Abstract
Factors affecting the long-term survival and growth of planted understorey species in re-introductions and restoration are poorly known. Seven understorey species were planted in jarrah forest restored after bauxite mining in the Southwest of Western Australia to test the effects of the competitive environment (both over- and understorey) and herbivory by large mammals on survival and growth over a 16-year period. Differences in the overstorey environment were achieved by planting the study species into restored sites differing in age (0, 1, 4 and 10 years of age). The understorey competitive environment was manipulated by removing other understorey species at the time of planting. Reducing both herbivory (using plant guards) and competition with other understorey plants significantly increased survival, spread and height growth of the study species. Planting into different ages of restored site significantly affected plant responses: survival and growth were maximal in either newly restored (year 0) or the oldest (10 years of age) sites. Across species, survival decreased to ~50 % after 2 years, but thereafter mortality was low. These data demonstrate survival and spread of planted species over a 16-year period indicating that planting nursery-raised plants can be a viable strategy for establishing long-term persistent plant populations in both newly restored and older restored sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Re-establishing understorey species is a challenge both for species re-introductions and the restoration of habitats disturbed by external processes (e.g., mining). While numerous studies have monitored plant survival following experimental plantings (e.g., Petersen and Philipp 2001; Mottl et al. 2006), the factors affecting the long-term survival and spread of reintroduced understorey species are poorly known (Van der Veken et al. 2007, 2012; Dalrymple et al. 2011; Godefroid et al. 2011; Drayton and Primack 2012; Maschinski and Haskins 2012).
Few studies on understorey re-establishment monitor species over long time frames. For example, in recent reviews of the available literature on plant establishment, the average duration of monitoring was 3–4 years (Dalrymple et al. 2011, 2012; Godefroid et al. 2011), although there are longer term exceptions (Guerrant Jr 2012). In addition, in a 15-year study of eight reintroduced understorey species in the USA, Drayton and Primack (2012) found that while most plants survived for 5 years after planting, the majority were absent when the plants were re-monitored after a further 10 years, i.e., early plant performance may not necessarily reflect longer term performance. However, Drayton and Primack (2012) did not identify the factors contributing to poor long-term survival. Based on a meta-analysis of published studies, Godefroid et al. (2011) reported that fencing (i.e., reduced herbivory) positively impacted short-term establishment and survival (<4 years post-planting) of plant re-introductions, as did removing surrounding competing vegetation. While the various reviews of establishment success are largely based on studies of the re-introduction of rare and threatened species into existing (relatively undisturbed) vegetation (Dalrymple et al. 2011, 2012; Godefroid et al. 2011), they highlight a need for longer term studies into factors influencing successful plant establishment.
Approximately, 600 ha of jarrah (Eucalyptus marginata) forest is mined (for bauxite) and restored each year in the Southwest of Western Australia by Alcoa of Australia Ltd. One of the restoration goals is the establishment of a species-rich understorey layer. The majority of the understorey species in restored sites originate from either the topsoil seed bank or the applied seed mix (Ward et al. 1996; Koch 2007). Species in the seed mix are characterised by easily collected seeds that have high germination and survival in the field. However, life-history characteristics of some jarrah forest understorey species make restoration challenging. The jarrah forest contains a number of re-sprouter species (sensu Bell 2001). These are long-lived species that can spread clonally and are resilient to disturbances such as fire but generally produce low numbers of seed. Many of these re-sprouter species are abundant in the understorey and are therefore priorities for re-establishment in restored sites. However, compared with re-seeder species, which are either easy to collect or have a large soil seed bank, they are under-represented within restored sites (Norman et al. 2006). As an example, in unmined forest, the re-sprouter Tetraria capillaris (Cyperaceae) occurs at densities of ~10,000 plants ha−1, whereas following bauxite mining and restoration, this species establishes at ≤5 plants ha−1. Due to minimal seed production, species such as T. capillaris are propagated either by tissue culture or cuttings and planted into newly restored sites (Koch 2007). These plants have high value in restored sites, both in terms of resilience of restored sites to disturbance and re-establishing a jarrah forest with a similar species mix to unmined forest. Furthermore, maximising survival is important for cost efficiency.
Many Western Australian re-sprouter species are also comparatively slow growing compared to re-seeder species (Pate et al. 1990; Bowen 1991; Bowen and Pate 1993). For example, lateral spread of T. capillaris following planting into restored areas is ~10 mm year−1 (Stanton-Clements et al. 2013). Consequently, we predict that establishment of these species will be negatively impacted by competition with more rapidly growing understorey re-seeder species (such as Acacia spp.). However, this remains to be tested.
