Abstract
This study was conducted in order to determine the role of the two seed morphs, observed for the first time in a desert population of the widespread Atriplex canescens (Amaranthaceae), in regard to their germination requirements, salinity tolerance and recovery after salt exposure during the seed germination stage. Seeds of the two produced colours (brown and black) were germinated in laboratory conditions under two photoperiods (12/12-h light and continuous dark), three alternating temperature regimes (15/25, 20/30 and 25/35 °C), and several salt concentrations (0, 100, 200, 400, 600, 800 mM NaCl), in order to check the salinity tolerance of each chromatic category and its recovery. Mean seed mass of brown seeds was significantly higher than that of black ones. Brown seeds did not show dormancy on the contrary of black seeds. For both colours, light and temperature did not affect seed germination. Very few seeds germinated when they were exposed to salinity, even at the lowest NaCl concentration; however, all seeds were able to recover their germinability once they were transferred to distilled water. Our results may indicate that both the coloured seed categories could remain viable in saline conditions and they will able to germinate once the salinity level decreases due to rains. Seed colour variation could be a survival strategy of A. canescens in the unpredictable desert environment where this species may grow, although the causes of the occurrence of this phenomenon in this species only for this observed desert population need to be further deepen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seed heterogeneity or heteromorphy, defined as the production of different types of seeds by a single individual, appears in many species of angiosperms (Matilla et al. 2005). Moreover, production of seeds with different morph, size, shape or testa colour within the same species (Santo et al. 2015a), especially in Amaranthaceae, Brassicaceae, Caryophyllaceae and Poaceae (Matilla et al. 2005), has been reported (Mira et al. 2011). Heteromorphy in seeds may affect physiological properties, being associated with ecological strategies that have evolutionary significance, including dormancy (Duràn and Retamal 1989), germination (Puga-Hermida et al. 1997) and longevity behaviour (Diederichsen and Jones-Flory 2005). Heteromorphic seeds showed also variation in dispersal ability as well as in their ability to persist in soil seed bank (El-Keblawy and Bhatt 2015). The production of heteromorphic diaspores is one of the most effective strategies for adaptation and increasing the reproductive success of desert plants, under unpredictable climatic conditions (Harper 1977). Previous studies reported that temporal and spatial environmental fluctuations favour bet-hedging strategies that allow the individuals to optimize their fitness in variable and unpredictable environmental conditions (Carter and Ungar 2003; Minissale et al. 2011; Bhatt et al. 2016).
Atriplex L. is one of the most important genera of Amaranthaceae and many Atriplex species show tolerance to salinity and drought in arid and desert regions (Busso and Bonvissuto 2009). Most of the members of the family Amaranthaceae produce dimorphic seeds, where seed morphs differ in germination characteristics and ability to tolerate salinity (Khan and Gul 1998). Consequently, seed dimorphism might help population survival by avoidance or tolerance of salinity in saline habitat conditions (Khan and Ungar 1986).
Atriplex canescens (Pursh) Nutt. (Amaranthaceae) is a wind-pollinated evergreen shrub native to the western and mid-western United States. This species is the most widespread North American saltbush, extending from Sonora, Mexico, to Alberta, Canada, and from sea level to 2500 m. It grows in sandy to heavy clay soils and under salinity regimes ranging from non-saline to sea-water saturated soils in the intertidal zones of desert estuaries (Glenn et al. 1996). This species is well adapted and naturalized also in the Middle East and in Egypt (Al-Turki et al. 2000). Winged fruits of A. canescens help seeds to be dispersed for long-distance by wind (Henrickson 1974). This species is considered an important food source for both domestic and wild animals in arid and semi-arid regions of the world due to the presence of higher protein content in its foliage (Ortíz-Dorda et al. 2005). Moreover, this species could be used as screens, hedges and barriers, especially on saline-sodic soils and has the potential to be used for conservation and recovery of eroded lands (Romero Paredes Rubio and Ramírez Lozano 2003). A. canescens has also been recommended as an excellent phytoremediation plant in saline-alkali and heavy-metal contaminated lands (Benzarti et al. 2013).
