Abstract
Heat wave frequency and intensity are predicted to increase. We investigated whether repeated exposure to heat waves would induce acclimation in Quercus rubra seedlings and considered [CO2] as an interacting factor. We measured gas exchange and chlorophyll fluorescence of seedlings grown in 380 (C A) or 700 (C E) μmol CO2 mol−1, and three temperature treatments (ambient, ambient +3 °C, and an ambient +12 °C heat wave every fourth week). Measurements were performed during the third and fourth +12 °C heat waves (July and August 2010) at Whitehall Forest, GA, USA. Additionally, previously unexposed seedlings were subjected to the August heat wave to serve as a control to determine acclimation of seedlings which were previously exposed. Seedlings with a history of heat wave exposure showed lower net photosynthesis (A net) and stomatal conductance (on average −47 and −38 %, respectively) than seedlings with no such history, when both were subjected to the same +12 °C heat wave. During both heat waves, A net significantly declined in the +12 °C treatment compared with the other treatments. Additionally, the A net decline during the August compared with the July heat wave was stronger in C E than in C A, suggesting that elevated [CO2] might have had a negative effect on acclimation capacity. We conclude that seedlings subjected to consecutive heat waves will moderate stomatal conductance outside the heat wave, to reduce water usage at lower temperatures, increasing survival at the expense of carbon assimilation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global climate change is expected to result in a rise in mean global temperature by the end of the century, due in part to a steady rise in atmospheric CO2 concentration ([CO2]) and other greenhouse gasses (IPCC 2013). Climate change has already resulted in increases in the number and intensity of heat waves (Rahmstorf and Coumou 2011), a trend considered very likely to continue in this century (Diffenbaugh and Ashfaq 2010; Lynn et al. 2007). Heat waves are attracting public and scientific interest as the number of extreme weather events has increased in recent decades (Coumou and Rahmstorf 2012). Examples include the 2003 European heat wave (Ciais et al. 2005), the 2009 Australian heat wave (Karoly 2009), and the 2011 heat wave in the Southern USA (Luo and Zhang 2012). The number of heat events longer than 10 days significantly increased in many regions in Pakistan from 1961 to 2009 (Zahid and Rasul 2012), and the number of summer heat waves in the Eastern USA increased by 20 % from 1949 to 1995 (Gaffen and Ross 1998). Modelling by Coumou and Robinson (2013) has shown that the global land area experiencing temperatures three or more standard deviations warmer than average local climatology will have doubled (covering ~10 %) by 2020 and quadrupled (~20 %) by 2040. In this manuscript, the term “heat wave” denotes a time period during which air temperature is not only significantly higher than its average over the past 30 years, but furthermore high enough to induce a distinct response from the studied object (in this case: tree seedlings). This definition is loosely based on Reichstein et al. (2013), who attempted to give a more general definition of climate extremes.
Changes in heat wave frequency and intensity will not happen overnight, and it is plausible that individuals and ecosystems will acclimate and eventually adapt to their altered thermal environment. Acclimation can be defined as environmentally induced changes in characteristics that result in an improved performance under the new growth regime (Berry and Björkman 1980). Indeed, species have already developed mechanisms to cope with extreme temperatures and heat waves throughout their evolution, and individuals may therefore be able to acclimate to conditions that are more extreme. In this manuscript, we discuss two plant strategies for coping with heat stress, namely thermal acclimation of carbon gain and water conservation. In a recent review, Way and Yamori (2014) argued that photosynthetic thermal acclimation should include adjustments of both the photosynthetic thermal optimum (T opt) and the photosynthetic rate at the growth temperature (A growth). Many studies have reported an increase in T opt (Sage and Kubien 2007), but A growth has both increased (e.g. Geum vernum, Anderson and Cipollini 2013) and decreased (e.g. Cucumis sativus, Li et al. 2013). Acclimation to a sustained growth temperature may differ from acclimation to short intense heat events, and plants have been observed to increase expression of heat-shock proteins to correct the structure of heat-damaged photosynthetic proteins during an intense heat period (Vierling 1991). For example, Huerta et al. (2013) found that Aloe barbadensis plants acclimated at higher temperatures expressed more heat-shock proteins prior to a 45 °C heat shock than nonacclimated individuals.