Sites restored after mining activities initially constitute a high light environment due to an absence of established plants (Koch 2007). Since the majority of studies of the re-establishment of understorey species involved planting beneath an established canopy (e.g., Van der Veken et al. 2007, 2012; Drayton and Primack 2012), it is unclear whether the high light post-mining environment is conducive for understorey plant establishment. In addition, the presence of an overstorey canopy can exert a significant moderating effect on the understorey micro-climate (von Arx et al. 2013). Consequently, we predict that plant survival would be lower in newly restored sites particularly in environments with a prolonged summer drought such as the Southwest of Western Australia.
Herbivores (e.g., macropods) can also have significant impacts on plant community composition in newly established mine restoration by preferentially grazing particular plant species (Koch et al. 2004; Parsons et al. 2006). In particular, in newly restored sites, herbivores can move unobstructed through large open areas containing highly visible plants and seedlings which are often nutrient enriched (Koch et al. 2004). Thus, strategies to either reduce herbivory directly (e.g., plant guards) or indirectly, by reducing the visibility of key understorey plants (e.g., by planting into more established vegetation), may be appropriate.
Therefore, an alternative to re-establishing all understorey species at the onset of restoration activities is to plant nursery-propagated plants once the establishing overstorey and understorey vegetation provide some degree of cover. This may have two advantages. First, species may be planted into a more benign environment (in terms of irradiance levels and temperature extremes; von Arx et al. 2013). Second, the already established plants may provide a positive ‘nurse’ plant effect (e.g., Close et al. 2010) via either physical (e.g., spines) or chemical (e.g., toxins) protection from herbivory or by simply reducing the visibility of newly established plants to herbivores (Smit et al. 2007). However, while this strategy might reduce herbivory, beneficial effects from nurse plant are not consistently observed in restoration studies since increased competition resulting from the close proximity of neighbours can outweigh facilitation effects (Gómez-Aparicio et al. 2004; Caldeira et al. 2014; Poulos et al. 2014): facilitation is most likely to be observed with increasing abiotic stress.
The aims of this study were to assess long-term (16 years) survival and spread of re-sprouter understorey species in jarrah forest restored after bauxite mining. In particular, for seven species, we tested the hypotheses that (1) reducing grazing by macropods increases plant survival and spread; (2) removal of initial inter-specific understorey competition (achieved by removing other understorey species at the time of planting) increases survival and spread and (3) planting into older restored sites (up to 10 years of age) increases plant survival and spread by reducing grazing pressure and providing a more benign environment compared with newly restored sites.
Materials and methods
Study site
The experiment was established at Alcoa of Australia’s bauxite mining operations in jarrah forest in the Southwest of Western Australia (32°35′06″ S, 116°06′44″ E) ca. 100–180 km south of Perth. The region has a Mediterranean-type climate with cool, wet winters and hot, dry summers. Average annual rainfall is 1200 mm with an average summer monthly maximum temperature of 28 °C and average winter minimum of 5 °C. The overstorey vegetation within areas that are mined is dominated by jarrah (E. marginata family Myrtaceae) with varying densities of marri (Corymbia calophylla family Myrtaceae). In addition, there is a small-tree component, with Allocasuarina fraseriana (Casuarinaceae), Banksia grandis (Proteaceae), Persoonia longifolia (Proteaceae) and Xylomelum occidentale (Proteaceae) being the most common species. The understorey consists of sclerophyllous shrubs up to 3 m tall (predominantly in the families Ericaceae, Fabaceae, Myrtaceae and Proteaceae) and herbs (predominantly in the families Restionaceae, Orchidaceae, Apiaceae, Liliaceae [sensu lato], Asteraceae and Cyperaceae).
Mine pits range in size from 1 to 20 ha and are adjacent to relatively intact native forest. Alcoa’s restoration goal is to establish a self-sustaining jarrah forest ecosystem that will fulfil pre-mining forest land uses. These include nature conservation, timber production, water catchments and recreation. The restoration process involves reshaping the mine pit to approximate the pre-mining topography, deep ripping to alleviate compaction and placement of fresh topsoil (Standish et al. 2015). Each restored pit has a low point designed to collect water to prevent water release into unmined forest. Fresh topsoil is sourced from areas located nearby (in the same forest type) that have been cleared of vegetation, while the restoration earthworks are being undertaken. Seeds of local plants are also spread over the restored mine pits. Seeds are collected within 20 km of the mined areas and stored at 4 °C prior to use. Planting of nursery grown plants is also carried out for re-sprouter species where direct seeding is not a viable establishment method. These plants are grown from cuttings or tissue culture. A fertiliser mix is then applied by helicopter in late winter or early spring after the completion of restoration activities (Daws et al. 2013).
