). Therefore, many species have the ability to produce new leaves, which are morphologically and physiologically adapted to the new light environment, to improve their whole-plant acclimation under such conditions (Avalos and Mulkey 1999; Ida and Kudo 2010; Ishii and Ohsugi 2011; Kamaluddin and Grace 1993; Kursar and Coley 1999; Shimizu et al. 2006; Yamashita et al. 2000).
However, not all species can produce new leaves after the growth irradiance changes. These include plants with a determinate habit, for which growth stops once a genetically pre-determined structure has completely formed. While the determinate growth habit can be found in tree species (e.g., Ishii and Ohsugi 2011), the phenomenon is more common and pronounced in epiphytes: plants produce a fixed number of leaves simultaneously in a growing season. For example, plants in the Orchidaceae usually produce one or two leaves every year (see Supplementary Material, Fig. S1), and members of this particular family alone contribute 68% of all species to the epiphytic group (Zotz 2013). The carbon gain of these epiphytic species with determinate growth relies solely upon the preexisting leaves, whereas understory tree seedlings or herbs may benefit from their new leaves produced after the change of growth irradiance caused by the formation of a tree-fall gap or seasonal canopy closure (Ida and Kudo 2010; Kamaluddin and Grace 1993; Kursar and Coley 1999; Shimizu et al. 2006).
Epiphytic plants are an essential component of tropical and some subtropical forest ecosystems (Benzing 1998). Few investigations have examined photosynthetic light acclimation in epiphytes (Maxwell et al. 1994, 1999; Pires et al. 2012). However, epiphytes have to undergo light acclimation if they are to adjust to abrupt changes in light intensity under the circumstances of host tree leaf flushing and shedding, or unpredictable gap formation and host branches falling. Since water availability is the greatest restriction to the growth of vascular epiphytes (Laube and Zotz 2003; Zotz et al. 2010), drought is the main cause of juvenile death (Mondragón et al. 2015; Padmawathe et al. 2004; Werner and Gradstein 2008; Winkler et al. 2007). However, most of the mortality for adult epiphytes is caused by host branches falling from the canopy onto the dark forest floor and the individual being incapable of adapting to the changed light environment (Cabral et al. 2015; Hietz 1997; Winkler et al. 2007; Zotz 1998; Zotz et al. 2005). Compared with seedlings and juveniles, adult plants can store more water, and they also have a smaller surface-to-volume ratio that reduces water loss (Schmidt and Zotz 2001; Winkler et al. 2007; Zotz 1998; Zotz et al. 2001). As a result, adult deaths are more likely linked to their weak ability to respond to irradiance changes rather than to drought stress sensitivity.
Considering the predominance of determinate growth in the epiphytic group, any changes in light intensity that occur after leaf maturation present a great challenge for those species in the highly dynamic epiphytic habitat. Determinate growth species provide excellent opportunities to study photosynthetic light acclimation in mature leaves, because debudding damage has to be involved in studies of indeterminate growth species in order to avoid the reallocation of nutrients from old leaves to young leaves (e.g., Brooks et al. 1994). In this study, we use an epiphytic orchid that produces a single leaf in each growing season, as a simplified model of determinate growth species, to investigate whether and how they adapt to altered light environments after leaf maturation. We hypothesized the mature leaves of determinate growth species acclimate to the changes in irradiance mainly via physiological adjustments rather than morphological adjustment, and the inability to produce new leaves makes them vulnerable when exposed to a new light environment.
Materials and methods
Plant materials and growth conditions
Pleione aurita P. J. Cribb & H. Pfennig is a perennial herb characterized by a single leaf attached to its annually renewed pseudobulb. In Spring, this species begins to produce new shoot(s) at the side of the leafless and rootless dormant pseudobulb. The base of the new shoot swells and the old pseudobulb shrinks during the developmental process. When plant growth ceases in Autumn, the single leaf withers, the old pseudobulb shrivels, and the roots die, leaving the new pseudobulb(s) to survive the cold and dry Winter (Cribb and Butterfield 1999, see Fig. 1). The leaf maturation period is approximately 70 days, and the entire leaf lifespan ranges from 180 to 200 days in the greenhouse.