Acclimation of a plant’s water status to higher temperatures has been studied less extensively, and no specific literature exists on the mechanistic background of water strategy responses to heat waves. The general assumption remains that individuals will strive to conserve water at higher temperatures by reducing stomatal conductance (Fischer et al. 1970) or adjusting osmotic balance (Lu and Zhang 1999), until potentially damaging temperatures and the need for evaporative cooling become more important than water conservation. In a heat wave experiment on Quercus rubra seedlings, Bauweraerts et al. (2013) observed that seedlings with a history of >50 °C heat wave exposure had greatly increased stomatal conductance, transpiration, and net photosynthesis (A net) during a >50 °C heat wave compared with measurements before and after the heat wave. In the same experiment, Ameye et al. (2012) found no such behaviour in these seedlings 1 month (and one >50 °C heat wave) earlier.
It has been shown that elevated [CO2] has the potential to stimulate A net at higher temperatures and mitigate damage at very high temperatures (>45 °C) (Sage and Kubien 2007). In a heat wave experiment on Larrea tridentata, Hamerlynck et al. (2000) found that elevated [CO2] protected photosynthetic activity and capacity during the heat wave. Bauweraerts et al. (2014), in their heat wave experiment, found that biomass of Q. rubra seedlings grown under elevated [CO2] (700 μmol CO2 mol−1) was not different between ambient +3 and +12 °C heat wave treatments, while biomass of seedlings grown under ambient [CO2] was 40 % lower in the +12 °C heat wave treatment compared with the ambient +3 °C treatment.
In this report, we examine the effect of consecutive severe (+12 °C) heat waves on gas exchange and chlorophyll a fluorescence of Q. rubra seedlings. Data were collected during the third and fourth +12 °C weeklong heat waves (July and August). Part of this dataset (gas exchange and chlorophyll a fluorescence data from July, and afternoon gas exchange data from August) has been published in Ameye et al. (2012) and Bauweraerts et al. (2013), where it was used to compare the response of two species (Q. rubra and Pinus taeda) to one heat wave. The current report compares these data to unpublished values recorded in August, to identify signs of acclimation in the evolution of a single species’ response to successive heat waves. Additionally, during the fourth heat wave, putative acclimation of physiology after repeated heat wave exposure is tested against controls without previous exposure. Elevated [CO2] is considered as a potential interacting factor in the framework of climate change. Our initial hypotheses with regard to the August acclimation experiment were as follows: (1) seedlings with a history of heat wave exposure will have higher A net and Φ PSII than seedlings with no history of heat wave exposure when they are subjected to the same heat wave; (2) elevated [CO2] will mitigate differences in A net and Φ PSII between seedlings with a history of heat wave exposure and seedlings with no history of heat wave exposure when they are subjected to the same heat wave. Our hypotheses with regard to the consecutive July and August heat waves experiment were as follows: (3) the difference in A net and effective PSII quantum yield (Φ PSII) between the severe heat wave treatment and the ambient temperature treatment will be smaller, because of acclimation, in the August heat wave than in the July heat wave; (4) elevated [CO2] will mitigate differences in A net and Φ PSII among the temperature treatments during both heat waves.
Materials and methods
Study site
The study site was located at Whitehall Forest, a research unit of the University of Georgia in Athens, GA (33°57′N, 83°19′W, altitude 230 m). Six treatment chambers, half-cylinder in shape and measuring 3.62 m length by 3.62 m width by 2.31 m height were constructed at the site. The chambers were constructed according to the method described in Boyette and Bilderback (1996), with lumber bases and PVC pipe frames supporting 0.15-mm-thick clear polyethylene film (GT Performance Film, Green-Tek Inc., Edgerton, WI, USA). The chambers were placed in an open field, spaced 3.7 m apart to minimise shading, and oriented facing south to maximise daily sun exposure.