Seed germination is one of the most important and vulnerable stages in the plant life cycle and is influenced by several factors, including light, temperature and salinity (Baskin and Baskin 2014). Light plays a crucial role in optimizing the time of seed germination (Baskin and Baskin 2014) and its presence could either inhibit germination completely (Benvenuti et al. 2004), partially (Zia and Khan 2004) or have no effect (Wei et al. 2008). Temperature can also interact with light, thereby modifying the seed sensitivity to this last factor (Sugahara and Takaki 2004). Salt stress can cause changes in the germination regulators balance, inducing a secondary physiological dormancy (Ungar 1978). In particular, salt may inhibit seed germination, either by creating a low osmotic potential, which prevents water uptake, or through the toxic effects of Na+ and Cl− ions on the metabolic processes (Kaya et al. 2006). Seeds that are unable to germinate at high salinity levels might survive during salt exposure and maintain the ability to germinate later (recovery), when salinity decreases due to various environmental events (Baskin and Baskin 2014; Murru et al. 2015). Seeds of several species treated with high salt concentrations germinated after the transfer to distilled water. However, the ability of seeds to recover is species-specific (Song et al. 2005).
Germination responses of dimorphic seeds have been tested in some Atriplex species, such as A. triangularis Willd. (Khan and Ungar 1984), A. patula L. (Ungar 1996), A. prostrata Bouchér ex DC. (Katembe et al.1998; Carter and Ungar 2003), A. rosea L. (Khan et al. 2004) and A. sagittata Borkh. (Mandak and Pysek 2005). Physiologic effects of activities of protective enzymes on seed germination in A. canescens under NaCl and Na2NO3 stress were investigated by Wang et al. (2011), mainly focusing on seedling growth of this species. Another study (Wang et al. 2010) investigated A. canescens seed germination but testing frozen or 10–22 months collected seeds, differently from the present study, in which we considered only freshly collected seeds. However, the role of different seed morphs of A. canescens has not been investigated so far, and its occurrence, in our knowledge, was not reported before. We hypothesized that the different seed colours in this species might have different germination requirements that may play a role in the survival of this species, in particular when it grows in harsh desert habitats. Moreover, in function of the germination response of the two seed colours, our results could give information about the potentialities for the cultivation of this species for food purpose also in hyper-salt soils, in function of seed morphs tolerance to salinity.
To test this hypothesis, we examine (1) whether the two seed chromatic categories of A. canescens display any difference in their germination behaviour relatively to light and temperature conditions, (2) whether the two seed morphs show differences in their salinity tolerance and (3) whether they can maintain the viability when exposed to high salinity stress and then recover their ability to germinate when incubated in distilled water.
Materials and methods
Seed collection and seed lot details
Fruits of A. canescens were collected during April 2014 from plants growing near Shahniya Nursery [Doha, Qatar (25°27′39″N–51°11′22″E)]. Seeds were randomly collected from 49 individuals, distant at least five metres among themselves, to represent the genetic diversity of the population in the collected seed lots. Seeds were removed from their wings, cleaned by hands, then separated into brown and black seeds and immediately stored in paper bags in the laboratory at room temperature (20 ± 2 °C) and total darkness for less than 1 month, before the starting of germination tests. The seed mass was determined by weighing three replicates, each of 50 seeds, for each seed chromatic category.
Effect of light and temperature on seed germination
To examine the effect of light and temperature requirements during germination, seeds of each colour were incubated in incubators (LMS, UK) at daily (12/12 h) temperature regimes of 15/25, 20/30 and 25/35 °C in 12 h dark/12 h light and continuous darkness. The lower temperature of each cycle corresponded to the night time, while the higher to the day time. Darkness was attained by wrapping, immediately after the seeds sown, two layers of aluminium foil around the Petri dishes, to stop a light response as the seeds imbibed water. Four replicates of 25 seeds each were used for each treatment and colour. Petri dishes were sealed with parafilm (Brand parafilm, Sigma-Aldrich, UK) to minimize evaporation of water and external contaminations. Germination tests were conducted in 9-cm tight-fitting Petri dishes containing one disc of Whatman No. 1 filter paper moistened with 10 ml of distilled water. Germinated seeds were counted and removed every day per 1 month in the light treatments and at the end of the experiment (after 30 days) in the dark treatments. Seeds were considered to be germinated with the emergence of the radicle (≥2 mm). At the end of germination tests, a cut-test with scalpel to evaluate the embryo status (living and therefore white and turgid or brown and therefore died) under a binocular microscope was carried out to evaluate the viability of ungerminated seeds.
Effect of salinity on seed germination
To assess the effect of seed morphs on salinity tolerance during germination, both brown and black seeds were sown under six different salinity concentrations (0, 100, 200, 400, 600 and 800 mM NaCl). Four replicates of 25 seeds each were used for each treatment. Seeds were germinated in 9-cm-diameter Petri dishes on two layers of Whatman No. 1 filter paper, moistened with 10 ml of the test solution. Petri dishes were sealed with parafilm and incubated at the alternating temperature regime of 25/35 °C in 12 h dark/12 h light and continuous darkness. This temperature was chosen because it corresponded to a high germination velocity for this species during preliminary experiments. For the dark treatment, the dishes were wrapped in aluminium foil to prevent any exposure to light. Petri dishes were then incubated in 12 h dark/12 h light and monitored per 30 days and the number of germinated seeds was recorded daily.