In the wilds of west Yunnan Province in southwestern China, this species grows epiphytically on tree trunks at elevations of 1400–2800 m (Chen et al. 2009). We collected dormant pseudobulbs from there in November 2014 and cultivated the plants in a greenhouse at Kunming Institute of Botany, Chinese Academy of Sciences (1990 m, E102°41′, N25°01′). Before planting, the original dry mass of each pseudobulb was estimated by multiplying fresh weight by the mean ratio of dry weight to fresh weight. Then the dormant pseudobulbs were grown in baskets containing a mixture of 70% bark (1 cm × 1 cm), 20% moss, and 10% humus. When the growth phase began in April 2015, conditions included temperature maintained at 18–30 °C, relative humidity above 60%, and watering at 3-day intervals.
Experimental treatments
To investigate leaf plasticity in response to light intensity, dormant pseudobulbs were exposed to one of three light intensities provided by shade nets before leaf flushing. Our preliminary experimental results had shown that this species ceased to develop new leaves and eventually died in full sunlight. Therefore, we set the test levels at 55% (high light, HL), 15% (medium light, ML), or 5% (low light, LL) of full sunlight, with photosynthetic photon flux density (PPFD) values of approximately 1100, 300, or 100 μmol photons m−2 s−1, respectively. To investigate the response of mature leaves to altered growth irradiance, fully expanded leaves that developed under HL and LL were transferred to the opposite condition (HL–LL or LL–HL).
Leaf photosynthesis, leaf anatomical structures, and chlorophyll and nitrogen contents were investigated at leaf full expansion (for LL, ML, and HL plants) and at 40 days after full expansion (for LL, LL–HL, HL, and HL–LL plants). The dry masses of old and new pseudobulbs from all treatments were obtained from the flowering stage until leaf shedding (Fig. 1). In order to avoid variations among the pseudobulbs, only adult individuals were used, and the dry mass of each new or old pseudobulb was expressed as the percentage of the dry mass of their original pseudobulb.
Development of Pleione aurita over a whole growing season. a Dormant pseudobulb, with two buds at side, b flowering stage, new roots are produced and leaf starts to expand, c full leaf expansion, old pseudobulb shrinks and roots elongate, d 40 days after full leaf expansion, old pseudobulb shrinks further and new pseudobulb swells, and e leaf shedding, leaf has turned yellow, old pseudobulb shrivels, and roots die, leaving a well-formed new pseudobulb. Bar 2 cm
Anatomical observations
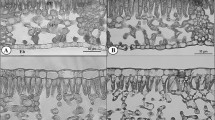
Leaf area was first measured with a LI-3100 m (Li-Cor, Lincoln, Nebraska, USA) after excision. The samples were either oven-dried at 80 °C for 48 h to determine dry mass or else fixed in an FAA solution (formalin, acetic acid, ethanol, and distilled water; 10:5:50:35, v:v:v:v) for anatomical observations. Specific leaf weight was expressed as leaf dry mass per unit area (LMA). Transverse sections of the fixed leaf materials were made with a cryostat microtome (CM3050S; Leica, Wetzlar, Germany). The slices were then photographed under a light microscope (DM2500; Leica, Wetzlar, Germany). Leaf thickness (LT), upper epidermal thickness (UET), lower epidermal thickness (LET), and mesophyll thickness (MT) were then obtained from those digital photographs with Image J software (National Institutes of Health, Bethesda, Maryland, USA). The plasticity index for each trait was calculated as (maximum mean–minimum mean)/maximum mean. For each treatment, six leaves from different plants were used for these anatomical observations.
