Abstract
A favorable leaf water balance can be achieved through coordinated variation in multiple leaf traits. However, the role of evolutionary history in producing this trait coordination is not well understood, especially in epiphytic species. To address these limitations, we measured 11 leaf traits for 19 Dendrobium species grown in the same environment, and used phylogenetically independent contrasts to test how the co-evolution of these traits has contributed to leaf water balance in epiphytes. Our results show that more closely correlated species had similar habitats, geographical distribution, and some leaf traits (leaf density, upper cuticle thickness, and stomatal index), whereas distantly related species did not exhibit such similarities. Species originating from forests below the altitude of 1,500 m exhibited smaller leaf area, stomatal area, and stomatal index, but thicker leaves than those from forests above the altitude of 1,500 m. Stomatal density was evolutionarily correlated with vein density, upper and lower epidermal thicknesses, and leaf density. Leaf thickness was significantly correlated with stomatal density and vein density, while leaf area was significantly correlated with epidermal cell thicknesses, stomatal density, and stomatal area. These results found that both the environment and evolutionary history significantly affected leaf functional traits and ecological characteristics in Dendrobium, and supported the hypothesis that co-evolution in leaf traits is an important contributor to leaf water balance in Dendrobium. This study provides new insights into the evolution of ecological strategies in epiphytic orchids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leaf water balance, or the balance between water supply to the leaf and evaporative loss, is collectively determined by many leaf traits (Brodribb and Jordan 2011). While the venation and stomata are the primary drivers of leaf water balance due to their roles in controlling hydraulic supply and evaporative loss, respectively, other leaf morphological and anatomical traits are also important contributors (Brodribb et al. 2007; Franks and Beerling 2009). A higher vein density increases the number of vascular pathways for water transport through the mesophyll, thereby increasing hydraulic conductivity and photosynthetic capacity (Sack and Frole 2006; Brodribb et al. 2007; Brodribb and Jordan 2011), while a greater stomatal density can increase both photosynthetic capacity and evaporation (Hetherington and Woodward 2003; Xu and Zhou 2008). Greater leaf thickness increases the mesophyll path length between the veins and stomata, decreasing the mesophyll water conductance, total hydraulic conductance, and photosynthetic capacity (Brodribb et al. 2007), while greater cuticular thickness is hypothesized to decrease cuticular water permeability and reduce evaporative water loss through the epidermis (Kerstiens 1996; Riederer and Schreiber 2001). Moreover, leaf size and density are easily measured proxies that are structurally, functionally, allometrically, and developmentally linked to a wide range of traits that influence leaf water balance (Niinemets and Sack 2006; Sack and Holbrook 2006; Dunbar-Co et al. 2009; Kembel and Cahill 2011). Through their effects on leaf water supply and evaporative loss, these traits are expected to be crucial determinants of overall leaf water balance.
Plants adapt to their habitat water availability through coordinated variation or trade-offs among multiple leaf traits related to water supply, maintenance, and evaporative loss (Franks and Beerling 2009; Brodribb and Jordan 2011). For example, small leaf size, thick and dense blades, and thick leaf cuticles often coordinate in dry habitats to minimize evaporation and maintain leaf moisture (Cunningham et al. 1999; Wright and Westoby 2002). Dense venation tightly correlates with high stomatal density under water deficit conditions to decrease water delivery resistance, and to improve leaf water conductance (Brodribb and Jordan 2011). Elucidating the functional and evolutionary drivers of coordination in these traits is fundamental to our understanding of plant adaptation to water stress (Zhang et al. 2012b).
Although many previous studies have explored the influence of trait coordination on leaf water balance, these studies are limited by the use of distantly related species, a failure to address the evolutionary as well as environmental drivers of trait coordination, and the under-representation of diverse life forms. Trait correlations among closely related species have not been frequently tested (Dunbar-Co et al. 2009), despite a growing realization that these correlations may provide more meaningful insights into plant adaptation than trends across broadly diverse species (Edwards 2006). While many studies address the environmental drivers of trait coordination, only a few studies investigate the phylogenetic drivers (Ackerly and Reich 1999; Santiago and Kim 2009; Zhang et al. 2012b). As both environment and phylogeny commonly affect leaf trait variation, phylogeny cannot be ignored, especially within closely related species (Ackerly 1999). Furthermore, while many previous studies have examined trait coordination in woody dicots, trait coordination in other life forms is poorly understood (Zhang et al. 2012b, 2014). Notably, the epiphytic life form is an especially valuable system for investigating leaf water balance because they experience greater water limitation than terrestrial plants (Zotz and Hietz 2001; Laube and Zotz 2003; Zotz and Bader 2009), and water scarcity has been found to be the primary constraint on their growth (Zotz and Hietz 2001; Laube and Zotz 2003). Epiphytes also contribute importantly to the biodiversity and nutrient and water cycling of forest ecosystems (Hsu et al. 2002; Stuntz et al. 2002). Therefore, in this study, we investigated the effect of phylogeny on the coordination of leaf morphological and anatomical traits that have an important influence on leaf water balance within a genus of epiphytes.