After 30 days, all seeds that failed to germinate under light (12 h light/12 h darkness) and dark treatments after being exposed to the different NaCl concentrations were transferred to distilled water. In particular, all seeds of each individual Petri dish were transferred to another Petri dish with distilled water. Germinated seeds were recorded per 10 days (recovery phase) of incubation in the 12/12 light photoperiod.
Data analysis
Final germination percentages were calculated as the mean of four replicates (±1 standard deviation, hereafter SD), while recovery percentages (hereafter RP) according to the following equation (Pujol et al. 2000): RP = {[(a−b)/(c−b)] × 100}, where a is the total number of seeds germinated in the salt solutions plus those that recovered to germination in the distilled water, b is the total number of seeds germinated in saline solutions, and c is the total number of seeds. The rate of germination was estimated by using the Timson’s index of germination velocity (TI) (Santo et al. 2015b):
TI = Σ G/t, where G is the percentage of seed germination at 1-day interval and t is the total germination period. Using this index, a higher value indicates more rapid germination. For all the data, the normality values were analysed by the Shapiro–Wilk test. Seed mass values, arcsine-transformed germination percentages of light and temperature experiments and log10-transformed TI were analysed by ANOVA and consequent Fisher’s least significant differences (LSD) post hoc test. Germination percentages in salt conditions and RP were analysed by the non-parametric Kruskal–Wallis test, followed by a Mann–Whitney U test, due the non-satisfaction of the ANOVA assumptions neither after arcsine transformation. All graphs were made using Sigmaplot 11.0 (Systat Software Inc., London, UK), while all the statistical analyses were carried out using the statistical software Statistica 7.0 for Windows (Software Statsoft Release 7).
Results
Mean seed mass
The one-way ANOVA showed highly significant differences (p < 0.001) among the two chromatic categories. Mean seed mass of brown seeds (1.06 ± 0.03 mg) was significantly higher (p < 0.05) than that of black seeds (0.85 ± 0.01 mg).
Effect of light and temperature on seed germination and its rate
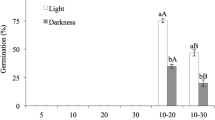
For both photoperiods (12 h dark/12 h light and 24 h dark), brown seeds showed a significantly higher (p < 0.05) final germination in all the three tested temperature regimes, respect to black seeds (Fig. 1). The three-way ANOVA showed a highly significant effect of seed morph (p < 0.001) and light (p < 0.01) on final germination, but not of temperature (p > 0.05) to which seeds were incubated. For each seed morph, significant differences (p < 0.05) in the final germination percentages were detected only at 25/35 °C between light- and dark-incubated seeds. However, none of the interactions (T × C; T× L; C× L; T× C × L) among the three factors were significant (p > 0.05, Table 1).
Final germination of brown and black seeds of Atriplex canescens a in light (12/12) and b dark (0/24) conditions. Data are the mean (±SD) of four replicates. Bars with the same letters are not significantly different at p < 0.05 (three-way ANOVA followed by Fisher’s least significant difference (LSD) post hoc test). Lower-case letters were used to compare final germination between the two colours at the same temperature in the same photoperiod condition (12/12 or 0/24), while capital letters were used to compare final germination between the two photoperiods (12/12 and 0/24) for the same colour
On the germination rate, both the seed colour and temperature had a significant effect (p < 0.01 and p < 0.001, respectively), as well as their interaction (p < 0.001, by three-way ANOVA).
Brown seeds germinated faster respect to black ones at all the three tested alternating temperatures (Fig. 2). Moreover, the germination rate ranged from TI values of ca. 12 at 15/25 °C to ca. 18 at 25/35 °C for brown seeds. Differently, for black seeds, no differences were observed among the TI values at the three temperatures (TI of ca. 10 for all, Fig. 2). All non-germinated seeds were cutted with a scalpel at the end of the germination tests and observed under a binocular microscope, resulting imbibed and no died seeds were recorded, showing as black seeds had from 40 to 50 % of dormant seeds, while brown ones, germinating with higher percentages, did not show the phenomenon of the seed dormancy.
