Abstract
“Grazing lawns” are well-known effects of large mammalian herbivores on a short grass sward that is maintained through a positive feedback effect; plants are re-grazed due to their increased palatability. However, there are few studies that have addressed the notion of “browsing lawns.” I simulated browsing and added nutrients to Acacia nigrescens, a palatable and dominant African tree, to examine the effects on the architecture of these trees in a greenhouse. I recorded investments in non-structural carbohydrates and crude protein between roots and stems as a possible mechanism behind such architectural changes. There was a significant decrease in mean branch internode length and an increase in the branching ratio with clipping; the last mentioned indicated that there was more extensive tree branching. There were no significant effects on plant architecture of nutrient addition. There were changes in non-structural carbohydrates and crude protein between the roots and stems due to clipping but not to nutrient addition. Non-structural carbohydrate concentrations decreased with clipping in the roots and increased in the stems. Crude protein decreased in the stems with clipping frequency but not in the roots. There was an increase in root:shoot ratio of crude protein with increasing clipping frequency. A. nigrescens performs like a browsing lawn because of the changes in tree architecture (mean branch internode length and branching ratio) that are consistent with clipping. Clipping but not nutrient addition was important for these trees. Clipping frequency was important for non-structural carbohydrate redistribution from roots to stems. A positive feedback could have long-term effects on the palatability of these trees which should cause large mammalian herbivores to consistently favor A. nigrescens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is considerable interest in the relationships between architecture, plant functional traits and the optimal structure of plants (e.g., Farnsworth and Niklas 1995; Barthelemy and Caraglio 2007; Lavorel et al. 2007; Ward et al. 2012). There is much debate as to the relative importance of optimal plant architecture based on hydraulic and mechanical constraints (West et al. 1999; Niklas and Spatz 2004). Plants are often phenotypically plastic and vary considerably in their architecture (Schlichting and Pigliucci 1998; Sultan 2003; Cromsigt and Kuiper 2011). This had led some researchers to be concerned that intraspecific variability is sufficient that functional traits have little role in causing an optimal plant architecture to exist (Albert et al. 2011; Ward et al. 2012; Du Toit and Olff 2014). However, others have noted that the optimal architecture depends on the situation, such as whether or not the plant occurs in shaded habitats (Donohue 2003) or is grazed or browsed (e.g., Archibald and Bond 2003; Scogings et al. 2013; Wigley et al. 2015).
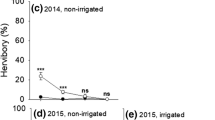
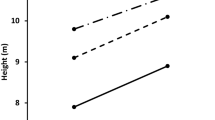
The optimal architecture for a plant is inherently different when plants are grazed and browsed (McNaughton 1984; Du Toit et al. 1990; Mopper et al. 1991; Ward 2010; Pastor and De Jager 2012). For example, “grazing lawns” are created by large mammalian herbivory on a short grass sward that is maintained through a positive feedback when the plants are re-grazed due to their increased palatability (McNaughton 1984; Waldram et al. 2008; Stock et al. 2010). There are several mechanisms that have been proposed for this positive feedback, including plants shunting nitrogen aboveground in response to grazing, or concentrating more nitrogen into less tissue (these grasses are usually shorter than grasses not found in “grazing lawns”) or the plants become more palatable because grazers alter soil nitrogen dynamics such as by defaecation and urine deposition (reviewed by Stock et al. 2010). In a similar fashion, browsers affect the architecture of trees (Martinez and Lopez-Portillo 2003; Johnston et al. 2007; Moncrieff et al. 2011; Pastor and De Jager 2012; Ward 2010; Ward et al. 2012). However, there are few studies that have addressed the notion of “browsing lawns” (also known as “browsing hedges” sensu Du Toit and Olff 2014) where trees are maintained in a positive feedback by browsing, with increased herbivory leading to increased investment in nitrogen by the plants, followed by increased herbivory due to the higher palatability of such plants (Fornara and Du Toit 2007, 2008; Du Toit and Olff 2014). Fornara and Du Toit (2007) found that there was intense year-round herbivory on Acacia nigrescens by giraffes Giraffa camelopardalis, elephants Loxodonta africana, kudus Tragelaphus strepsiceros, impalas Aepyceros melampus, and steenbok Raphicerus campestris that resulted in a change in this tree’s architecture at their study site in the central Kruger Park, South Africa (Fig. 1). In particular, giraffes had a very heavy effect on the architecture of A. nigrescens trees in South Africa (Fornara and Du Toit 2007; Moncrieff et al. 2011) (Fig. 1a, b).