The epiphytic life form, which is characteristic of tropical and subtropical forests, evolved independently in many plant families, but more than 80 % of all epiphytic species are monocotyledons (Zotz 2013). Dendrobium, one of the most diverse epiphytic genera, is mainly distributed in tropical and subtropical sparse evergreen broad-leaved forests, mountain forests, and mountain valleys (Stern et al. 1994; Ji 1999). China is home to 76 species and 2 varieties of this genus, all of which live epiphytically in tree trunks and/or on rock surfaces, and exhibit distinctive ecological diversification (Ji 1999). In natural conditions, Dendrobium species are thought to be highly drought tolerant due to having smaller and thicker leaves compared to many other dicotyledons (Blonder et al. 2012). These species also have relatively low stomatal density compared to dicotyledons (Kelly and Beerling 1995). However, no studies have examined their coordination in the leaf traits that maintain leaf water balance.
For these reasons, we performed the experiment with all species growing in the same environment and used evolutionary comparative methods to examine trait coordination in leaf water balance within a phylogenetic context across 19 Dendrobium species from diverse habitats. We hypothesized that (1) species from different habitats have different leaf attributes; (2) phylogeny would have a significant effect on leaf attributes within Dendrobium; and (3) significantly correlated evolution would occur in different leaf traits to maintain leaf water balance, due to their shared selective pressure of water limitation.
Materials and methods
Study site and plant materials
This study was conducted in an orchid garden in Xishuangbanna Tropical Botanical Garden (XTBG; 21°41′N, 101°25′E; elevation 570 m), which is located in SW Yunnan Province, China. In this area, the climate is dominated by the southwest Asian monsoon, with a well-defined contrast between wet and dry seasons. The mean annual temperature is 21.7 °C, with the monthly mean temperature ranging from 14.8 °C (January) to 25.5 °C (July). Mean annual precipitation is approximately 1,560 mm, with about 85 % occurring during the wet season (May–October). In this orchid garden, the woody dicotyledonous species Lagerstroemia villosa Wall. ex Kurz, Melia toosendan Sieb. et Zucc., Litsea glutinosa (Lour.) C.B. Rob., Litsea liyuyingi Lion, Phoebe lanceolata (Wall. ex Nees) Nees, Gardenia sootepensis Hutchins., and Mesua ferrea L. are used as the host plants for the tested Dendrobium species.
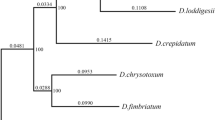
To minimize the confounding effects of environment, we measured leaf traits for 19 species of Dendrobium that have grown in this orchid garden for many years. Each species was represented by at least six individuals distributed randomly throughout the garden. Among 19 species, voucher specimens of 15 species have been collected and deposited in the Herbarium of XTBG (HITBC) by previous researchers, and the others were identified by an orchid specialist at XTBG. We preserved the specimens of these species in HITBC (details are presented in Table S1). The sampled species were widely distributed across the Dendrobium phylogeny (Fig. 1) and represented a wide range of plant functional attributes (Tables 1, 2).
In order to explore the effects of the original habitats on leaf traits, we collected data on the native ecological information of the 19 Dendrobium species, including altitude, habitat, and climatic zone in China and abroad from Flora of China (Ji 1999). Detailed species and their ecological information are listed in Table S2. These species were divided into three groups: group 1 including the species present in tropical sparse evergreen broad-leaved forests with maximum altitude <1,500 m, group 2 including the species present in tropical and subtropical sparse evergreen broad-leaved forests and mountain forests with maximum altitude >1,500 m, and group 3 containing the species present in tropical and subtropical mountain valleys and dense forests with maximum altitude >1,500 m.
The measurements were conducted in the wet season (from July to September), and 6–10 healthy plant individuals of each species were randomly selected as experimental materials, depending on the number of healthy individuals present. From each individual, 2–5 fresh, undamaged, and mature leaves were harvested, sealed in plastic bags, and then immediately taken to the laboratory near the orchid garden for leaf trait measurements (sample sizes detailed in Table S3). This sample size meets the statistical and experimental needs for investigations (Baraloto et al. 2010).