Germination rate of brown and black seeds of Atriplex canescens (A) at the three tested alternating temperatures (15/25, 20/30 and 25/35 °C) in the light (12/12 h). Data are the mean (±SD) of four replicates. Bars with the same letters are not significantly different at p < 0.05 (two-way ANOVA followed by Fisher’s least significant difference (LSD) post hoc test). Lower-case letters were used to compare germination rate between the two colours at the same temperature, while capital letters were used for each colour to compare the germination rate between the three temperatures
Effect of salinity on seed germination
For both brown and black seeds, the highest germination (95.0 ± 4.1 and 62.5 ± 11.9 % in the light, for the two colours, respectively) occurred in distilled water (0 mM NaCl) in the two tested photoperiods and significantly (p < 0.001, by Kruskal–Wallis test) decreased (germination percentages not higher than 10 % also at the lowest NaCl concentration) with increasing salinity for both colours (Figs. 3, 4).
Final germination of brown and black seeds of Atriplex canescens in different NaCl concentrations (0–800 mM), both a in light (12/12 h) and b dark (0/24 h) conditions. Kruskal–Wallis test was conducted to detect significant differences among germination percentages of the two colours in light and in the dark. Bars with different letters are significantly different at p < 0.05 (by Mann–Whitney U test). Data are the mean (±SD) of four replicates
Cumulative germination percentages in the light (12/12) at the tested alternating temperature regime (25/35 °C), under different saline conditions (0–800 mM NaCl) and following transfer to distilled water (recovery phase, indicated by the shaded area in the graph) for brown and black seeds of Atriplex canescens. Each point represents the mean (±1SD) of four replicates. Error bars are not showed for clarity of the graphs and avoid overlaps, but they did not exceed 14 %. In the figure are represented, both for salt phase (white) and recovery phase (shaded) area only the period time in which variations in seed germination were recorded
At 0 mM NaCl, significantly higher (p < 0.05) germination percentages were detected for brown seeds respect to the black ones, both in the 12/12 photoperiod than in the total darkness. In the light, brown seeds were able to germinate up to 200 mM (6.25 ± 2.5 %), but in the dark they germinated only up to 100 mM (2.5 ± 2.9 %). Black seeds germinated up to 600 mM NaCl (1.2 ± 2.5 %) in the light, but their germination in the dark did not exceed 100 mM (1.2 ± 2.5 %) of salinity (Fig. 3). However, the germination observed at the two salt tolerance limits for the two colours (up to 200 mM for brown seeds and 600 mM for black) was not statistically different (p > 0.05).
Germination recovery
Brown seeds recovered their germination also when subjected to the highest NaCl concentration (800 mM) in the previous NaCl phase. Black seeds showed a recovery performance after the salt exposure similar to that detected from brown seeds, although with lower values respect to brown seeds (Fig. 4). Significant differences (p < 0.05, by Kruskal–Wallis test) were detected among the RP under different NaCl concentrations for both brown and black seeds in the light (12/12; Table 2).
For each colour and under the two tested photoperiods, RP decreased with increasing salt concentration to which seeds were exposed in the previous NaCl phase. Similarly, in the total darkness (0/24), RP of each colour detected at different salinities differed significantly (p < 0.01, by Kruskal–Wallis test). In the light cycle (12/12), at each NaCl concentration, the recovery percentages of brown seeds were significantly higher (p < 0.05, by Mann–Whitney U test) respect to that of black seeds. In the darkness (0/24), significant differences among RP of the two seed colours were detected only at 100, 200 and 400 mM NaCl, while RP of the two chromatic categories were statistically similar (p > 0.05, by Mann–Whitney U test) at the highest salinities (600 and 800 mM NaCl; Table 2).
Discussion
The present study investigated the germination requirements of the two seed morphs (brown and black) produced by A. canescens. These two distinct types of seeds differed in their mass, dormancy, final germination and germination rate. Seed germination of A. canescens was affected by seed size, indeed brown seeds (with higher seed mass) showed significantly higher germination in all the tested temperatures compared to black seeds (lower seed mass). These findings suggest that increasing seed mass has a positive effect on A. canescens seed germination. Similar results have been reported for other species where larger seeds showed better germination respect to smaller ones (Shaukat et al. 1999; Kidson and Westoby 2000). The variation in germination response of different colours and seed masses may have ecological significance in desert conditions and it might allow the species to colonize different habitats and expand their geographic distribution limit (Mendes-Rodrigues et al. 2011; Ranieri et al. 2012). Smaller seeds, due to their dimensions, may be facilitated in their dispersal respect to larger seeds (Kigel 1995) and may be dispersed to farther distances. In species with heteromorphic seeds is well known that differences in seed dispersal occur among seed categories in the same species (Matilla et al. 2005). In the case of A. canescens, black seeds may have an advantage in their dispersal by anemochory respect to brown seeds, although their final germination is lower. The difference in seed mass might help in wider distribution during the seed germination time and enhances the chances of survival under unpredictable desert conditions by favouring the formation of soil seed banks (Baskin and Baskin 2014. Generally, small seeds tend to occupy deeper soil horizons, thereby avoiding predation and becoming more persistent in the soil seed bank (Matilla et al. 2005).