Experimental design
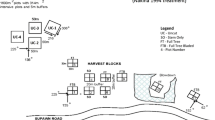
In early winter (July) 1997, seven understorey plant species (Table 1) produced at Alcoa’s Marrinup Nursery were planted into a total of 12 restored mine pits. For the species produced by tissue culture, between 3 and 24 clones were used to produce the plants used in this experiment. The 12 pits comprised 3 newly restored pits (restoration activities completed in 1997), 3 pits restored in 1996 (1-year old), 3 pits restored in 1993 (4-year old) and 3 pits restored in 1988 (10-year old). In this context, completion of restoration activities means that all management-related activities had been undertaken, i.e., fresh topsoil had been placed, and seeding had been completed. Consequently, for the 1, 4 and 10-year-old sites, under- and overstorey species were already present at the time the experiment was established (Fig. 6b, c). For the restoration completed in 1997, seeding had taken place, and initial germinants were starting to emerge when planting of the seven study species took place in winter 2007.
Within each pit, a 36 m × 19 m plot was established and divided into four 6 m × 19 m blocks, with 4 m between blocks. The plot was randomly located within each pit except that the water holding low point within each pit was avoided. Each block was randomly allocated to one of the following treatments: (1) no plant guards with understorey competition (control), (2) no plant guards, understorey competition removed, (3) plant guards with understorey competition and (4) plant guards, understorey competition removed. Within each block, a total of 20 individual plants of each of the seven species were planted representing an overall total of 6720 plants.
The plant guards, which were left in place for the duration of the experiment, were constructed from transparent green plastic sleeves (Nurserymen’s Supplies, Kewdale, Perth, Australia). The sleeves were punctured by eight holes that allowed some airflow and held in place by three jarrah stakes (750 mm high). While the plastic sleeves disintegrate over time (Stanton-Clements et al. 2013), the jarrah stakes were still in place in 2014. In the competition removal treatments, all pre-existing understorey vegetation within the appropriate 6 m × 19 m blocks was manually removed (as a once off treatment) at the time of planting. In the case of the newly established restored pits, where there was minimal vegetation at the time of planting, newly emerging understorey vegetation was removed over the course of the first 12 months of the experiment but not thereafter.
Plants were monitored in winter 1999, 2004, 2008 and 2013. Plant height and survival were monitored on all four occasions, while plant spread (measured at the widest point across the plant) was recorded in 2004, 2008 and 2013. The proportion of live plants that exhibited visible evidence of having been grazed was recorded in 1999, 2008 and 2013.
Characterisation of understorey and overstorey environments at experimental sites
The environments of the four ages of restored sites were characterised both in terms of the percentage cover in the understorey layer and leaf area index (predominately driven by the overstorey environment). Understorey cover within the trial sites was not monitored throughout the course of the experiment and was only measured in 2013. Specifically, in August 2013, within each 6 m × 19 m block, three 2 m × 2 m quadrats were randomly positioned, and the identity and percentage cover was determined for each individual plant. Based on visual assessment, understorey plants were evenly distributed across the treatment blocks. Consequently, for each of the plus and minus competition removal treatments a total of six plots were measured per mine pit. However, the understorey has been monitored annually for a range of similar sites restored between 1987 and 1997. In these sites, 20 m × 20 m monitoring plots have been established, and the identity and percentage cover for every individual plant was recorded in each of five 4 m × 4 m subplots. Data from these plots were used to generate a general time line of changes in the understorey environment.
Prior to 2006, leaf area index maps produced from Landsat images are available for the northern jarrah forest (including the area where the current experiment took place; Mauger et al. 2013). For the 12 study sites, average leaf area index per year for the period since the restoration was established up until 2005 was calculated by overlaying the trial locations with the leaf area index maps using ArcGIS 9 (ESRI, Redlands, California). In addition, in winter 2013, 24 vertical photographs were taken within each site using a Nikon Coolpix 4500 and analysed using digital cover photography (Macfarlane et al. 2007).