Plant material
Seeds of Quercus rubra L. were planted on 1 December 2009 in 0.5-L pots in potting medium (Fafard Nursery Mix, Conrad Fafard Inc., Agawam, MA, USA). The seeds were obtained from a wild collection in Tennessee (Louisiana Forest Seed Company, 135 Lecompte, LA, USA). This provenance is in the southernmost region of the species range (Little 1971).
In February 2010, the seedlings were transplanted to 8 L pots (cylindrical in shape; 22 cm diameter, 22 cm height) in the same potting medium. The pots were watered three times a day to saturation until treatments started. In April, May, June, and July, each pot was fertilised with 30 g of 15-9-12 extended release fertiliser (Osmocote plus #903286, Scotts-Sierra Horticultural Products, Marysville, OH, USA). In May, 0.04 mL Imidacloprid was applied topically to the soil in each pot to prevent insect infestation (Bayer Advanced 12 months tree and shrub insect control, Bayer, Monheim am Rhein, Germany).
Experimental design and monitoring
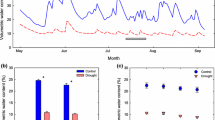
The setup of this experiment was based on Wertin et al. (2010). Each treatment chamber was assigned one of six treatment combinations. These combinations were produced by two factors: atmospheric [CO2] and chamber temperature. The [CO2] treatments were ambient [CO2] (C A, 380 μmol CO2 mol−1) or elevated [CO2] (C E, 700 μmol CO2 mol−1). The temperature treatments were ambient (T A0), ambient +3 °C (T A3), and a heat wave elevating ambient by 12 °C every fourth week (T HW12). Each applied heat wave lasted for 1 week, and the heat wave treatment scheme was repeated every 4 weeks throughout the growing season (Fig. 1a). The elevated temperature treatments T A3 and T HW12 represented the same applied heat sum and corresponded to an average increase of 3 °C over a 4-week period and the entire growing season (May through September). In this context, the heat treatments may also be considered as differences in temperature variability. Thus, the experiment was a complete factorial of 2 [CO2] × 3 temperature treatments.
a Chamber temperature treatment scheme for the elevated temperature treatments T A3 and T HW12. While T A3 is set to constantly raise chamber temperature 3 °C above the ambient temperature treatment T A0, T HW12 is set to raise chamber temperature 12 °C above T A0 for 1 week during every 4-week period. Shaded areas represent the temperature increment above the T A0 temperature. The two shaded areas are of equal size, meaning that the average temperature increments over the 4-week period are equal. b Average diurnal temperature profile in the chambers at three temperature treatments T A0, T A3, and T HW12, as well as outside of the treatment chambers over the course of the entire experiment from 2 May through 2 October (156 days). Temperatures were measured 1 m above the ground
Fifteen Q. rubra seedlings were placed in each chamber. The seedlings were randomly assigned to one of three blocks per species per chamber. Pots were evenly spaced within the chamber. To minimise any potential chamber effect, the treatment combinations, blocks, and pots within blocks were randomly rotated among the chambers every 4 weeks, and chamber CO2 and temperature conditions were adjusted accordingly. Treatments were initiated on 2 May 2010.