Fornara and Du Toit (2008) speculated that the increased availability of nutrients, perhaps due to dung and urine deposition, resulted in an increased availability of nutrients to A. nigrescens. In their study, they found that A. nigrescens resprouted from the base and produced an average of 3.7 stems in heavily browsed areas, while they produced only 1.86 stems on average in lightly browsed areas. They recognized that they had not examined the effects of non-structural carbohydrate redistribution between shoots and roots in this species. Changes in the responses to soil nutrients may lead to changes in investment in non-structural carbohydrates (Fornara and du Toit 2008). Changes in distribution of non-structural carbohydrates may lead to changes in the architecture of the plant because plants typically invest more in roots as a consequence of herbivory of the shoots (Scogings and Macanda 2005). This is a potential mechanism that affects the control of the positive feedback between browsers and trees. Cromsigt and Kuiper (2011) consider “browsing lawns” to be more likely to occur in resource-rich environments where patch selection is high and individual tree preference is low (because it would generally reduce the availability of palatable species in the patch). However, I note that A. nigrescens is often a dominant tree where it occurs, making the availability of other species less important. Thus, the relative resource richness of the habitat may be moot (Fornara and Du Toit 2007; Ward personal observation).
In a controlled greenhouse experiment, I set out to examine the following:
-
1)
Does clipping frequency affected the architecture of the plants?
-
2)
Does fertilization (sensu the concerns raised by Cromsigt and Kuiper 2011) with nitrogen, phosphorus, and nitrogen + phosphorus (and controls) affect regrowth patterns of these trees?
-
3)
Does the allocation of non-structural carbohydrates (and crude protein) in roots and stems create a mechanism that affects responses to clipping and/or nutrient addition in these trees?
Materials and methods
Acacia nigrescens Oliv. (common name = knob thorn) is an upright and single-stemmed tree that reaches 10–15 m in height, although it may reach 30 m on occasion (Smit 1999). This thorny tree derives its common name from the persistent prickles that occur on the trunk as well as on the branches.
I used A. nigrescens trees that were three-years old at the start of this experiment. I employed a completely crossed simulated herbivory (by clipping) and nutrient addition experimental design, with each treatment combination replicated eight times.
Bins with a 95 L capacity (top diameter 0.45 m and height 0.6 m) were filled with Umgeni sand aggregate (alluvial sand from the Umgeni river, near Pietermaritzburg, South Africa). Each bin was planted with a single tree and fitted with a micro-jet water irrigation system and operated for half an hour every day with the capacity to deliver 20 L h−1.
Nutrient treatments included nitrogen (N), phosphorus (P), nitrogen + phosphorus (N + P), and controls (no nutrients). The amount of nitrogen was 16 g of N and 12 g of P for each plant in the respective treatments on a six-monthly basis over 18 months.
I simulated herbivory on these plants by either clipping them once at the beginning of the experiment (July 2011), twice (after 6 months in January 2012), or complete controls (unclipped). All measurements were taken in December 2012. I clipped only shoots that might have been exposed to ungulate browsers at the edge of the canopy. Following Fornara and Du Toit (2007), I used a Giraffe Browsing Unit (GBU) to simulate mass loss due to browsing damage. This GBU was equivalent to the length of a shoot pruned or leaf-stripped by a giraffe in a single “bite” and, on average, was 14.4 cm of shoot measured directly into the canopy from the shoot’s terminal end (Fornara and Du Toit 2007). Twelve GBUs were clipped per plant in the single- and twice-clipped treatments.