Statistical analysis
Analyses for the effects of the age of restored sites at the time of planting, plant guards, competition and time since planting on (1) plant survival, (2) spread and (3) height were undertaken using linear mixed-effects models in the lme4 package (Bates et al. 2011) for R (R Development Core Team 2009). Survival was analysed using a binomial link function using the glmer function, and spread and height were analysed using the lmer function. Restoration age at the time of planting, the use of plant guards, time and competition were treated as fixed effects and site (pit) and subject (individual plant) as random effects. To assess the effect of ‘species’ on the response variables, models were run with and without species as a random factor, and the change in log-likelihood between models was assessed using a χ 2 test.
Lepidosperma squamatum and T. capillaris were the two most heavily grazed species. For these two species, the effect of the age of restored sites at the time of planting on the proportion of plants exhibiting visible evidence of grazing 2 years after planting (i.e., in 1999) was assessed using glmer with a binomial link function. In addition, the effect of time since planting on the proportion of plants that exhibited visible evidence of grazing was assessed using glmer with a binomial link function. In both grazing-related analyses, site (pit) was included as a random effect.
Results
Effects of age of restored sites at time of planting
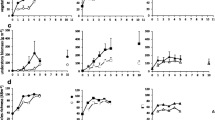
There was a significant effect of the age of restored sites at the time of planting on the three response variables (Table 2). Survival, height growth and spread were highest for plants in newly established restored sites and decreased for 1- and 4-year-old restored sites and then increased for 10-year-old restoration (Fig. 1). There was also a significant interaction between the presence of plant guards and the age of restored sites at the time of planting: guards had a greater effect on plant responses in younger restored sites (Table 2).
Effects of plant guards and understorey competition
Seven years after planting, the use of plant guards had a positive significant effect on survival and height for all seven species (Fig. 2; Table 2). In addition, guards had a positive effect on the lateral spread of the majority of the seven species, with Adenanthos barbiger and Hibbertia commutata being exceptions and spreading to a lesser extent when plant guards were present (Fig. 2c). These generally beneficial effects of plant guards persisted throughout the 16 years of the experiment with the guard × time interaction being non-significant for two of the three response variables (Table 2).
Effect of plant guards on a survival, b height and c lateral growth of seven understorey species monitored 7 years after planting (2004). Error bars are +1 SE of the mean. Also shown in a is the percentage of non-guarded plants exhibiting visible evidence of having been grazed when plants were assessed in winter 1999 (open circles): error bars are ±1 SE of the mean
Removing understorey competition had a significant positive effect on plant survival, height and spread 7 years after the start of the experiment (Fig. 3). However, in the overall analysis including data from the entire 16-year duration of the experiment, only plant height and spread were significantly affected by removing competition (Table 2). The non-significant effect on survival may reflect a reduction in the competition effect over time: the competition × time interaction was significant for plant spread and marginally significant for survival (Table 2). There was also a significant guards × competition interaction for all three response variables: plant guards had a greater effect when competition (and barriers to visibility and access) had been removed (Table 2).
Effect of time and age of restored sites on herbivory
The level of herbivory varied significantly between species: 2 years after planting the percentage of plants grazed ranged from 45.6 to 0.2 % for L. squamatum and Lechanaultia biloba, respectively (Fig. 2a). For the two most heavily grazed species (T. capillaris and L. squamatum), the combined percentage of plants grazed declined significantly with time since planting, from 47 % 2 years after planting to 29 % after 16 years (General Linear Mixed Model, P < 0.05; Fig. 4a). In addition, the initial degree of grazing was highest (74 %) for plants in newly restored sites (year 0) and was lower for plants established in older restored sites reaching 31 % for plants established in 10-year-old restored sites (General Linear Mixed Model, P < 0.05; Fig. 4b).
The effect of (a) time since planting and (b) the age of restoration that plants were established into, on the percentage of plants of Lepidosperma squamatum and Tetraria capillaris combined, that exhibited visible evidence of having been grazed. For the effect of restoration age at the time of planting, grazing was assessed 2 years after planting of the understorey species, i.e., in winter 1999. Error bars are ±1 SE of the mean
Long-term plant performance
Plant survival declined rapidly over the first 2 years of the experiment (Fig. 5a). However, after this time, ongoing mortality was low with survival averaging ~35 % across species and treatments after 16 years (Fig. 5a). Plant spread and height also increased significantly with time (Fig. 5b, c; Table 2). There were also considerable differences in survival and growth responses between species which were reflected in the significant species effect in the linear mixed models (Table 2). For example, plant survival after 7 years ranged from 21.9 to 71.2 %, for Dampiera linearis and L. squamatum, respectively (Figs. 2, 3), and survival after 16 years ranged from 9.7 to 71.2 % for Loxocarya cinerea and L. squamatum, respectively.