Leaf trait measurements
In the laboratory, we randomly divided the leaves of each species into two parts. The first part included six leaves from different individuals per species to measure anatomical traits. We then numbered each of the other leaves belonging to the second part, and immediately assessed its fresh mass (FM). Saturated mass (SM) was determined after immersing the leaf in distilled water for 24 h. After measuring the saturated mass, each leaf was then assessed for leaf area (LA) with a Li-Cor 3000A area meter (Li-Cor Inc., Lincoln, NE, USA) and blade thickness (BT) with a vernier caliper (precision 0.01 mm; Guanglu, Guilin, China), then dried for 72 h at 70 °C until it reached a constant mass to obtain dry mass (DM). Relative water content (RWC, %) was calculated as (FM–DM)/(SM–DM) × 100. Leaf tissue density (LD, kg m−3) was calculated as leaf biomass per unit volume, with leaf volume calculated as the product of LA and BT. These blade thicknesses were only used for calculating LD, but not considered to be the same as the leaf thicknesses (LT) measured from microscope sections.
To characterize leaf anatomy, we cut the leaves of the first part in half lengthwise, then hand-cut transverse sections at the midpoint of one halves, stained the sections for 2 min with 0.1 % toluidine blue, rinsed them with distilled water, and photographed these sections at 200× (for measuring leaf thickness) and 400× magnification (for measuring tissue and epidermal thicknesses) under a DM2500 light microscope (Leica Inc., Bensheim, Germany). Leaf thickness (LT), upper cuticle thickness (UCT), upper epidermal thickness (UET), and lower epidermal thickness (LET) were then measured from the digital photographs with the software ImageJ v.1.48 (http://rsbweb.nih.gov/ij/).
The abaxial midpoints of the other halves of the reserved leaves were then pasted onto pellucid enamels, and then transferred to glass slides after drying. The stomatal prints on the enamels were photographed under the DM2500 light microscope, and stomatal traits were measured with ImageJ software. Stomatal density (SD) was measured as the number of stomata per unit area, and was calculated as the mean value of 60 digital images from each species (10 images per leaf). Stomatal length (SL) and width (SW) were averaged from 60 randomly selected stomata for each species. The stomatal area (SA) was estimated by the formula 1/4 × π × SL × SW (Sack et al. 2003) and the stomatal index (SI) was estimated as [S/(E + S)] × 100, where S and E are the numbers of stomata and surrounding epidermal cells, respectively, within a given area (Royer 2001).
After measuring the stomata, we completely removed the enamel and used the same half of the leaves to measure vein density. The leaves were boiled for 20 min in 5–7 % NaOH aqueous solution and soaked for 30 min in distilled water, then bleached for 20 min in 5 % sodium hypochlorite and soaked for another 30 min in distilled water. The leaves were then stained for 2 min with 1 % toluidine blue, mounted on slides, and photographed. Total vein length was measured manually with ImageJ software and vein density (VD) was calculated as total vein length per area.
Phylogenetic tree
A phylogram (Fig. 1) was reconstructed based on a concatenated molecular sequence data of four gene regions: Internal Transcribed Spacers (ITS), rbcL, the chloroplast gene trnK–matK, and the chloroplast gene ycf5. These gene sequences were downloaded from GenBank (http://www.ncbi.nlm.nih.gov), and their accession numbers are presented in Table S1. Bulbophyllum odoratissimum was chosen as the Outgroup because of its close relationship to Dendrobium (Freudenstein and Rasmussen 1999). Multiple alignments were automatically performed using CLUSTALW in MEGA v.5.0 (Higgins et al. 1994) and slight corrections were performed manually. Phylogenetic analysis for the matrix was carried out by Bayesian analyses in MrBayes v.3.2 (Ronquist and Huelsenbeck 2003). These analyses used the best-fit models selected by Akaike information criterion (Akaike 1974) using the software Modeltest v.3.7 (Posada and Crandall 1998). The GTR (generalized time reversible) + I (Invariant Sites) + G (Gamma shape) model best fits these data. In the Bayesian analyses, trees were generated by running Metropolis-coupled Monte Carlo Markov (MCMC) chains and sampling one tree every 100 generations for 1,000,000 generations, starting with a random tree. The posterior probability (PP) was used to estimate nodal robustness.
Data analysis
Before analysis, all data were log10 transformed to improve normality and homoscedasticity. Comparison of leaf traits among different native habitats was conducted by a one-way ANOVA, with the groups based on ecological data as the independent factor, and all leaf traits as the dependent variables.