Leaf gas exchange and chlorophyll fluorescence measurements
The rate of CO2 assimilation (P n) was measured with a portable photosynthesis system (LI-6400; Li-Cor, Lincoln, Nebraska, USA). Recordings of that rate in response to incident PPFD were made between 2000 and 0 μmol photons m−2 s−1, when the relative humidity was approximately 80%. The atmospheric CO2 concentration was maintained at 400 μmol mol−1 by a CO2 injector system (LI-6400-01; Li-Cor, Lincoln, Nebraska, USA), and the light saturation point (LSP) was calculated from the light response curve, as described by Walker (1989).
Chlorophyll fluorescence was measured simultaneously with the gas exchange using a leaf chamber fluorometer (LI-6400-40, Li-Cor, Lincoln, Nebraska, USA). Maximal quantum yield of PSII (F v/F m) was measured before dawn. The fluorescence parameters F s, \(F^{\prime}_{\text{m}}\), and \(F^{\prime}_{\text{o}}\) were determined as previously described (Baker and Rosenqvist 2004). Values for \(F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}}\) (light-adapted maximum quantum yield of PSII), qP (coefficient of photochemical quenching), and ΦPSII (quantum yield of PSII photochemical quenching) were calculated according to the method of Genty et al. (1989). Six leaves from different plants were used for the all of these measurements.
Leaf chlorophyll and nitrogen contents
Leaf chlorophyll (Chl) was extracted in N,N-dimethylformamide in volumetric flasks for 48 h in the dark. Absorbance was measured at 647.0 and 664.5 nm with a spectrophotometer (UV-2550; Shimadzu, Kyoto, Japan) to quantify Chl a and b, following the method of Inskeep and Bloom (1985). Leaf nitrogen content (N) was determined using a carbon–nitrogen analyzer (Vario MAX CN; Elementar, Germany).
Data analysis
One-way ANOVA was used to examine significant differences among treatments at full leaf expansion or 40 days after that, with means discriminated by LSD multiple comparison tests. Two-way ANOVA (with initial and final light environment as the main factors) was used to analyze the effects of initial and final light environment on the leaf anatomy and physiology. All analyses were conducted with the SPSS 16.0 program (SPSS Inc., Chicago, Illinois, USA).
Results
Leaf plasticity in response to light intensity
When compared with medium light (ML) and low light (LL) plants, the plants of Pleione aurita developed under high light (HL) had relatively higher values for leaf mass per unit area (LMA), leaf thickness (LT), mesophyll thickness (MT), chlorophyll a:b (Chl a:b) ratio, and leaf nitrogen (N), but lower values for leaf area per unit pseudobulb dry mass (LA/PM), chlorophyll content (Chlarea and Chlmass), and the Chlarea:N ratio. However, epidermal parameters, such as upper epidermal thickness (UET) and lower epidermal thickness (LET), did not differ significantly among treatments (Table 1). These two traits also had a relatively lower plasticity index (Table 1). Because no significant differences in epidermal thickness were observed among treatments, the thicker HL leaves mainly resulted from the thicker mesophyll layer, as reflected by their MT values.
The plants under HL treatment had the highest values for PPFD-saturated photosynthetic rate (P n) and light saturation point (LSP) at the stage of leaf full expansion (Table 1; Fig. 2a), as well as highest values for non-photochemical quenching (NPQ), coefficient of photochemical quenching (qP), and quantum yield of PSII photochemical quenching (ΦPSII), but lowest values for light-adapted maximum quantum yield of PSII (\(F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}}\)) (Fig. 3).
Responses of non-photochemical quenching (NPQ), light-adapted maximum quantum yield of PSII (\(F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}}\)), coefficient of photochemical quenching (qP), and effective quantum yield of PSII (ΦPSII) to incident photosynthetic photon flux density (PPFD) for leaves of Pleione aurita grown under different light levels, measured at full leaf expansion (a, c, e, and g) and 40 days after full leaf expansion (b, d, f, and h). Data are mean ± SE (n = 6)
Response of mature leaves to altered growth irradiance
The epidermal parameters UET and LET did not differ significantly among treatments after plants were transferred to their new lighting conditions. The transferred plants showed no significant difference in thickness of leaf or mesophyll when compared with their HL or LL controls (Table 2).