Following the methods of Fornara and Du Toit (2007), I measured total tree height, stem diameter at the base of the tree, aboveground dry biomass, belowground dry biomass, mean thorn length (five thorns per tree), mean inter-thorn distance (five thorns per tree), mean length of the first three branch internodes, mean new shoot length (three shoots per tree), mean shoot diameter (three shoots per tree), total length of all shoots, and length of longest shoot. The shoot diameter was measured with vernier calipers at the cut section. Composite shoot length was measured exactly using string, which allowed me to follow all shoot curvatures and all terminal shoots included in the GBU. I measured mean leaf weight (five leaves per tree). The leaves are double pinnately compound and differ from those of many Acacia species because they are not fine leaved (Fornara and Du Toit 2007). They have 4–6 leaflets with oblique bases. I did not measure condensed tannins or total polyphenolics because these have been shown to be relatively low in A. nigrescens (Du Toit 2003).
Branching ratio (Archibald and Bond 2003) was also measured and indicates whether branch growth is in the form of branch elongation or lateral branching. For each branch unit, the ratio between the total length of all shoots and the length of the longest shoot was calculated and expressed as a mean of values collected after two growing seasons. The higher the ratio, the more numerous were the side branches and therefore the greater the degree of tree branching.
Total non-structural carbohydrates were analyzed as reducing sugars following quantitative hydrolysis to monosaccharides through a carefully controlled acid-hydrolysis procedure (Marais 1979). The reducing sugars formed during hydrolysis were determined quantitatively by a modified Nelson-Somogyi method (Marais et al. 1966) and recorded in %. Crude protein was analyzed by the automated Dumas dry combustion method using a LECO CNS 2000 (Leco Corporation, Michigan, USA; Matejovic 1996) and measured in %.
I tested all parameters for normality and homogeneity of variance. To control for comparison-wise error, I first performed a MANOVA of all architecture variables before subsequently examining the significant univariate statistics for those parameters and used a Bonferroni adjustment of α to correct for Type I error.
Results
I used a MANOVA to test the overall effects of nutrients, clipping, and nutrient X clipping interaction on the architecture parameters mentioned above (Methods). I found no significant interaction effect (Wilks’ λ = 0.436, F = 0.988, p = 0.506) and no significant nutrient effect (Wilks’ λ = 0.649, F = 1.011, p = 0.457). However, there was a significant effect of clipping frequency (Wilks’ λ = 0.630, F = 1.690, p = 0.043).
Of the significant univariate effects of clipping frequency, there was a significant effect on branch internode length (F = 3.914, p = 0.024—Fig. 2a) and on the branching ratio (F = 9.701, p < 0.001—Fig. 2b). Mean branch internode length was the greatest for unclipped trees and it declined with increasing clipping frequency. Branching ratio was the lowest when trees were unclipped and there were similar values for trees that were clipped once and twice. No significant effects were found on any of the other architecture parameters. This meant that clipped trees formed a dense cluster of branches (reduced branch internode length and increased branching ratio). No evidence of resprouting was found.
Changes in tree sapling architecture due to changes in a mean branch internode length (in cm), which declined with increasing clipping frequency (post hoc tests, p < 0.05) and b branching ratio, which was the lowest in the control treatment (post hoc test, p < 0.05) and did not differ between those clipped once or twice (post hoc test, p > 0.05). Closed circles represent means and vertical lines represent 95 % confidence intervals (CI)
There was a significant decrease in % non-structural carbohydrates (NSC) in the roots with increasing clipping frequency (F = 20.066, p < 0.001—Table 1a; Fig. 3a) and a corresponding increase in NSC with increasing clipping frequency in the shoots (F = 34.835, p < 0.001—Table 1b; Fig. 3b). Thus, NSCs were shunted from the roots to the shoots with increasing clipping frequency.