Phylogenetically independent contrasts (PICs) are designed to detect correlated evolution or a relationship between evolutionary changes in two traits (Felsenstein 1985; Price 1997). Species means and PICs produce identical correlations when the phylogenetic effect is strong, while differences in the correlations identified by these methods are biologically informative, reflecting correlations changing as an adaptive radiation proceeds; contrasts, as a means of investigating past history, play more useful role than species values (Price 1997).
To evaluate the evolutionary history of these leaf traits, we first tested for a phylogenetic signal in each trait using the K-statistic, which is based on a “Brownian motion model” of trait evolution, to measure how the sum of variation in a quantitative trait relates to the expected value, with testing the significance level by comparing the variance of standardized contrasts to random values which were obtained by mixing trait data across the tips of the tree (Blomberg et al. 2003). The K metric can be used to assess phylogenetic conservatism, or the tendency for more closely related species to possess similar trait values due to inheritance from a common ancestor, with K > 1 indicating that a trait is more conserved than expected from Brownian motion (Blomberg et al. 2003). K < 1 indicates that a trait is significantly less conserved than expected from Brownian motion, and instead demonstrates significant lability, while K = 1 shows that a trait value is as expected from a Brownian-motion model (Blomberg et al. 2003). The K-statistic and associated p values were estimated using the “picante” package in R program v.3.01 (Kembel et al. 2010).
We tested node contrasts of leaf traits using PICs by the “analysis of traits (AOT)” module in Phylocom software (Webb et al. 2008). Contrasts in traits among descendants of each node are statistically independent in the phylogeny. These contrasts were calculated as trait difference between two sister species-pairs at the tips, and were subsequently weighted for an internal node average. Then they were divided by the expected amount of change, which was calculated as the square root of the branch length separating the two taxa. At last, these contrasts provided N-1-independent data points, with each representing an evolutionary divergence (Ackerly 1999; Ackerly and Reich 1999), where N is the number of tips in the phylogeny (N = 19). We explored node contrasts correlations to evaluate whether a selected trait was consistently correlated with divergence of another trait during their evolution, and considered statistically significant correlations between contrasts as evidence for correlated evolution of corresponding leaf traits (Kembel and Cahill 2011).
Contrasts correlations were evaluated with “Pearson” product-moment regression in R statistical program. A principal component analysis (PCA) was also performed with the “prcomp” function of the “vegan” package in R program to analyze the associations among the 11 studied leaf traits.
Results
All leaf traits varied significantly across Dendrobium species (Table 1). Leaf area (LA) had the biggest variation (CV = 66.6 %), while leaf relative water content (RWC) had the smallest variation (CV = 3.4 %). In addition, leaf density (LD) also varied greatly (CV = 42.5 %). Different Dendrobium species also varied considerably in anatomical traits; upper epidermal thickness (UET) varied the least (CV = 33.9 %), while vein density (VD) varied the most (CV = 56.1 %). The maximum variation for stomatal traits was stomatal density (SD; CV = 57.5 %), and the minimum was stomatal index (SI; CV = 22.9 %).
Habitat had a significant effect on leaf attributes in Dendrobium (Table 2). Species from tropical sparse evergreen broad-leaved forests with maximum altitude <1,500 m (group 1) had significantly lower LA, SA, and SI, but much larger LT than those from tropical and subtropical sparse evergreen broad-leaved or mountain forests with maximum altitude >1,500 m (group 2) or from tropical and subtropical mountain valleys and dense evergreen broad-leaved forests with maximum altitude >1,500 m (group 3). However, all included leaf traits in the latter two groups were not statistically different (Table 2). Based on the information about native habitats and the phylogenetic tree of Dendrobium, we found that the closely related species were distributed in more similar native habitats than those distantly related species (Fig. 1).
In the PCA of species values, the first two principle components explained 38.0 and 17.7 %, respectively, of the total variation (Fig. 2). The first PC axis was positively correlated with UET, LET, and SA, but it was negatively correlated with LD, VD, UCT, and SD (Table 3). The second PC axis was positively correlated with LD, but it was negatively correlated with LT (Table 3). Species from group 1 were separated from groups 2 and 3 along the PC axis (Fig. 2). This indicates that species in the forests below the altitude of 1,500 m are associated with a higher capacity for water storage and retention (higher LT) while those in the forests above the altitude of 1,500 m were associated with a higher rate of water loss (larger LA, SA and SI).