Leaf nitrogen and chlorophyll contents were higher in HL–LL leaves than in the HL control, perhaps because the simulated shade environment slowed the progress of leaf aging. Moreover, LSP values remained stable in HL–LL leaves but increased in LL–HL leaves after the transfers were made (Table 2).
We observed a strong carry-over effect of the initial light environment on morphological parameters such as leaf thickness and mesophyll thickness, whereas the leaf nitrogen and chlorophyll contents were influenced by both initial and final light environment or their interaction (Table 3).
At 40 days after full leaf expansion, PPFD-saturated P n was highest for plants under HL conditions, although this value was lower when compared with P n measured at full leaf expansion (Fig. 2). Those HL plants also decreased in P n after being transferred to LL, while the opposite trend was noted for LL–HL plants. Consequently, those two treatments did not differ significantly in their P n values at 40 days after the transfers (Fig. 2b).
Forty days after the transfers, LL–HL plants had lower F v/F m values than other treatments (Table 2), suggesting slight photoinhibition after the shade plants were transferred to the sun. However, we found it remarkable that NPQ values were the highest in these LL–HL plants (Fig. 3b). \(F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}}\) was greatest in LL leaves and lowest in LL–HL leaves (Fig. 3d). Values for qP were lower in LL leaves and higher in LL–HL leaves (Fig. 3f), while ΦPSII was similar for all treatments except LL condition (Fig. 3h).
The impact of light level on biomass accumulation in pseudobulbs
Approximately 25% of the dry matter in an old pseudobulb was consumed during the flowering stage. By the time the leaf had fully expanded, only about 20% of the original dry mass remained. Light levels had little impact on this process (Fig. 4a). Once a leaf was fully developed, the dry mass of the old pseudobulb decreased only slightly until leaf shedding (Fig. 4a). However, light intensity had a dramatic impact on dry mass accumulation in the newly produced pseudobulbs. For example, the mass of the new pseudobulb was less than 10% of the old pseudobulb at the flowering stage. After the leaves were fully expanded, the highest new pseudobulb dry mass across treatments was observed in the HL plants, where values were approximately 40% of that recorded for the original pseudobulb (Fig. 4b). At 40 days after full leaf expansion, HL–LL plants had lower new pseudobulb dry mass than the HL control, while LL–HL plants had higher values than the LL control (Fig. 4b). At the leaf shedding stage, HL plants had the highest increment of dry mass, accounting for more than 50% of their original pseudobulb weight, while LL plants lost two-thirds of the original weight (Fig. 4b). The LL plants also had a longer leaf lifespan (~200 vs. ~180 days for HL plants), a larger photosynthetic area (leaf area/pseudobulb dry mass), and a similar dark respiration rate (data not shown) to that of HL plants. All these facts indicated that a reduction in Pn by LL plants led to less dry mass accumulation in those new pseudobulbs.
Discussion
Plasticity of the single leaf in response to light intensity
Pleione aurita exhibited multiple adaptions to light level. When compared with plants grown under lower light levels, plants exposed to stronger irradiance had thicker leaves, higher leaf nitrogen, and lower values for leaf area/pseudobulb dry mass ratio and chlorophyll content. These responses are similar to those previously described (Anderson et al. 1995; Boardman 1977), and demonstrate that such high plasticity can enable plants to function optimally even in contrasting light environments (e.g., Yang et al. 2014). The determinate growth species studied here is no different from earlier reports that leaf anatomy is dependent on light-level conditions when leaves are being formed and only weakly influenced by changes in light intensity after full expansion (carry-over effect) (Brooks et al. 1994; Niinemets et al. 2006; Oguchi et al. 2003, 2005; Sims and Pearcy 1992).