Effect of plant guards and competition on a survival, b plant height and c plant lateral spread over time. Data are for all seven species pooled. For comparison, plant height and spread are included for the start of the experiment. These measurements are based on 20 individuals of each species recorded at the nursery just prior to planting. Error bars are ±1 SE of the mean
Characterisation of the understorey and overstorey environments at experimental sites
Sites used for this experiment were restored between 1987 and 1997. For restored sites, the understorey plant cover, for all species present, increases rapidly after establishment reaching a maximum of ~80 % cover by an age of 3 years. After year 5, cover starts to decline reaching ~50 % by year 13 and then remaining at this level to 26 years after establishment. This age (26 years) represents the maximum potential age of restored sites at the end of the 16-year experiment (Fig. 6b). The initial establishment environments for the seven study species planted into the with competition treatments, therefore represent cover values of 0 (but rapidly increasing), ~32 (rapidly increasing), ~80 and ~70 % (with an ongoing decline) cover for 0, 1, 4 and 10-year-old restored sites, respectively. For the competition removal treatment, initial cover values were 0 % for all four ages of restored site. At 16 years after planting (i.e., at the end of the experiment), all cover values in the study sites were consistent with the long-term trend for restored sites (Fig. 6b). However, there was no apparent trend of plots, where the understorey had been removed, having lower percentage cover than plots where the understorey had been left intact. Thus, after 16 years, the understorey had recovered to a similar level despite being initially removed. Indeed, for two of the four ages of restoration (1 and 4 years), cover after 16 years was higher in the understorey removal treatments (Fig. 6a).
a Monitoring periods of the study species planted into restored areas of four different ages in relation to changes over time in trajectories of b total understorey plant cover and c overstorey leaf area index. For understorey cover (b), each data point (filled circles) represents the mean cover value from at least 20, 20 m × 20 m monitoring plots in restored sites, while the leaf area index values in c are from the 12 experimental sites used in this study. Also shown in b are percentage cover values for the competition (i.e., understorey left intact; filled triangles) and competition removal (i.e., understorey initially removed; open triangles) treatments from the 12 experimental sites assessed in winter 2013. Error bars are ±1 SE of the mean
Overstorey, as assessed by leaf area index, increased rapidly between years 0 and 7 reaching a maximum of 2.2. This level was then maintained throughout the course of the experiment (Fig. 6c).
Discussion
We found that survival and growth of our study species were enhanced by reducing herbivory, removing other understorey species and planting into either the youngest or oldest restored sites. Macropod grazing was a key factor in all these responses: plant guards directly reduced grazing impacts and enhanced plant responses, but grazing also modified the response to planting into different ages of restored site and to competition with other understorey species. Thus, due to significantly lower herbivory, plant survival and growth were as high in the competitive environment of older restored sites as in newly restored sites which have high resource availability. Likewise, the benefit of reduced competition from other understorey species was partly offset by increased grazing, presumably due to the study species being more visible to macropods.
The seven study species differed in their susceptibility to grazing, with several, e.g., L. cinerea, exhibiting minimal grazing damage. However, the use of plant guards had a significant positive effect on survival and height growth of all species and on lateral spread of five species throughout the experiment. Positive effects of plant guards have been reported previously in jarrah forest restoration. For example, Stanton-Clements et al. (2013) reported that survival and spread of T. capillaris to 7 years of age were increased using plant guards. While a key mechanism for a plant guard effect is a reduction in grazing (e.g., Stanton-Clements et al. 2013), this is unlikely to explain the positive effects for minimally grazed species (e.g., H. commutata; Fig. 2a). Plastic sleeve plant guards can also affect the micro-climate for plant growth. For example, Close et al. (2009) examined seedling survival and growth of native tree species with different plant guards in Perth Western Australia and showed that plastic guards raised the air temperature by an average of 6.7 °C, while light levels inside guards were twofold lower. Thus, these guards may, in addition to reducing herbivory, provide an enhanced micro-climate for plant growth, although it is of note that the largest positive effect of guards was observed for L. squamatum and T. capillaris which were also the two most heavily grazed species (Fig. 2a).