Investment in % non-structural carbohydrates (NSC) in a roots, which was the highest in the control treatment (post hoc test, p < 0.05) and did not differ between those clipped once or twice (post hoc test, p > 0.05) and b shoots, which increased with clipping frequency (post hoc tests, p < 0.05). Closed circles represent means and vertical lines represent 95 % confidence intervals (CI)
There was a significant decrease in % crude protein (CP) in the shoots with increasing clipping frequency (F = 4.122, p = 0.020—Fig. 4a) but no change in CP with increasing clipping frequency in the roots (F = 1.570, p = 0.215). I also found that there was a change in the root:shoot ratio of CP (F = 5. 735, p = 0.005—Table 1c; Fig. 4b). CP was higher in the roots than in the shoots in all cases. There were no changes in CP due to fertilization in roots, shoots, or root:shoot ratios nor any significant interaction effect between fertilization and clipping frequency (p > 0.05).
Discussion
The most important results in this study were that clipped trees formed a dense cluster of branches (reduced branch internode length and increased branching ratio). This result was similar to that provided by Martinez and Lopez-Portillo (2003) who found that honey mesquites Prosopis glandulosa torreyana produced short spiny branches and compact crown cover when browsed by jackrabbits Lepus californicus, while unbrowsed plants produced long shoots and had open crown covers. However, there was no change in the height of the trees, unlike those reported on by Fornara and Du Toit (2007) (Fig. 1). A similar result to that of Martinez and Lopez-Portillo (2003) was found by Wigley et al. (2015) who found that trees from iMfolozi Game Reserve in KwaZulu-Natal, South Africa, invested more in structural defences and a lower bite-size index (contributing to increased ramification) after herbivory. Staver et al. (2012) also found that herbivory-adapted species tend to have “cage-like” architecture (as did Archibald and Bond (2003) for Acacia karroo) and thicker bark. Staver et al. (2012) found that there was increased investment in non-structural carbohydrates in the roots relative to the shoots in certain herbivory-adapted species such as Acacia karroo, A. nilotica, and A. gerrardii.
My study was a relatively short-term one (18 months); it may take many years for the trees to resemble those shown in Fig. 1. Interestingly, nutrient addition had no significant effect on A. nigrescens, contra the claims of Cromsigt and Kuiper (2011). Nonetheless, non-structural carbohydrates (NSC) were redistributed in a way that was consistent with a response to the effects of herbivory.
Pastor and De Jager (2012) used simulation models to predict changes in the geometry of plant canopies caused by both mammalian browsers and by changes in soil fertility that may result in a feedback on the population dynamics of the browsers themselves. They found that this may even result in complex dynamics such as population oscillations in mammalian browsers. These results are consistent with the “browsing lawns” described by Fornara and Du Toit (2007) and further elucidated in the current study. Similar results were obtained by Ward (2010) for a dominant desert tree, Acacia raddiana, in Israel.
Redistribution of non-structural carbohydrates and nitrogen (crude protein)
Fornara and Du Toit (2008) indicated that the redistribution of non-structural carbohydrates (NSC) was related to regrowth and resprouting abilities and inferred that these NSC were related to the availability of soil nutrients. A number of other studies have shown that disturbances such as browsing (e.g., Van Der Heyden and Stock 1995, 1996; Canadell and Lopez-Soria 1998; Canham et al. 1999; Cherbuy et al. 2001) and fire (e.g., Schutz et al. 2009; Wigley et al. 2009) have resulted in redistribution of non-structural carbohydrates (NSC) from roots to stems. Although we have found elsewhere (Hean and Ward 2012) that fire and herbivory are not substitutable in a number of Acacia species (fire results in a decrease in architecture parameters, while herbivory mostly results in an increase), I found that simulated herbivory (clipping) resulted in a decline in root NSC (Fig. 4) and a concomitant increase in stem NSC (Fig. 4). Fornara and Du Toit (2008) speculated that the increased availability of nutrients, perhaps due to dung and urine deposition, resulted in an increased availability of nutrients to A. nigrescens. However, I found that there was no significant effect of nutrients and that clipping only resulted in redistribution of NSC. Interestingly, more NSC were in the stems of A. nigrescens trees that were clipped twice than those clipped once only, despite the fact that I measured these trees almost one year after clipping them the second time. My results indicate that plants may be at least partly independent of soil nutrients (Chapin et al. 1990) because supplementation with key nutrients did not alter their investments in NSC or alter their growth patterns.