To maintain [CO2] at the desired levels, a nondispersive infrared CO2-sensor (Model GMT222, Vaisala Inc., Woburn, MA, USA) continuously measured [CO2] inside each chamber and directly controlled a solenoid valve which released CO2 into the chambers as necessary from a cylinder of industrial grade compressed 100 % CO2 (Airgas National Welders, Toccoa, GA, USA). An oscillating fan was installed in each chamber to disperse the CO2 evenly throughout the chamber. A datalogger (23X, Campbell Scientific, Logan UT, USA) was used to monitor air temperature with a thermocouple inside each chamber and outside 1.45 m south of the chambers. Each thermocouple was housed in a ventilated radiation shield (Model SRS100, AmbientWeather, Chandler, AZ, USA) mounted on a pole 1 m above ground level. The datalogger was programmed to compare chamber air temperature with outside temperature at a 1-min interval, and control air conditioners (FAM186R2A, Frigidaire, Augusta, GA, USA) and resistance heaters (3VU33A, Dayton Electric, Niles, IL, USA) to maintain the treatment target air temperatures. Air conditioners and resistance heaters were controlled using a simple on/off signal, meaning that there was some lag and overshoot in the temperature control, though this effect was negligible.
Soil water content in the pots was measured with soil moisture probes (ECH2O EC5, Decagon Devices, Pullman, WA, USA), which were inserted to a depth of 9 cm, and recorded every 10 min with the datalogger. Probes were inserted in two pots per chamber. To maintain the soil at field capacity (ca. 51 %), plants were provided with three watering events of 8 min per day, during each of which 84 mL (=252 mL in total) was applied via an automated irrigation system and drip emitters (Supertif—PLASTRO, Kibbutz Gvat D. N. Ha’Amakim, Israel). Drip emitters were attached right above the soil surface, on the opposite side of the pot from the soil moisture sensors.
From 2 May to 2 October 2010, ambient temperature outside the chambers averaged 29.5 °C. Over the same time period, the elevated temperature treatments T A3 and T HW12 averaged 3.2 and 2.9 °C, respectively, above the ambient temperature treatment T A0 (Fig. 1b). Mean treatment [CO2] was 390 µmol mol−1 for C A and 715 µmol mol−1 for C E. Mean soil water content was 50 % v/v and did not significantly differ among [CO2] × temperature treatments. Air temperatures were slightly higher (0.2–2 °C) during the July heat wave compared with the August heat wave (Table 1). Temperatures were equal between the [CO2] treatments.
Leaf gas exchange and chlorophyll fluorescence parameters
Leaf gas exchange parameters were measured using a portable photosynthesis system (Model LI-6400XT, LiCor Biosciences, Lincoln, NE, USA) fitted with a fluorescence cuvette (6400-40 Leaf Chamber fluorometer, LiCor Biosciences, Lincoln, NE, USA). Light-saturated net photosynthesis (A net, μmol CO2 m−2 s−1), stomatal conductance (g s, mol H2O m−2 s−1), and transpiration rate (E, mmol H2O m−2 s−1) were simultaneously measured. Intrinsic water use efficiency (WUEi) was calculated per individual plant as the ratio of A net to g s. The temperature of the cuvette was set to match the temperature in the treatment chamber. The light source of the cuvette was maintained at 1,500 μmol PAR m−2 s−1 and [CO2] was maintained at treatment conditions, either 380 or 700 μmol CO2 mol−1 for C A and C E, respectively. Relative humidity in the cuvette was maintained near 50 %. Leaf chlorophyll a fluorescence was measured immediately after gas exchange on the same leaves. Light-acclimated PSII efficiency (F v′/F m′ = 1 − F 0′/F m′), effective PSII quantum yield (Φ PSII = 1 − F s/F m′) and photochemical quenching, which relates F v′/F m′ to Φ PSII and is a nonlinear measure for the proportion of open PSII centres (q P = [F m′ − F s]/[F m′ − F 0′]), were calculated from steady-state fluorescence (F s), maximum fluorescence after a saturating light flash (F m′ after 1 s 7,000 μmol m−2 s−1), and minimum fluorescence after a far-red pulse (F 0′ after 3 s 9 μmol m−2 s−1 peaking at 740 nm). Measurements were performed on sunny or mostly sunny days. All measurements were conducted on foliage of the most recent fully developed flush.