The reduction in competition from other understorey plants also enhanced height growth and lateral spread of the study species (Table 2) and improved survival after 7 years (Fig. 3a). Similarly, Van der Veken et al. (2012) found that initially removing understorey competition improved survival for three of four understorey herbs transplanted into established woodland, and in a meta-analysis, Godefroid et al. (2011) reported that removing existing ground-later vegetation improved survival. Interestingly, the significant interaction between understorey competition and plant guards in our study indicates that plant guards have a smaller positive effect when the understorey layer is intact. This suggests that when the understorey layer is intact, the study species were less visible and/or accessible to herbivores. Similarly, Stanton-Clements et al. (2013) reported that planting Acacia seedlings next to newly established T. capillaris reduced grazing pressure over time because plants were less visible to herbivores, although Acacia ‘nurse’ plants were less effective than using plant guards. Consequently, this raises the challenge that while minimising competition with existing vegetation has beneficial effects for plant establishment, the presence of pre-existing plants may also have beneficial effects such as reducing herbivory. For example, in a study in montane rainforest, Anthelme et al. (2014) reported that transplanted palm seedlings planted adjacent to the highly competitive environment of grass tussocks exhibited both higher survival and less herbivory than plants in an essentially competition free environment in the open. Resolving where particular restoration projects fall on this competition–facilitation spectrum may lead to strategies that improve both plant survival and spread.
The initial benefit of reduced competition was sustained throughout the entire 16-year course of the experiment. We do not know when the initial differences in cover between the competition removal treatments disappeared, although there was no longer any trend in understorey cover between the two treatments when sites were monitored after 16 years (Fig. 6). This equalisation in understorey environments over time is consistent with the significant competition × time interaction (for survival and plant spread) which indicates that the effect of initially removing competition declined over the 16-year course of the experiment. Nonetheless, the sustained benefit on plant growth, even though understorey cover levels had equalised between the two treatments during the experiment, reinforces the initial benefit of plants ‘hitting the ground running’: even after 16 years, plants establishing in the initially more competitive environment had failed to ‘catch up’ with plants in the competition removal treatment.
Plant establishment and growth were highest when plants were established into either newly restored sites or older restored sites (10 years of age). These are contrasting environments: one has high irradiance and minimal competition and the other has a closed canopy and presumably high competition from both established overstorey and understorey (Fig. 6a, b). One possible explanation for these divergent responses is that newly restored sites provide an optimal site for maximising initial establishment and growth (i.e., high light, water and nutrient availability; Koch and Samsa 2007; Grant et al. 2007), albeit with the downside of a higher grazing pressure (Koch et al. 2004; Fig. 4b). However, as the restored sites mature, the understorey cover, and by implication the degree of competition with other understorey plants, increases to a maximum at ~6 years, before declining by year 10 and beyond (Fig. 6a). Thus, in this experiment, competition with established understorey plants was likely lowest at years 0 and 10: planting in years 1 and 4 meant that plants were establishing into a highly competitive understorey environment with rapidly increasing understorey cover. Conversely, planting after 10 years had the advantage of a reduced and declining understorey layer. In addition, grazing pressure was lower when planting into the 10-year-old restored sites (Fig. 4b), probably because these sites are more difficult for macropods to traverse and understorey plants are less visible, due to the presence of large quantities of senesced understorey species (especially Acacia species; Grant et al. 2007). This effect of reduced grazing on plants established into older restored sites was also supported by the significant guards × restoration age interaction which indicated that guards had a greater positive effect for plants established into younger restored sites.
Drayton and Primack (2012) reported that of the eight herb species they planted, six had completely disappeared after 15 years. Van der Veken et al. (2012) found that for four planted woodland herbs, survival averaged 42 % after 7 years, and Mottl et al. (2006) reported survival of 57 % across 24 species of herb over a 7-year period. In our current study, average survival after 16 years was 35 and 50 % when competition and grazing were reduced: these survival values compare favourably in the context of the wider literature. Nonetheless, these overall survival values mask considerable differences between species which were also apparent after 7 (Figs. 2, 3) and 16 years. In particular, survival after 16 years for L. cinerea and D. linearis was only 9.7 and 12.9 %, respectively. While reasons for differential survival across these seven species are unknown, it is possible that the species with lower survival require fire or more open sites for successful establishment: leaf area index values for these study sites (Fig. 6c) are higher than the typical range observed in jarrah forest that exhibits old growth characteristics (typical range of 1.27–1.80; Macfarlane et al. 2010). For these poorer performing species, planting into restored sites may not be an efficient use of resources without further investigation into reasons for poor performance.
Guerrant Jr (2013) indicated that while initial survival of planted understorey species may be low, low early survival is not a reliable indicator of impending failure. Similarly, in our current study, the majority of plant mortality occurred within 2 years of planting, with longer term survival being relatively constant over time (Fig. 5). While initial mortality may reflect factors such as transplant shock, plant damage at the time of planting or high initial grazing, a failure to undertake longer term monitoring may inflate the apparent importance of these factors and falsely influence perceptions of the success of species re-establishment. In conclusion, our current study demonstrates the value of longer term monitoring in indicating species persistence and spread and identifying the enduring positive effects of plant guards and reduced initial understorey competition on plant performance.