Contra the results of Scogings et al. (2013) who found no significant effect on the nutrients of leaves of trees with and without herbivory, I found that repeated clipping negatively affected the concentration of crude protein (which is nitrogen multiplied by 6.25 to convert it to %) in the shoots in this study (Fig. 4a). Wigley et al. (2015) also found that leaf N was higher in Acacia grandicornuta trees with herbivory than without. However, Wigley et al. (2015) found no significant difference for any of the other six species that they studied at the same site. I also found that there was an increase in crude protein in A. nigrescens with clipping in root:shoot ratios (but not in the roots) (Fig. 4b). Here too there was no effect of fertilization. The decline in shoot crude protein indicates that there is insufficient nitrogen for regrowth after repeated loss of shoot material (see also Cherbuy et al. 2001). The increased root:shoot ratio with repeated clipping suggests that more nitrogen is manufactured in the roots in response to repeated clipping (Kim et al. 1991) but that this is insufficient to match the decline in shoot nitrogen. Indeed, a similar response was detected by Thornton and Millard (1997) who found that an increase in the frequency of defoliation of two grass species, Lolium perenne and Festuca rubra, had little effect on the uptake of nitrogen by roots which was subsequently supplied to new leaves. However, these authors found that there was a decrease in the ability of these grasses for the remobilization of nitrogen with repeated clipping. This resulted in a reduction in the growth rate of new leaves. A similar pattern appears evident in the shoots of A. nigrescens in this study.
Conclusions
The dominance of A. nigrescens may result from the fact that there is a positive feedback between herbivory and palatability (as indexed by crude protein or nitrogen), which could ultimately lead to its increase in abundance (Fornara and Du Toit 2007, 2008). The change in aboveground architecture is consistent with the results obtained by Fornara and Du Toit (2007). The redistribution of non-structural carbohydrates from belowground to aboveground with increased clipping suggests a mechanism for the creation of the “bushy” structure of these trees.
Cromsigt and Kuiper (2011) have suggested that these “browsing lawns” should develop in relatively resource-rich environments where plants can invest in tolerance traits. Furthermore, they consider that herbivores should show strong patch selection, but at the same time they should be quite unselective for individual plants within the patch. Cromsigt and Kuiper (2011) believe that the concept of a “browsing lawn” may be overstated because many of the examples used are from relatively nutrient-poor systems with strong selection for individual palatable trees (e.g., Fornara and Du Toit 2007). This may result in switches in the palatability of trees, such that unpalatable species replace palatable ones (see e.g., Bond and Loffell 2001; Gordijn et al. 2012). Their claims may require a group-selectionist argument (community vs. individual) and may result in debates about what a “relatively” resource-rich and “relatively” resource-poor environment really is. The current study shows that nutrients play an unimportant role and that it is clipping per se that is a key to our understanding of a positive feedback between herbivores and trees.