In the present study, seed dormancy was detected for about 50 % of black seeds, respect to the non-dormant brown ones. In some heteromorphic species, heterogeneity is of special relevance, since the morphs are ecologically distinct, helping the plants to cope with spatio-temporal variability of habitats (Matilla et al. 2005) and seed dormancy among different seed categories may occur as observed also for the seed categories of A. sagitatta (Venable et al. 1995). Our results indicate that some of the black seeds may germinate when environmental conditions are favourable, while about the 50 % remains dormant and contributes in the formation of a persistent soil seed bank. On the contrary, all the brown seeds germinate when water availability and requirements for the seed germination of the species are present in the habitat. However, differences in dormancy among seed categories of heteromorphic species are not always present, as observed for Cirsium vulgare (Savi) Ten. (Islam et al. 2009) and Silene diclinis (Lag.) M.Laínz (Mira et al. 2011), in which different colours were not associated with this aspect.
Both the types of A. canescens seeds germinated well at all temperatures. These tested temperature ranges perhaps allow the seeds to germinate in the winter (November–March) when temperatures are slightly lower and chances of rainfalls are higher in the natural desert habitat where seeds were collected (temperatures between November and March in the collecting area vary between 20.1 and 25.9 °C) (Islam et al. 2009). In A. canescens, we observed a faster germination in larger seeds (brown) than in smaller (black) and this pattern was different to that detected in A. sagitatta, in which larger seeds germinated slower respect to smaller ones (Venable 1985). Kigel (1995) asserted that germination is rapid and dormancy low in far-dispersed diaspores and vice versa, although we observed exactly the inverse in A. canescens, suggesting that this pattern may be species-specific.
Both coloured seeds of A. canescens germinated well in both light and darkness indicating that they are neutral photoblastic and therefore not photo-inhibited. This indicates that A. canescens seeds have equal chance of germination if they remain on the soil surface or buried in soil seed bank. However, if they are buried too deep in soil probably they might exhaust their resources before seedlings could emerge. On the basis of the different seed dormancy detected in our study, we speculate that A. canescens black seeds maintain a persistent soil seed bank, while brown seeds form only a transient soil seed bank. Therefore, black seeds persist ungerminated for a longer period in the soil than brown seeds. However, only within field studies, this pattern might be fully investigated.
Both brown and black seeds germinated very fast (within a week) and better in distilled water. Many studies report that percentages of germination decreased with increased salinity stress and the highest germination occurs in the absence of NaCl in the substrate both for glycophytes and halophytes (Khan and Ungar 1984; Baskin and Baskin 2014; Santo et al. 2014a, b). In particular for halophytes, this pattern indicates as the halophilous character is an ecological avoiding mechanism to avoid the ecological competition with other species. In fact, this is an adaptive strategy of halophyte plants because the salt content of the soil is reduced only for a short duration. This characteristic is similar to other desert halophytes such as Salsola imbricata Forssk. (Zaman et al. 2010), Salsola rubescens Franch. (El-Keblawy et al. 2013), Halocnemum strobilaceum (Pall.) Bieb. and Halopeplis perfoliata (Forssk) Bunge ex Schweinf (El-Keblawy and Bhatt 2015). Very few seeds of A. canescens were able to germinate with NaCl in the substrate, indicating that A. canescens seeds cannot tolerate the high salinity during the germination stage and seeds will not able to germinate in natural conditions until the salts are dissolved by rains. Generally, salinity enforce the dormancy in seeds and therefore they remain ungerminated under saline conditions, but once the salinity stress is alleviated, they retain their capacity to germinate (Ungar 1991) and this was also the case of A. canescens. Seeds of several species treated with high salinity levels recovered their germination following transfer to distilled water; however, the temperature regimen to