References
Anthelme F, Gomez-Aparicio L, Montufar R (2014) Nurse-based restoration of degraded tropical forests with tussock grasses: experimental support from the Andean cloud forest. J Appl Ecol 51:1534–1543
Bates D, Maechler M, Bolker B (2011) Lme4: linear mixed-effects models using S4 classes. R package version 0.999375-42. http://CRAN.Rproject.org/package=lme4. Accessed March 2013
Bell DT (2001) Ecological response syndromes in the flora of southwestern Western Australia: fire resprouters versus reseeders. Bot Rev 67:417–440
Bowen BJ (1991) Fire response within the family Proteaceae: a comparison of plants displaying the seeder and resprouter mode of recovery. PhD Thesis, University of Western Australia, Perth
Bowen BJ, Pate JS (1993) The significance of root starch in post-fire shoot recovery of the resprouter Stirlingia latifolia R. Br. (Proteaceae). Ann Bot 72:7–16
Caldeira MC, Ibanez I, Nogueira C, Bugalho MN, Lecomte X, Moreira A, Pereira JS (2014) Direct and indirect effects of tree canopy facilitation in the recruitment of Mediterranean oaks. J Appl Ecol 51:349–358
Close D, Ruthrof K, Turner S, Rokich D, Dixon K (2009) Ecophysiology of species with distinct leaf morphologies: effects of plastic and shadecloth tree guards. Restor Ecol 17:33–41
Close DC, Davidson NJ, Churchill KC, Corkrey R (2010) Establishment of native Eucalyptus pauciflora and exotic Eucalyptus nitens on former grazing land. N For 40:143–152
Dalrymple SE, Stewart GB, Pullin AS (2011) Are re-introductions an effective way of mitigating against plant extinctions? CEE review 07-008 (SR32). Collaboration for Environmental Evidence. www.environmentalevidence.org/SR32.html. Accessed June 2014
Dalrymple SE, Banks E, Stewart GB, Pullin AS (2012) A meta-analysis of threatened plant reintroductions from across the globe. In: Maschinski J, Haskins KE (eds) Plant reintroduction in a changing climate: promises and perils, the science and practice of ecological restoration. Island Press, Washington, DC, pp 31–50
Daws MI, Standish RJ, Koch JM, Morald TK (2013) Nitrogen and phosphorus fertilizer regime affect jarrah forest restoration after bauxite mining in Western Australia. Appl Veg Sci 16:610–618
Drayton B, Primack RB (2012) Success rates for reintroductions of eight perennial plant species after 15 years. Restor Ecol 20:299–303
Godefroid S, Piazza C, Rossi G, Buord S, Stevens A-D, Aguraiuja R, Cowell C, Weekley CW, Vogg G, Iriondo JM, Johnson I, Dixon B, Gordon D, Magnanon S, Valentin B, Bjureke K, Koopman R, Vicens M, Virevaire M, Vanderborght T (2011) How successful are plant species reintroductions? Biol Conserv 144:672–682
Gómez-Aparicio L, Zamora R, Gómez JM, Hódar JA, Castro J, Baraza E (2004) Applying plant facilitation to forest restoration: a meta-analysis of the use of shrubs as nurse plants. Ecol Appl 14:1128–1138
Grant CD, Ward SC, Morley SC (2007) Return of ecosystem function to restored bauxite mines in Western Australia. Restor Ecol 15:S94–S103
Guerrant EO Jr (2012) Characterising two decades of rare plant reintroductions. In: Maschinski J, Haskins KE (eds) Plant reintroduction in a changing climate: promises and perils, the science and practice of ecological restoration. Island Press, Washington, DC, pp 9–29
Guerrant EO Jr (2013) The value and propriety of reintroduction as a conservation tool for rare plants. Botany 91:v–x
Koch JM (2007) Restoring a jarrah forest understorey vegetation after bauxite mining in Western Australia. Restor Ecol 15:S26–S39
Koch JM, Samsa GP (2007) Restoring jarrah forest trees after bauxite mining in Western Australia. Restor Ecol 15:S17–S25
Koch JM, Richardson J, Lamont BB (2004) Grazing by kangaroos limits the establishment of the grass trees Xanthorrhoea gracilis and X. preissii in restored bauxite mines in eucalypt forest of southwestern Australia. Restor Ecol 12:297–305
Macfarlane C, Grigg A, Evangelista C (2007) Estimating forest leaf area using cover and fullframe fisheye photography: thinking inside the circle. Agric For Meteorol 146:1–12