Principal Component (PC) Analysis for 11 leaf traits of 19 Dendrobium species. Loading of species distribute in different habitats (triangles species belonging to group 1; circles species belonging to group 2; stars species belonging to group 3) along PC axis are presented in Panel (b). Abbreviations for leaf traits and plant species are defined in Table 1 and Table S2, respectively
Although all leaf traits varied strongly across Dendrobium species (Table 1), LD, UCT, and SI exhibited statistically significant phylogenetic signals (K > 1), indicating strong phylogenetic conservatism (Table 3). However, K values of the other leaf traits were significantly lower than expected from Brownian motion, showing that these traits exhibit significant lability (Table 3).
SD, LT, and VD were significantly correlated after correcting for phylogeny (Fig. 3). Both SD and SA were evolutionarily correlated with UET and LET (Fig. 4). Additionally, as shown in Table 4, LA was positively correlated with UET, LET, and SA, and negatively correlated with SD. However, LD was only significantly correlated with SD (Table 4).
Discussion
This study shows that both habitat and phylogeny affect the variation of leaf traits within Dendrobium, and that significant correlated evolution has occurred in most of the tested leaf traits to maintain leaf water balance.
Leaf functional traits in relation to the native habitat
In this study, we found that the native habitats had significant effects on the variations in leaf area (LA), stomatal area (SA), stomatal index (SI), and leaf thickness (LT): species from the forests below the elevation of 1,500 m (group 1) had significantly lower LA, SA, and SI but larger LT than those from the forests above 1,500 m (groups 2 and 3). However, the tested leaf traits were not significantly different in all of the forests above the altitude of 1,500 m (Table 2). PCA results also showed that species from group 1 were separated from groups 2 and 3 along the PC axis (Fig. 2b). LA, SA, and SI are all linked to leaf stomatal conductance and water loss (Scoffoni et al. 2011; Sack et al. 2012), while LT is related to leaf water storage (Watkins and Cardelús 2012). Smaller leaves have more rapid cooling by thinner vapor boundary layer than larger leaves, so as to reduce the cooling by evaporation (Nicotra et al. 2008). Smaller stomata can close quickly in water-deficient conditions also to reduce water loss by transpiration (Xu and Zhou 2008). Species with thicker leaves and greater water storage capacity can maintain more stable hydraulic functioning during drought periods, and hence often occur in dry habitats (Cunningham et al. 1999; Wright and Westoby 2002). In Southwest China, mean annual temperature decreases with increasing altitude while mean annual precipitation is curvilinearly related to altitude, increasing up to mid-altitudes but then decreasing with further increase toward mountaintop altitudes (Zhang et al. 2011). This may explain why the Dendrobium species from the forests below the altitude of 1,500 m exhibited more obvious drought-tolerant characteristics than those from forests above the altitude of 1,500 m, as the leaves in areas below mid-altitude would suffer more risk of water stress and overheating.
Our phylogenetic tree of the tested Dendrobium species showed that more closely related species are distributed in much more similar native habitats than the distantly related species are (Fig. 1). Habitats and climatic zones induce plant accommodation, and then adaptive radiation in Dendrobium (Conran et al. 2009). Phenotypic divergence and speciation in such adaptive radiation are ultimately caused by divergent natural selection arising from differences in habitat and competition between species, which may explain why more closely related species tend to be distributed in more similar native habitats than those distantly related species are (Hodges and Derieg 2009).
Phylogenetic effects on leaf functional characters
Consistent with our second hypothesis, phylogeny had a significant effect on leaf characters within Dendrobium. Two pieces of evidence support the existence of such an effect: closely related species have similar native habitats (Fig. 1) and the distribution of species clusters on the PC graph (Fig. 2). As all plants used in this study were cultivated in the same environment, this would reduce plasticity compared to field plants (e.g., Givnish et al. 2004). However, all leaf traits still varied considerably across species as their CV (coefficient of variation) values were high (Table 1), suggesting that a strong genetic component influences this trait variation (Dunbar-Co et al. 2009).
Leaf density (LD), upper cuticle thickness (UCT), and SI showed significant phylogenetic conservatism as phylogenetic signals K > 1 (Table 3), suggesting that their variation reflects relatively ancient rather than recent divergences (Felsenstein 1985; Ackerly 1999). Additionally, SI and relative water content (RWC) contributed little to total variations (Fig. 2; Table 3), also indicating the stability of these two traits in adaptation.
Combining phylogenetic signals with PCA results, phylogeny has a significant effect on leaf attributes within Dendrobium. LD, RWC, UCT, and SI showed observable phylogenetic conservatism. The other leaf traits examined here showed significant lability, perhaps because of adaptive evolution.