References
Albert CH, Grassein F, Schurr FM, Vieiledent G, Violle C (2011) When and how should intraspecific variability be considered in trait-based plant ecology? Persp Plant Ecol Evol Syst 13:217–225
Archibald S, Bond WJ (2003) Growing tall vs. growing wide: tree architecture and allometry of Acacia karroo in forest, savanna, and arid environments. Oikos 102:3–14
Barthelemy D, Caraglio Y (2007) Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Ann Bot 99:375–407
Bond WJ, Loffell D (2001) Introduction of giraffe changes acacia distribution in a South African savanna. Afr J Ecol 39:286–294
Canadell J, Lopez-Soria L (1998) Lignotuber reserves support regrowth following clipping of two Mediterranean shrubs. Funct Ecol 12:31–38
Canham CD, Kobe RK, Latty EF, Chazdon RL (1999) Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121:1–11
Chapin FS, Schulze ED, Mooney HA (1990) The ecology and economics of storage in plants. Ann Rev Ecol Syst 21:423–447
Cherbuy B, Joffre R, Gillon D, Rambal S (2001) Internal remobilization of carbohydrates, lipids, nitrogen and phosphorus in the Mediterranean evergreen oak Quercus ilex. Tree Physiol 21:9–17
Cromsigt JPGM, Kuiper DPJ (2011) Revisiting the browsing lawn concept: evolutionary interactions or pruning herbivores? Persp Plant Ecol Evol Syst 13:207–215
Donohue K (2003) Setting the stage: phenotypic plasticity as habitat selection. Int J Plant Sci 164:S79–S92
Du Toit JT (2003) Large herbivores and savanna heterogeneity. In: Du Toit JT, Rogers K, Biggs H (eds) The Kruger experience: ecology and management of savanna heterogeneity. Island Press, Washington, DC, pp 292–309
Du Toit JT, Olff H (2014) Generalities in grazing and browsing ecology: using across-guild comparisons to control contingencies. Oecologia 174:1075–1083
Du Toit JT, Bryant JP, Frisby K (1990) Regrowth and palatability of Acacia shoots following pruning by African savanna browsers. Ecology 71:140–154
Farnsworth KD, Niklas KJ (1995) Theories of optimization, form and function in branching architecture in plants. Funct Ecol 9:355–363
Fornara DA, Du Toit JT (2007) Browsing lawns? Responses of Acacia nigrescens to ungulate browsing in an African savanna. Ecology 88:200–209
Fornara DA, Du Toit JT (2008) Responses of woody saplings exposed to chronic mammalian herbivory in an African savanna. Ecosci 15:129–135
Gordijn PJ, Rice E, Ward D (2012) The effects of fire on woody plant encroachment are exacerbated by succession of trees of decreased palatability. Persp Plant Ecol Evol Syst 14:411–422
Hean J, Ward D (2012) Fire and herbivory are not substitutable: evidence from regrowth patterns and changes in physical and chemical defences in Acacia seedlings. J Veg Sci 23:13–23
Johnston DB, Cooper DJ, Hobbs NT (2007) Elk browsing increases aboveground growth of water-stressed willows by modifying plant architecture. Oecologia 154:467–478
Kim TH, Ourry A, Boucaud J, Lemaire G (1991) Changes in source-sink relationship for nitrogen during regrowth of lucerne (Medicago sativa L.) following removal of shoots. Austr J Plant Physiol 18:593–602
Lavorel S, Diaz S, Cornelissen JHC, Garnier E, Harrison SP, McIntyre S, Pausas JG, Perez-Harguindeguy N, Roumet C, Urcelay C (2007) Plant functional types: are we getting any closer to the Holy Grail? In: Canadell JG, Pataki DDE, Pitelka LF (eds) Terrestrial ecosystems in a changing world. Springer, Berlin, pp 171–186
Marais JP (1979) Evaluation of acid hydrolysis procedures for the rapid determination of total non-structural carbohydrates in plant species. Agrochemophys 11:1–3
Marais JP, De Wit JL, Quicke GV (1966) A critical evaluation of the Nelson-Somogyi method for the determination of reducing sugars. Anal Biochem 15:373–381
Martinez AJ, Lopez-Portillo J (2003) Growth and architecture of small honey mesquites under jackrabbit browsing: overcoming the disadvantage of being eaten. Ann Bot 92:365–375
Matejovic I (1996) The application of Dumas method for determination of carbon, nitrogen, and sulphur in plant samples. Rostlinna Vyroba 42:313–316