Macfarlane C, Bonda C, White DA, Grigg AH, Ogdena GN, Silberstein R (2010) Transpiration and hydraulic traits of old and regrowth eucalypt forest in southwestern Australia. For Ecol Manag 260:96–105
Maschinski J, Haskins KE (eds) (2012) Plant reintroduction in a changing climate: promises and perils, the science and practice of ecological restoration. Island Press, Washington, DC
Mauger GW, Grigg AH, Croton JT (2013) Preparation of a long time series of leaf area index grids for the northern jarrah forest, Western Australia. Alcoa World Alumina Australia, Environmental Research Bulletin No. 42. ISSN 1320-4807
Mottl LM, Mabry CM, Farrar DR (2006) Seven-year survival of perennial herbaceous transplants in temperate woodland restoration. Restor Ecol 14:330–338
Norman MA, Koch JM, Grant CD, Morald TK, Ward SC (2006) Vegetation succession after bauxite mining in Western Australia. Restor Ecol 14:278–288
Parsons MH, Koch JM, Lamont BB, Vlahos S, Fairbanks MM (2006) Planting density effects and selective herbivory by kangaroos on species used in restoring forest communities. For Ecol Manag 229:37–49
Pate JS, Froend RH, Bowen BJ, Hansen A, Kuo J (1990) Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of S. W. Australia. Ann Bot 65:585–601
Petersen PM, Philipp M (2001) Implantation of forest plants in a wood on former arable land: a ten year experiment. Flora 196:286–291
Poulos JM, Rayburn AP, Schupp EW (2014) Simultaneous, independent, and additive effects of shrub facilitation and understory competition on the survival of a native forb (Penstemon palmeri). Plant Ecol 215:417–426
R Development Core Team (2009) R 2.9.0. The R Foundation for Statistical Computing, Vienna
Smit C, Vandenberghe C, Den Ouden J, Müller-Schärer H (2007) Nurse plants, tree saplings and grazing pressure: changes in facilitation along a biotic environmental gradient. Oecologia 152:265–273
Standish RJ, Daws MI, Gove AD, Didham RK, Grigg AH, Koch JM, Hobbs RJ (2015) Long-term data suggest jarrah-forest establishment is resistant to climate variability. J Ecol 103:78–89
Stanton-Clements EM, Koch JM, Daws MI (2013) Effectiveness of plant guards in reducing grazing of Tetraria capillaris in restored bauxite mines in Western Australia. S Afr J Bot 87:4–8
Van der Veken S, Rogister J, Verheyen K, Hermy M, Nathan R (2007) Over the (range) edge: a 45-year transplant experiment with the perennial forest herb Hyacinthoides non-scripta. J Ecol 95:343–351
Van der Veken S, De Frenne P, Baeten L, Van Beeka E, Verheyen K, Hermya M (2012) Experimental assessment of the survival and performance of forest herbs transplanted beyond their range limit. Basic Appl Ecol 13:10–19
von Arx G, Pannatier EG, Thimonier A, Rebetez M (2013) Microclimate in forests with varying leaf area index and soil moisture: potential implications for seedling establishment in a changing climate. J Ecol 101:1201–1213
Ward SC, Koch JM, Ainsworth GL (1996) The effect of timing of rehabilitation procedures on the establishment of a jarrah forest after bauxite mining. Restor Ecol 4:19–24
Willyams D (2005) Tissue culture of geophytic rush and sedge species for revegetation of bauxite mine sites in the northern jarrah forest of Western Australia. In: Bennett IJ, Bunn E, Clarke H, McComb JA (eds) Contributing to a sustainable future. Proceedings of the Australian Branch of the IAPTC&B, Perth, Western Australia, pp 226–241
Acknowledgments
Matthew Daws is a paid employee of Alcoa of Australia Ltd., and John Koch is a former employee of Alcoa. Dr. Sam Ward, Larry Hantler, Alex Ruschmann, Cameron Richardson and Tim Morald provided assistance with either initial trial establishment or ongoing monitoring.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jodi Price.
Rights and permissions
About this article
Cite this article
Daws, M.I., Koch, J.M. Long-term restoration success of re-sprouter understorey species is facilitated by protection from herbivory and a reduction in competition. Plant Ecol 216, 565–576 (2015). https://doi.org/10.1007/s11258-015-0459-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-015-0459-7