The leaf-level N redistribution and non-photochemical quenching enhancement
Nitrogen is preferentially allocated to chlorophyll-related light harvesting in the acclimation to shade and to CO2 fixation in the acclimation to sun (Hikosaka and Terashima 1995; Matsubara et al. 2009). Even though our HL plants had the lowest nitrogen content and Chlarea:N ratio 40 days after full leaf expansion, they still maintained the highest photosynthetic rate. This indicated that more N was being utilized for CO2 fixation-related enzymes in those HL leaves, while the nitrogen was being re-distributed to light harvesting after these HL leaves were move to LL condition. The redistribution of N between light harvesting and CO2 fixation within the preexisting leaf facilitates the leaf-level acclimation to the altered environment.
Dissipation of excess light energy is vital for plants living epiphytically because they frequently experience water stress concomitantly with high irradiance, which restricts CO2 assimilation and can cause photoinhibition (Zotz and Tyree 1996). The enhancement of non-photochemical quenching (NPQ) in the LL–HL plants diminished the production of reactive oxygen species that not only cause direct photoinactivation to photosynthetic apparatus but also inhibit the repair of photodamaged PSII (Jahns and Holzwarth 2012; Lawlor and Tezara 2009). Therefore, the sole preexisting leaf of P. aurita can respond to abrupt increased growth light intensity by the enhancement of NPQ.
Anatomical constraint for photosynthetic light acclimation
We found here that the photosynthetic rate rose in LL–HL plants without an accompanying increase in the thickness of leaf or mesophyll. The anatomy of mature leaf usually serves as a constraint for photosynthetic acclimation (Oguchi et al. 2003, 2005). Our LL–HL plants probably enlarged their area of chloroplasts facing the intercellular space per unit leaf area to enable mature leaves to increase photosynthetic rate after light conditions improved. However, the limited mesophyll thickness likely prevented further increases in the total area of chloroplasts facing the intercellular space, which in turn would prevent further increases in photosynthetic rate (Oguchi et al. 2003, 2005, 2006). Therefore, due to the mesophyll thickness constraint, the sole preexisting leaf in our study could not fully acclimate to a new light environment as newly produced leaves would do. Our results are consistent with other studies suggesting that determinate growth species have lower light acclimation potential than indeterminate growth species because of their lower leaf turnover (Ishii and Ohsugi 2011; Kursar and Coley 1999).
The effects of light environment on biomass accumulation and plant survival
From the whole-plant perspective, the adjustments in physiology by preexisting leaves did not appear to be sufficient for coping with different light environment scenarios. This could be inferred from the fact that only plants under HL throughout the experiment had a positive annual biomass gain (Fig. 4b). In contrast to species that can produce new leaves in an altered environment, the carbon gain of determinate growth species relies solely upon the preexisting leaves. We found that, even though photosynthesis increased when LL plants were transferred to HL conditions, the maximum rate of CO2 assimilation was lower than its HL control. Irrespective of a shorter leaf lifespan and lower leaf area, a brighter environment promoted photosynthesis in leaves and dry mass accumulation in pseudobulbs in the HL plants. Therefore, photosynthetic rate reduction must be one main reason of less dry mass accumulation in pseudobulbs in the shaded treatments.
In its native habitat, the epiphytic orchid examined here occurs exclusively in evergreen tree species, usually Rhododendron or Lithocarpus (personal observation). This indicates the growth of the tested species requires a relatively stable environment. A recent study showed that deciduous trees host fewer individuals and less diverse epiphytes than evergreen trees (Einzmann et al. 2015). Water stress is no doubt one of the most important factors that restrict the establishment of epiphytes in the deciduous trees; however, the seasonally light environment changes caused by host tree phenology must present another challenge for those determinate growth species in the epiphytic habitat.