which seeds were exposed may greatly influence the recovery percentages (Pujol et al. 2000; Gulzar et al. 2001; El-Keblawy et al. 2007). The ability of a species to tolerate high salinities is reflected on the maximum salt concentration at which seeds may germinate, and to have the possibility of recovery after NaCl exposure (Ungar 1982). Seeds of both colours were able to recover their germination once they were transferred to distilled water, however, for both the two chromatic categories, we observed a higher germination recovery for seeds exposed to lower NaCl concentrations, respect to those under higher salt concentrations. Our results indicate that although A. canescens seeds have the ability to remain viable in saline conditions, in the field they will be able to germinate once the salinity level decreases by rainfall. However, it is unclear whether dimorphic seeds of A. canescens differ in persistence in the soil seed bank. Evolution may have arrived to a trade-off with respect to seed size, since the small seeds are more suited to dispersion, whereas larger seeds favour establishment and adaptation to a particular ecosystem. The germination recovery of black seeds is largely reduced by salinity stress suggesting that the brown seeds of A. canescens have the ability to better maintain their viability under salinity stress, which allows the species to withstand unfavourable periods of environmental stress. However, difference in ability to recover their germination could be related with the variation in seed mass. Previous studies also reported that large seeds are less sensitive to salinity stress than the small seeds such as in Atriplex rosea (Khan et al. 2004), Suaeda aralocaspica (Bunge) Freitag and Schutze (Wang et al. 2008), Chenopodium album L. (Yao et al. 2010) and Atriplex centralasiatica (Xu et al. 2011). In conclusion, this study has allowed to enlarge the knowledge of A. canescens seed ecology, in particular investigating the phenomenon of heteromorphy observed in this species in a population from a desert habitat. It is not known what causes this heterogeneity in this species, but it may be an important strategy for the survival of plant species in adverse and variable ecosystems (Egli 1998). The occurrence of the heteromorphy phenomenon in one desert population of A. canescens is similar to that observed by Santo et al. (Santo et al. 2015a) for Brassica insularis Moris in a small islet in South Sardinia (Italy) which was probably due to an evolutionary divergence process. More information on chemical properties of the seed testa and genetic analysis on plant individuals of this desert population of A. canescens are needed in order to address the colour differences and occurrence of heteromorphy found in this population. Further studies are necessary to better investigate seedlings of the two chromatic categories and their growth in the field and under salinity conditions, possibly evaluating also inter-population variability.
References
Al-Turki TA, Omer S, Ghafoor A (2000) A synopsis of the genus Atriplex L. (Chenopodiaceae) in Saudi Arabia. Feddes Repert 111:261–293
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn. Academic Press, San Diego
Benvenuti S, Dinelli G, Bonetti A (2004) Germination ecology of Leptochloa chinensis: a new weed in the Italian rice agro-environment. Weed Res 44:87–96
Benzarti M, Ben Rejeb K, Debez A, Abdelly C (2013) Environmental and economical opportunities for the valorisation of the genus Atriplex: new insights. Crop Improv 6:441–445
Bhatt A, Santo A, Gallacher D (2016) Seed mucilage effect on water uptake and germination in five species from the hyper-arid Arabian desert. J Arid Env 128:73–79
Busso CA, Bonvissuto GL (2009) Soil seed bank in and between vegetation patches in arid Patagonia, Argentina. Env Exp Bot 67:188–195
Carter CT, Ungar IA (2003) Germination response of dimorphic seeds of two halophyte species to environmentally controlled and natural conditions. Can J Bot 81:918–926
Diederichsen A, Jones-Flory L (2005) Accelerated aging tests with seeds of 11 flax (Linum usitatissimum) cultivars. Seed Sci Technol 33:419–429
Doucet C, Cavers PB (1997) Induced dormancy and colour polymorphism in seeds of the bull thistle Cirsium vulgare (Savi) Ten. Seed Sci Res 7:399–407
Duràn JM, Retamal N (1989) Coat structure and regulation of dormancy in Sinapis arvensis L. seeds. J Plant Phys 135:218–222