Correlated evolution among leaf traits
The relatively low values for stomatal density (SD) and vein density (VD) in Dendrobium compared to other angiosperms (Kelly and Beerling 1995; Sack et al. 2012) suggest that this group endures much more serious water stress (Xu and Zhou 2008). Although the values for SD and VD in Dendrobium were lower, the coordination between these two traits showed similar patterns to other angiosperms. These common trends indicate that leaf water balance indeed exists in Dendrobium.
The variation of leaf traits in Dendrobium showed a strong influence of co-evolution on leaf water balance. The significant positive correlation that we found between SD and VD (Figs. 2, 3) is consistent with previous findings that these traits show covariance and react in a similar way to environmental changes (Uhl and Mosbrugger 1999). This covariation is likely to be a general trend in terrestrial plants (Brodribb and Jordan 2011; Zhang et al. 2012b). This result was also consistent with our third hypothesis that stomata and venation would be evolutionarily correlated in Dendrobium to achieve a favorable leaf water balance, since they are both associated with hydraulic conductance, water use efficiency, and photosynthesis (Sack and Frole 2006; Brodribb and Jordan 2011). The homeostatic balance between liquid and gas phase hydraulic conductance can be achieved by a close coupling of VD and SD in a water-stressed environment (Brodribb and Jordan 2011). Therefore, correlated evolution between venation and stomata plays an important role in optimizing the trade-off between photosynthetic benefit and evaporative cost.
Our results showed LT was negatively correlated with SD and VD (Figs. 2, 3). This result supported our third hypothesis that the evolutionary correlation of LT with other leaf traits can influence leaf water balance. Previous studies have also suggested phylogenetically coordinated patterns between stomatal traits and LT (Beerling and Kelly 1996), and between LT and VD (Scoffoni et al. 2011). Thick leaves can slow down water losses by increasing the path length of the transpiration stream from the vein end to the site of evaporation, and support large water storage (Brodribb et al. 2007; Scoffoni et al. 2011). High LT is linked with high mesophyll water transport resistance and low total hydraulic conductance (Sack and Frole 2006). So a thick leaf, low VD, and low SD are all responses to low water availability, suggesting that they act to balance water supply and loss. In addition, as thicker leaves have a higher construction cost than thinner leaves, they should have a longer lifespan to maximize nutrient use before their death (Wright and Cannon 2001; Wright and Westoby 2002). Increasing leaf longevity is used to efficiently maximize the duration of nutrient retention in nutrient-poor habitats (Wright and Cannon 2001; Wright and Westoby 2002). Therefore, increasing LT would be important for the adaptation of Dendrobium species to the low availability of water and nutrients in an epiphytic habitat.
Leaf epidermis will also play a role in leaf water balance. SD and SA were significantly correlated with upper and lower epidermal thicknesses (Figs. 2, 4). This result suggests tight evolutionary correlations between stomata and epidermis. A study using Paphiopedilum also found correlated evolution between SD and upper epidermal thickness, and between stomatal size and lower epidermal thickness (Zhang et al. 2012b). Although cuticular thickness alone is not a good predictor of a species’ drought tolerance, because it is not always correlated with cuticular water permeability (Riederer and Schreiber 2001), water loss can be controlled by the physical barriers presented by epidermal structures, such as the cuticle and the epidermis (Kerstiens 1996; Riederer and Schreiber 2001). Consequently, in Dendrobium, the correlated evolution between leaf epidermical thickness and stomata would be a credible means by which plants cope with water-stressed habitats.
Previous studies suggest that leaf size has structural, functional, allometric, and developmental linkages with many leaf traits (Niinemets and Sack 2006; Sack and Holbrook 2006; Dunbar-Co et al. 2009; Kembel and Cahill 2011). In this study, LA was negatively correlated with SD and positively correlated with SA, upper, and lower epidermal thicknesses (Table 4). Dunbar-Co et al. (2009) also found that with increasing LA, SD declined in Hawaiian Plantago species. With increasing leaf size, stomata and epidermal cells may be spaced farther apart due to allometry, thus increasing epidermal cell size and decreasing SD (Sack et al. 2012). The negative correlation between LA and SD may be due to hydraulic causes. Smaller leaves support higher SD, because smaller leaves have a higher major vein density, which helps reducing their vulnerability to water loss by increasing their hydraulic conductance (Scoffoni et al. 2011; Sack et al. 2012). Smaller leaves also show thinner vapor boundary layer, which reduces their risk of overheating (Nicotra et al. 2008).