Compared with the predictability of such seasonal changes, the unpredictable change of light environment is much harder for epiphytic plants to deal with in the arboreal habitat. Abrupt changes in the microenvironment and light conditions can be fatal to these plants. For example, transplanting epiphytic seedlings to a higher position on the host tree can lead to greater mortality (Wagner et al. 2013). Therefore, when conducting demographic studies of epiphytic plants, the fallen individuals that encounter a completely different environment from the arboreal habitat are generally counted as dead (e.g., Hietz 1997; Winkler et al. 2007). In some cases, mortality due to branch fall can be substantial (Cervantes et al. 2005). In fact, although these individuals have a low chance of survival, they can remain alive even for several years (Matelson et al. 1993). In a deciduous forest, although area-based photosynthetic rate of an understory herb decreased with the light-level reduction caused by seasonal canopy closure, net assimilation at whole-plant level was maintained at a high level owing to the increase in the total leaf area (Ida and Kudo 2010). However, those fallen epiphytes may not react as fast as this understory herb because their determinate growth habit limits their leaf area increase. The annual loss of biomass due to inefficient carbon assimilation in the LL treatment might explain why fallen epiphytes could remain alive on the forest floor for several years before the ultimate fate of death. In a study that compares light acclimation of determinate and indeterminate growth trees, the authors suggest that successive leaf production in indeterminate growth species plays an important role in reducing sapling mortality under altered light environment (Ishii and Ohsugi 2011). Our results demonstrate that the vulnerability of determinate growth species to changes in growth irradiance is linked to insufficient carbon gain.
Conclusion
The sole mature leaf of Pleione aurita exhibits multiple physiological modifications in its response to changes in irradiance. However, due to the mesophyll thickness constraint, the physiologically acclimated preexisting leaf is not as efficient as a newly produced leaf in carbon assimilation. This inability to produce new leaves when exposed to a new light environment limits light acclimation potential of determinate growth plants, because physiological modifications by the preexisting leaves are not adequate to obtain enough carbon at the whole-plant level. Our results highlight the importance of new leaf production in photosynthetic light acclimation, and indicate that determinate growth species require a stable environment, making those plants more vulnerable to changes in growth irradiance.
References
Anderson JM, Chow WS, Park YI (1995) The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46:129–139. doi:10.1007/bf00020423
Avalos G, Mulkey SS (1999) Photosynthetic acclimation of the liana Stigmaphyllon lindenianum to light changes in a tropical dry forest canopy. Oecologia 120:475–484. doi:10.1007/s004420050880
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621. doi:10.1093/Jxb/Erh196
Benzing DH (1998) Vulnerabilities of tropical forests to climate change: the significance of resident epiphytes. Clim Change 39:519–540. doi:10.1023/A:1005312307709
Boardman N (1977) Comparative photosynthesis of sun and shade plants. Annu Rev Plant Phys 28:355–377
Brooks JR, Hinckley TM, Sprugel DG (1994) Acclimation responses of mature Abies amabilis sun foliage to shading. Oecologia 100:316–324. doi:10.1007/bf00316960
Cabral JS, Petter G, Mendieta-Leiva G, Wagner K, Zotz G, Kreft H (2015) Branchfall as a demographic filter for epiphyte communities: lessons from forest floor-based sampling. PLoS ONE 10(6):e0128019. doi:10.1371/journal.pone.0128019
Cervantes SE, Graham EA, Andrade JL (2005) Light microhabitats, growth and photosynthesis of an epiphytic bromeliad in a tropical dry forest. Plant Ecol 179:107–118. doi:10.1007/s11258-004-5802-3