Egli DB (1998) Seed biology and the yield of grain. CAB International, Walllingford
El-Keblawy A, Bhatt A (2015) Aerial seed bank affects germination in two small-seeded halophytes in Arab Gulf desert. J Arid Env 117:10–17
El-Keblawy A, Al-Ansari F, Hassan N, Al-Shamsi N (2007) Salinity, temperature and light affect germination of Salsola imbricate. Seed Sci Technol 35:272–281
El-Keblawy A, Bhatt A, Gairola S (2013) Perianth colour affects germination behaviour in wind-pollinated Salsola rubescens in Arabian deserts. Botany 92:69–75
Glenn EP, Pfister R, Brown JJ, Thompson TL, O’Leary JW (1996) Na and K accumulation and salt tolerance of Atriplex canescens (Chenopodiaceae) genotypes. Am J Bot 83:997–1005
Gulzar S, Khan MA, Ungar IA (2001) Effect of salinity and temperature on the germination of Urochondra setulosa (Trin.) CE Hubbard. Seed Sci Technol 29:21–29
Harper JL (1977) Population biology of plant. Academic Press, London
Henrickson J (1974) Saline habitats and halophytic vegetation of the Chihuahuan desert region. Transactions of the Symposium on the biological resources of the Chihuahuan desert region, United States and Mexico. Sul Ross State University, Alpine, pp 289–314
Islam MD, Kubo I, Ohadi M, Alili AA (2009) Measurement of solar energy radiation in Abu Dhabi, UAE. Appl Energy 86:511–515
Katembe WJ, Ungar IA, Mitchell JP (1998) Effect of salinity on germination and seedling growth of two Atriplex species (Chenopodiaceae). Ann Bot 82:167–175
Kaya MD, Okçu G, Atak M, Çıkılı Y, Kolsarıcı Ö (2006) Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur J Agron 24:291–295
Khan MA, Gul B (1998) High salt tolerance in germinating dimorphic seeds of Arthrocnemum indicum. Int J Plant Sci 12:826–832
Khan MA, Ungar IA (1984) The effect of salinity and temperature on the germination of polymorphic seeds and growth of Atriplex triangularis Willd. Am J Bot 71:481–489
Khan MA, Ungar IA (1986) Inhibition of germination in Atriplex triangularis seeds by application of phenols and reversal of inhibition by growth regulators. Bot Gaz 147:148–151
Khan MA, Gul B, Weber DJ (2004) Temperature and high salinity effects in germinating dimorphic seeds of Atriplex rosea. West N Am Nat 64:193–201
Kidson R, Westoby M (2000) Seed mass and seedling dimensions in relation to seedling establishment. Oecologia 125:11–17
Kigel J (1995) Seed germination in arid and semiarid regions. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker, New York
Mandak B, Pysek P (2005) How does seed heteromorphism influence the life history stages of Atriplex sagittata (Chenopodiaceae). Flora 200:516–526
Matilla A, Gallardo M, Puga-Hermida MI (2005) Structural, physiological and molecular aspects of heterogeneity in seeds: a review. Seed Sci Res 15:63–76
Mendes-Rodrigues C, Oliveira PE, Ranal MA (2011) Seed germination and seedling growth of two pseudo-bombax species (Malvaceae) with contrasting habitats from Brazilian cerrado. Rev Biol Trop 59:1915–1925
Minissale P, Santo A, Sciandrello S (2011) Analisi geobotanica del SIC “Capo Murro di Porco, Penisola della Maddalena e Grotta Pellegrino” (Siracusa, Sicilia). Fitosociologia 48:77–98
Mira S, Gonzàles-Benito E, Ibars AM, Estrelles E (2011) Dormancy release and seed ageing in the endangered species Silene diclinis. Biodivers Conserv 20:345–358
Murru V, Santo A, Piazza C, Hugot L, Bacchetta G (2015) Seed germination, salt-stress tolerance, and the effect of nitrate on three Tyrrhenian coastal species of the Silene mollissima aggregate (Caryophyllaceae). Botany 93:1–12
Ortíz-Dorda J, Martínez-Mora C, Correal E, Simón B, Cenis JL (2005) Genetic structure of Atriplex halimus populations in the Mediterranean Basin. Ann Bot 95:827–834
Puga-Hermida MI, Gallardo M, Rodriguez-Gaelo MC, Mutilla AJ (1997) The heterogeneity of tumip-tops (Brassica rapa) seeds inside the silique affects germination, the activity of the final step of the ethylene pathway and ABA and polyamine content. Funct Plant Biol 30:767–775
Pujol JA, Calvo JF, Ramirez-Diaz L (2000) Recovery of germination from different osmotic conditions by four halophytes from southeastern Spain. Ann Bot 85:279–286
Ranieri BD, Pezzini FF, Garcia QS, Chautems A, França MGC (2012) Testing the regeneration niche hypothesis with Gesneriaceae (tribe Sinningiae) in Brazil: implications for the conservation of rare species. Austral Ecol 37:125–133
Romero Paredes Rubio JI, Ramírez Lozano RG (2003) Atriplex canescens (Purch, Nutt), como fuente de alimento para las zonas áridas. Cienc UANL 6:85–92