Additionally, LD was related to SD (Table 4). Similar to our results, Zhang et al. (2012a) reported that LD was correlated positively with SD in Chinese karst species, whereas Witkowski and Lamont (1991) pointed out that LT and LD often vary independently in response to resource gradients in sclerophylls of Mediterranean California and Western Australia. This positive correlation between LD and SD may also reflect leaf hydraulic balance, since dense leaves increase resistance to the diffusion of water and CO2, which can be ameliorated by increasing SD to maintain a high photosynthetic rate (Poorter et al. 2009; Sack and Frole 2006). However, independence between LD and LT may be due to their different determinants of leaf anatomical components. Variation in LD is the result of variations in inclusions in the cells, thickness and density of the cuticle and cell walls, and extent and abundance of air spaces, fiber caps, and vascular bundles. In contrast, LT varies with leaf shape, placements of veins, width, and number of layers of mesophyll and epidermis (Witkowski and Lamont 1991).
Conclusions
Both the native environment and evolutionary history significantly affected leaf functional traits and ecological characteristics in the studied Dendrobium species. More closely correlated species have similar habitats, geographical distribution, and share certain leaf traits (leaf density, upper cuticle thickness, and stomatal index), whereas such similarities were not observed among more distantly related species. Species native to forests below the altitude of 1,500 m exhibited more obvious drought-tolerant leaf characteristics than those from forests above the altitude of 1,500 m. We also found a correlated evolution between stomatal density and vein density, reflecting a balance between water supply and loss. Leaf anatomical and morphological traits such as leaf thickness, leaf epidermis, leaf area, and leaf density play an important role in regulating leaf water balance in Dendrobium. These results provide novel and robust insights into the evolution of ecological strategies in epiphytic orchids.
References
Ackerly DD (1999) Comparative plant ecology and the role of phylogenetic information. In: Press M, Scholes JD, Barker MG (eds) Physiological plant ecology. Blackwell Science, Oxford, pp 391–414
Ackerly DD, Reich PB (1999) Convergence and correlations among leaf size and function in seed plants: a comparative test using independent contrasts. Am J Bot 86:1272–1281
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Baraloto C, Paine CET, Patiño S, Bonal D, Hérault B, Chave J (2010) Functional trait variation and sampling strategies in species-rich plant communities. Funct Ecol 24:208–216
Beerling DJ, Kelly CK (1996) Evolutionary comparative analyses of the relationship between leaf structure and function. New Phytol 134:35–51
Blomberg SP, Garland JR, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Blonder B, Buzzard V, Simova I et al (2012) The leaf-area shrinkage effect can bias paleoclimate and ecology research. Am J Bot 99:1756–1763
Brodribb TJ, Jordan GJ (2011) Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytol 192:437–448
Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol 144:1890–1898
Conran JG, Bannister JM, Lee D (2009) Earliest orchid macrofossils: early Miocene Dendrobium and Earina (Orchidaeae: Epidendroideae) from New Zealan. Am J Bot 96:466–474
Cunningham SA, Summerhayes B, Westoby M (1999) Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol Monogr 69:569–588
Dunbar-Co S, Sporck MJ, Sack L (2009) Leaf trait diversification and design in seven rare taxa of the Hawaiian Plantago radiation. Int J Plant Sci 170:61–75
Edwards EJ (2006) Correlated evolution of stem and leaf hydraulic traits in Pereskia (Cactaceae). New Phytol 172:479–489
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Franks PJ, Beerling DJ (2009) CO2-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 7:227–236
Freudenstein JV, Rasmussen FN (1999) What does morphology tell us about orchid relationships? A cladistic analysis. Am J Bot 86:225–248
Givnish TJ, Montgomery RA, Goldstein G (2004) Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses, and whole-plant compensation points. Am J Bot 91:228–246
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Hodges SA, Derieg NJ (2009) Adaptive radiation: from field to genomic studies. PNAS 106:9947–9954
Hsu C, Horng F, Kuo C (2002) Epiphyte biomass and nutrient capital of a moist subtropical forest in north-eastern Taiwan. J Trop Ecol 18:659–670
Ji ZH (1999) Flora of China. In: Ji ZH, Chen XQ, Lang KY, Zhu GH (eds). Subtrib. Dendrobiinae Lindl., Dendrobium Sw. Science Press of China, Beijing, pp 67–146
Kelly CK, Beerling DJ (1995) Plante life form, stomatal density and taxonomic relatedness: a reanalysis of Salisbury (1927). Funct Ecol 9:422–431