Chen X, Cribb PJ, Gale SW (2009) Pleione D. Don. In: Wu ZY, Raven PH, Hong DY (eds) Flora of China, vol 25. Missouri Botanical Garden Press, St.Louis, pp 325–333
Cribb P, Butterfield I (1999) The Genus Pleione, 2nd edn. Royal Botanic Gardens, Kew, Richmond
Einzmann HJR, Beyschlag J, Hofhansl F, Wanek W, Zotz G (2015) Host tree phenology affects vascular epiphytes at the physiological, demographic and community level. Aob Plants 7. doi:10.1093/aobpla/plu073
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Hietz P (1997) Population dynamics of epiphytes in a Mexican humid montane forest. J Ecol 85:767–775. doi:10.2307/2960600
Hikosaka K, Terashima I (1995) A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18:605–618. doi:10.1111/j.1365-3040.1995.tb00562.x
Ida TY, Kudo G (2010) Seasonal patterns of carbon assimilation and allocation of a summer-green forest herb, Parasenecio auriculata (Senecioneae; Asteraceae). Plant Ecol 210:181–193. doi:10.1007/s11258-010-9748-3
Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol 77:483–485. doi:10.1104/Pp.77.2.483
Ishii H, Ohsugi Y (2011) Light acclimation potential and carry-over effects vary among three evergreen tree species with contrasting patterns of leaf emergence and maturation. Tree Physiol 31:819–830. doi:10.1093/treephys/tpr079
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193. doi:10.1016/j.bbabio.2011.04.012
Kamaluddin M, Grace J (1993) Growth and photosynthesis of tropical forest tree seedlings (Bischofia javanica Blume) as influenced by a change in light availability. Tree Physiol 13:189–201
Kursar TA, Coley PD (1999) Contrasting modes of light acclimation in two species of the rainforest understory. Oecologia 121:489–498. doi:10.1007/s004420050955
Laube S, Zotz G (2003) Which abiotic factors limit vegetative growth in a vascular epiphyte? Funct Ecol 17:598–604. doi:10.1046/j.1365-2435.2003.00760.x
Lawlor DW, Tezara W (2009) Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann Bot-Lond 103:561–579. doi:10.1093/aob/mcn244
Matelson TJ, Nadkarni NM, Longino JT (1993) Longevity of fallen epiphytes in a neotropical montane forest. Ecology 74:265–269. doi:10.2307/1939523
Matsubara S, Krause GH, Aranda J, Virgo A, Beisel KG, Jahns P, Winter K (2009) Sun-shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Funct Plant Biol 36:20–36. doi:10.1071/FP08214
Maxwell C, Griffiths H, Young AJ (1994) Photosynthetic acclimation to light regime and water stress by the C3-CAM epiphyte Guzmania monostachia: gas-exchange characteristics, photochemical efficiency and the xanthophyll cycle. Funct Ecol 8:746–754. doi:10.2307/2390234
Maxwell K, Marrison JL, Leech RM, Griffiths H, Horton P (1999) Chloroplast acclimation in leaves of Guzmania monostachia in response to high light. Plant Physiol 121:89–95. doi:10.1104/pp.121.1.89
Mondragón D, Valverde T, Hernández-Apolinar M (2015) Population ecology of epiphytic angiosperms: a review. Trop Ecol 56:1–39
Niinemets U, Cescatti A, Rodeghiero M, Tosens T (2006) Complex adjustments of photosynthetic potentials and internal diffusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant Cell Environ 29:1159–1178. doi:10.1111/j.1365-3040.2006.01499.x
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ 26:505–512. doi:10.1046/j.1365-3040.2003.00981.x
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927. doi:10.1111/j.1365-3040.2005.01344.x
Oguchi R, Hikosaka K, Hiura T, Hirose T (2006) Leaf anatomy and light acclimation in woody seedlings after gap formation in a cool-temperate deciduous forest. Oecologia 149:571–582. doi:10.1007/s00442-006-0485-1
Padmawathe R, Qureshi Q, Rawat GS (2004) Effects of selective logging on vascular epiphyte diversity in a moist lowland forest of Eastern Himalaya, India. Biol Conserv 119:81–92. doi:10.1016/j.biocon.2003.10.024
Pires MV, de Almeida AAF, Abreu PP, Silva DD (2012) Does shading explain variation in morphophysiological traits of tropical epiphytic orchids grown in artificial conditions? Acta Physiol Plant 34:2155–2164. doi:10.1007/s11738-012-1016-9