Santo A, Mattana E, Frigau L, Bacchetta G (2014a) Light, temperature, dry after-ripening and salt stress effects on seed germination of Phleum sardoum (Hackel) Hackel. Plant Species Biol 29:300–305
Santo A, Mattana E, Hugot L, Spinosi P, Bacchetta G (2014b) Seed germination and survival of the endangered psammophilous Rouya polygama (Apiaceae) in different light, temperature and NaCl conditions. Seed Sci Res 24:331–339
Santo A, Mattana E, Bacchetta G (2015a) Inter- and intra-specific variability in seed dormancy loss and germination requirements in the Lavatera triloba aggregate (Malvaceae). Plant Ecol Evol 148:100–110
Santo A, Mattana E, Grillo O, Bacchetta G (2015b) Morpho-colorimetric analysis and seed germination of Brassica insularis Moris (Brassicaceae) populations. Plant Biol 17:335–343
Shaukat SS, Siddiqui ZS, Aziz S (1999) Seed size variation and its effects on germination, growth and seedling survival in Acacia nilotica subsp. indica (Benth.) Brenan. Pak J Bot 31:253–263
Song J, Feng G, Tian C, Zhang F (2005) Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed-germination stage. Ann Bot 96:399–405
Sugahara VY, Takaki M (2004) Effect of light and temperature on seed germination in guava (Psidium guajava L.-Myrtaceae). Seed Sci Technol 32:759–764
Ungar IA (1978) Halophyte seed germination. Bot Rev 44:233–264
Ungar IA (1982) Germination ecology of halophytes. In: Sen DN, Rajpurchit KS (eds) Contributions to the ecology of halophytes. Junk, The Hague
Ungar IA (1991) Ecophysiology of vascular halophytes. CRC Press, Boca Raton
Ungar IA (1996) Effects of salinity on seed germination, growth, and ion accumulation of Atriplex patula (Chenopodiaceae). Am J Bot 83:604–607
Venable DL (1985) The evolutionary ecology of seed heteromorphism. Am Nat 126:577–595
Venable DL, Dyreson E, Morales E (1995) Population dynamic consequences and evolution of seed traits of Heterosperma pinnatum (Asteraceae). Am J Bot 82:410–420
Wang L, Huang Z, Baskin CC, Baskin JM, Dong M (2008) Germination of dimorphic seeds of the desert annual halophyte Suaeda aralocaspica (Chenopodiaceae), a C4 plant without Kranz anatomy. Ann Bot 102:757–769
Wang J, Zhang W, Liu G (2010) Effects of seed germination in three different treatments of Atriplex canescens under NaCl stress. Acta Agricul Boreali-Occid Sin 1:023
Wang J, Juanjuan L, Zhang W, Wenhui X (2011) Effects of activities of protective enzymes on seed germination in Atriplex canescens under NaCl and Na2NO3 stress. Sci Silvae Sin. http://en.cnki.com.cn/Article_en/CJFDTOTAL-LYKE201102023.htm
Wei Y, Dong M, Huang Z, Tan D (2008) Factors influencing seed germination of Salsola affinis (Chenopodiaceae), a dominant annual halophyte inhabiting the deserts of Xinjiang, China. Flora 203:134–140
Xu J, Yin H, Yang L, Xie Z, Liu X (2011) Differential salt tolerance in seedlings derived from dimorphic seeds of Atriplex centralasiatica: from physiology to molecular analysis. Planta 233:859–871
Yao S, Lan H, Zhang F (2010) Variation of seed heteromorphism in Chenopodium album and the effect of salinity stress on the descendants. Ann Bot 105:1015–1025
Zaman S, Padmesh S, Tawfiq H (2010) Seed germination and viability of Salsola imbricata Forssk. Int J Biodivers Conserv 2:388–394
Zia S, Khan MA (2004) Effect of light, salinity, and temperature on seed germination of Limonium stocksii. Can J Bot 82:151–157
Acknowledgments
The authors thank Dr. John Albert Malton for the linguistic revision of the manuscript. This work was partially supported by a grant from the Qatar National Research Fund, QNRF (Grant # 5-260-1-053). The authors would also like to acknowledge Dr. Yousef Al Horr, Dr. Esam Elsarrag (Gulf Organization for Research and Development) and Dr. Ali A. El-Keblawy (Dept of Applied Biology, Faculty of Science and Sharjah Research Academy, University of Sharjah, Sharjah, UAE) for their unlimited support. We thank the Associate Editor Thomas Abeli and the two anonymous reviewers for their suggestions which helped us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Communicated by Thomas Abeli.
Rights and permissions
About this article
Cite this article
Bhatt, A., Santo, A. Germination and recovery of heteromorphic seeds of Atriplex canescens (Amaranthaceae) under increasing salinity. Plant Ecol 217, 1069–1079 (2016). https://doi.org/10.1007/s11258-016-0633-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-016-0633-6