Kembel SW, Cahill JF Jr (2011) Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS ONE 6:e19992
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464
Kerstiens G (1996) Cuticular water permeability and its physiological significance. J Exp Bot 47:1813–1832
Laube S, Zotz G (2003) Which abiotic factors limit vegetative growth in a vascular epiphyte? Funct Ecol 17:598–604
Nicotra AB, Cosgrove MJ, Cowling A, Schlichting CD, Jones CS (2008) Leaf shape linked to photosynthetic rates and temperature optima in South African Pelargonium species. Oecologia 154:625–635
Niinemets Ü, Sack L (2006) Structural determinants of leaf light-harvesting capacity and photosynthetic potentials. Progress in botany. Springer, Berlin, pp 385–419
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Price T (1997) Correlated evolution and independent contrasts. Philos Trans R Soc Lond B Biol Sci 352:519–529
Riederer M, Schreiber L (2001) Protecting against water loss: analysis of the barrier properties of plant cuticles. J Exp Bot 52:2023–2032
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Royer DL (2001) Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev Palaeobot Palynol 114:1–28
Sack L, Frole K (2006) Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 87:483–491
Sack L, Holbrook NM (2006) Leaf hydraulics. Annu Rev Plant Biol 57:361–381
Sack L, Cowan PD, Jaikumar N, Holbrook NM (2003) The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant Cell Environ 26:1343–1356
Sack L, Scoffoni C, McKown AD, Frole K, Rawls M, Havran JC, Tran H, Tran T (2012) Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat Commun 3:837
Santiago LS, Kim SC (2009) Correlated evolution of leaf shape and physiology in the woody Sonchus alliance (Asteraceae: Sonchinae) in Macronesia. Int J Plant Sci 170:83–92
Scoffoni C, Rawls M, McKown A, Cochard M, Sack L (2011) Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol 156:832–843
Stern WL, Morris MW, Judd WS (1994) Anatomy of the thick leaves in Dendrobium section Rhizobium (Orchidaceae). Int J Plant Sci 155:716–729
Stuntz S, Simon U, Zotz G (2002) Rainforest air-conditioning: the moderating influence of epiphytes on the microclimate in tropical tree crowns. Int J Biometeorol 46:53–59
Uhl D, Mosbrugger V (1999) Leaf venation density as a climate and environmental proxy: a critical review and new data. Palaeogeogr Palaeoecol 149:15–26
Watkins JE Jr, Cardelús CL (2012) Ferns in an angiosperm world: cretaceous radiation into the epiphytic niche and diversification on the forest floor. Int J Plant Sci 173:695–710
Webb CO, Ackerly DD, Kembel SW (2008) Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24:2098–2100
Witkowski ETF, Lamont BB (1991) Leaf specific mass confounds leaf density and thickness. Oecologia 88:486–493
Wright IJ, Cannon K (2001) Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Funct Ecol 15:351–359
Wright IJ, Westoby M (2002) Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytol 155:403–416
Xu ZZ, Zhou GS (2008) Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot 59:3317–3325
Zhang SB, Slik JW, Zhang JL, Cao KF (2011) Spatial patterns of wood traits in China are controlled by phylogeny and the environment. Global Ecol Biogeogr 20:241–250
Zhang JL, Poorter L, Cao KF (2012a) Productive leaf functional traits of Chinese savanna species. Plant Ecol 213:1449–1460
Zhang SB, Guan ZJ, Sun M, Zhang JJ, Cao KF, Hu H (2012b) Evolutionary association of stomatal traits with leaf vein density in Paphiopedilum, Orchidaceae. PLoS One 7:e40080
Zhang SB, Sun M, Cao KF, Hu H, Zhang JL (2014) Leaf photosynthetic rate of tropical ferns is evolutionarily linked to water transport capacity. PLoS ONE 9:e84682
Zotz G (2013) The systematic distribution of vascular epiphytes-a critical update. Bot J Linn Soc 171:453–481
Zotz G, Bader MY (2009) Epiphytic plants in a changing world-global: change effects on vascular and non-vascular epiphytes. Progress in botany. Springer, Berlin, pp 147–170
Zotz G, Hietz P (2001) The physiological ecology of vascular epiphytes: current knowledge, open questions. J Exp Bot 52:2067–2078
Acknowledgments
We would like to thank Nissa Kreidler and Julie Anberree Lebreton for helping to troubleshoot complications with translation. We would also like to thank Xiao-Jing Wang, Yan Dai, Hong Ma, and Hui Jiang for helping to collect materials and measure leaf traits. This study was supported by the National Science Foundation of China (No. 31170315 and No. 31370362) and the Natural Science Foundation of Yunnan Province (No. 2013FA044).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K.-F. Cao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, M., Yang, SJ., Zhang, JL. et al. Correlated evolution in traits influencing leaf water balance in Dendrobium (Orchidaceae). Plant Ecol 215, 1255–1267 (2014). https://doi.org/10.1007/s11258-014-0383-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-014-0383-2