McNaughton SJ (1984) Grazing lawns: animals in herds, plant form, and coevolution. Am Nat 124:863–886
Moncrieff GR, Chamaille-Jammes S, Higgins SI, O’Hara RB, Bond WJ (2011) Tree allometries reflect a lifetime of herbivory in an African savanna. Ecology 92:2310–2315
Mopper S, Maschinski J, Cobb N, Whitham TG (1991) A new look at habitat structure: consequences of herbivore-modified plant architecture. In: Bell SS, McCoy ED, Mushinsky H (eds) Habitat structure. Chapman and Hall, London, pp 260–280
Niklas KJ, Spatz HC (2004) Growth and hydraulic (not mechanical) constraints govern the scaling of tree height and mass. Proc Nat Acad Sci USA 101:15661–15663
Pastor J, De Jager NP (2012) Simulated responses of moose populations to browsing-induced changes in plant architecture and forage production. Oikos 122:575–582
Schlichting CD, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sinauer Associates, Sunderland, MA
Schutz AE, Bond WJ, Cramer MD (2009) Juggling carbon: allocation patterns of a dominant tree in a fire-prone savanna. Oecologia 160:235–246
Scogings P, Macanda M (2005) Acacia karroo responses to early dormant season defoliation and debarking by goats in a semi-arid subtropical savanna. Plant Ecol 179:193–206
Scogings P, Mamashela TC, Zobolo AM (2013) Deciduous sapling responses to season and large herbivores in a semi-arid African savanna. Austral Ecol 38:548–556
Smit GN (1999) Acacias of South Africa. Briza Press, Pretoria
Staver AC, Bond WJ, Cramer MD, Wakeling JL (2012) Top-down determinants of niche structure and adaptation among African Acacias. Ecol Lett 15:673–679
Stock WD, Bond WJ, Van De Vijver CADM (2010) Herbivore and nutrient control of lawn and bunch grass distributions in a southern African savanna. Plant Ecol 206:15–27
Sultan SE (2003) Phenotypic plasticity in plants: a case study in ecological development. Evol Dev 5:25–33
Thornton B, Millard P (1997) Increased defoliation frequency depletes remobilization of nitrogen for leaf growth in grasses. Ann Bot 80:89–95
Van Der Heyden F, Stock WD (1995) Nonstructural carbohydrate allocation following different frequencies of simulated browsing in three semi-arid shrubs. Oecologia 102:238–245
Van Der Heyden F, Stock WD (1996) Regrowth of a semiarid shrub following simulated browsing: the role of reserve carbon. Funct Ecol 10:647–653
Waldram MS, Bond WJ, Stock WD (2008) Ecological engineering by a mega-grazer: white rhino impacts on a South African savanna. Ecosyst 11:101–112
Ward D (2010) The effects of apical meristem damage on growth and defences of two Acacia species in the Negev desert. Evol Ecol Res 12:589–602
Ward D, Shrestha MK, Golan-Goldhirsh A (2012) Evolution and ecology meet molecular genetics: adaptive phenotypic plasticity in two isolated Negev desert populations of Acacia raddiana at either end of a rainfall gradient. Ann Bot 109:247–255
West GB, Brown JH, Enquist BJ (1999) The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284:1677–1679
Wigley BJ, Cramer MD, Bond WJ (2009) Sapling survival in a frequently burnt savanna: mobilization of carbon reserves in Acacia karroo. Plant Ecol 203:1–11
Wigley B, Bond WJ, Fritz H, Coetsee C (2015) Mammal browsers and rainfall affect Acacia leaf nutrient content, defense, and growth in South African savannas. Biotropica 47:190–200
Acknowledgments
I would like to thank Alison Young for her assistance in the greenhouse and Tamanna Patel and Kayleigh Muller for their laboratory assistance. The National Research Foundation of South Africa funded this study. I thank the anonymous reviewers for their useful comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest for this manuscript.
Additional information
Communicated by E. T. F Witkowski.
Rights and permissions
About this article
Cite this article
Ward, D. Clipping frequency but not nutrients affect the architecture and non-structural carbohydrates of a browsing lawn. Plant Ecol 217, 21–29 (2016). https://doi.org/10.1007/s11258-015-0555-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-015-0555-8