Schmidt G, Zotz G (2001) Ecophysiological consequences of differences in plant size: in situ carbon gain and water relations of the epiphytic bromeliad, Vriesea sanguinolenta. Plant Cell Environ 24:101–111. doi:10.1046/j.1365-3040.2001.00658.x
Shimizu M, Ishida A, Tange T, Yagi H (2006) Leaf turnover and growth responses of shade-grown saplings of four Shorea rain forest species to a sudden increase in light. Tree Physiol 26:449–457
Sims DA, Pearcy RW (1992) Response of leaf anatomy and photosynthetic capacity in Alocasia macrorrhiza (Araceae) to a transfer from low to high light. Am J Bot 79:449–455. doi:10.2307/2445158
Wagner K, Bogusch W, Zotz G (2013) The role of the regeneration niche for the vertical stratification of vascular epiphytes. J Trop Ecol 29:277–290. doi:10.1017/S0266467413000291
Walker DA (1989) Automated measurement of leaf photosynthetic O2 evolution as a function of photon flux density. Philos Trans R Soc B 323:313–326. doi:10.1098/rstb.1989.0013
Walters RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56:435–447. doi:10.1093/jxb/eri060
Werner FA, Gradstein SR (2008) Seedling establishment of vascular epiphytes on isolated and enclosed forest trees in an Andean landscape, Ecuador. Biodivers Conserv 17:3195–3207. doi:10.1007/s10531-008-9421-5
Winkler M, Hulber K, Hietz P (2007) Population dynamics of epiphytic bromeliads: life strategies and the role of host branches. Basic Appl Ecol 8:183–196. doi:10.1016/j.baae.2006.05.003
Yamashita N, Ishida A, Kushima H, Tanaka N (2000) Acclimation to sudden increase in light favoring an invasive over native trees in subtropical islands, Japan. Oecologia 125:412–419. doi:10.1007/s004420000475
Yang SJ, Sun M, Zhang YJ, Cochard H, Cao KF (2014) Strong leaf morphological, anatomical, and physiological responses of a subtropical woody bamboo (Sinarundinaria nitida) to contrasting light environments. Plant Ecol 215:97–109. doi:10.1007/s11258-013-0281-z
Zotz G (1998) Demography of the epiphytic orchid, Dimerandra emarginata. J Trop Ecol 14:725–741. doi:10.1017/S0266467498000534
Zotz G (2013) The systematic distribution of vascular epiphytes-a critical update. Bot J Linn Soc 171:453–481. doi:10.1111/Boj.12010
Zotz G, Tyree MT (1996) Water stress in the epiphytic orchid, Dimerandra emarginata (G. Meyer) Hoehne. Oecologia 107:151–159. doi:10.1007/Bf00327898
Zotz G, Hietz P, Schmidt G (2001) Small plants, large plants: the importance of plant size for the physiological ecology of vascular epiphytes. J Exp Bot 52:2051–2056. doi:10.1093/jexbot/52.363.2051
Zotz G, Laube S, Schmidt G (2005) Long-term population dynamics of the epiphytic bromeliad, Werauhia sanguinolenta. Ecography 28:806–814. doi:10.1111/j.2005.0906-7590.04292.x
Zotz G, Bogusch W, Hietz P, Ketteler N (2010) Growth of epiphytic bromeliads in a changing world: the effects of CO2, water and nutrient supply. Acta Oecol 36:659–665. doi:10.1016/j.actao.2010.10.003
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31670342, 31370362, 31170315), the Natural Science Foundation of Yunnan Province (2013FA044), and the National Key Project of the Ministry of Science and Technology of China (2015BAD10B03).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure S1
Determinate growth habit in family Orchidaceae. (a) Laelia anceps; (b) Flickingeria albopurpurea; (c) Bulbophyllum odoratissimum;(d) Cattleya sp.; (e) Coelogyne ovalis; (f) Epigeneium amplum. For a, b, and c, only one leaf is produced every year; for d, e, and f, two leaves expand simultaneously each year. Bar = 2 cm TIFF 3762 kb)
Rights and permissions
About this article
Cite this article
Zhang, W., Huang, W. & Zhang, SB. The study of a determinate growth orchid highlights the role of new leaf production in photosynthetic light acclimation. Plant Ecol 218, 997–1008 (2017). https://doi.org/10.1007/s11258-017-0747-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-017-0747-5