Heat wave acclimation experiment
To determine whether seedlings were acclimating to repetitive exposure to +12 °C heat waves, three individuals from the T A0 treatment were placed in the T W12 treatment chamber right before the August heat wave (20–26 August 2010). Leaf gas exchange and chlorophyll a fluorescence measurements were conducted on these seedlings as well as on three seedlings that had been grown in the T HW12 treatment. These measurements took place 3 h before the consecutive heat waves experiment measurements (1200 and 1500 hours, respectively) on 19, 23 and 26, and 31 August (before, during, and after the +12 °C heat wave, respectively). This experiment allowed us to investigate whether seedlings with no previous exposure history (N-PEH) to a +12 °C heat wave performed similarly during a heat wave to those which were previously exposed (PEH). This factor was denoted as exposure history.
Consecutive heat waves experiment
Leaf gas exchange and chlorophyll a fluorescence measurements were conducted prior to, during, and after two midsummer heat wave treatment periods (20–26 July and 20–26 August, 2010, respectively). Measurements were conducted at noon on four randomly selected seedlings (different from the N-PEH and PEH seedlings) from each of the six treatment combinations. Different individuals in T HW12 treatment (i.e. PEH) than those used in the heat wave acclimation experiment were selected to avoid any artefacts caused by mechanical perturbation due to earlier handling of the seedlings. The rationale behind measuring the T HW12 seedlings again at this time is that photosynthetic activity can show a strong diurnal profile. Because of the 3 h difference between the two measurement sessions, it was decided that additional measurements in the T HW12 treatment were necessary. For the July heat wave treatment period, measurements were made over 10 days: 3 days before the start of the heat wave (13, 16, and 19 July), 4 days during the heat wave (20, 22, 24, and 26 July), and 3 days after the heat wave (27 and 29 July, and 1 August). For the August heat wave treatment period, measurements were made over 9 days: 2 days before the start of the heat wave (18 and 19 August), 4 days during the heat wave (20, 23, 24, and 26 August), and 3 days after the heat wave (27, 29, and 31 August).
Data analysis
For the acclimation experiment, leaf gas exchange and chlorophyll a fluorescence were analysed using a repeated-measures analysis of variance (RMANOVA) with [CO2] (2 levels; C A, C E) and previous +12 °C heat wave exposure history (2 levels; PEH, N-PEH) as the fixed effects, and treatment period (3 levels; PRE, DUR, POST) as the repeated effect. For the consecutive heat wave experiment, parameters were analysed using an RMANOVA with [CO2] (2 levels) and growth temperature (3 levels; T A0, T A3, T HW12) as the fixed effects, and treatment period (3 levels) and heat wave number (2 levels; July, August) as the repeated effects. Tree (n = 3 or 4) was included as a random effect in all analyses. Student’s t tests were used to compare between-treatment combinations of each parameter. Statistical tests were performed using PROC MIXED model analyses in SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The covariance structure with the lowest fit statistic (either −2 res log likelihood, AICc or BIC) was selected for the mixed model; all null model χ 2 tests yielded P < 0.05.
Results
Heat wave acclimation experiment
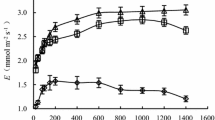
Previous exposure to +12 °C heat waves had a significant effect on A net (Table 2). During all treatment periods, N-PEH seedlings had equal or higher A net compared with PEH seedlings (+47 % across [CO2] treatments and periods; Fig. 2). Furthermore, A net of N-PEH seedlings was 22 % lower during the heat wave than before, while A net of PEH seedlings was 16 % higher during the heat wave than before (averaged across [CO2] treatments; both P < 0.05), indicated by an exposure history × treatment period interaction.
Mean (±SE, n = 3) net photosynthesis (A net) and transpiration (E) of Q. rubra seedlings grown under two [CO2] treatments: 380 (C A) and 700 (C E) μmol CO2 mol−1, and two temperature treatments: ambient (T A0), and +12 °C heat wave (T HW12). Before the start of the August heat wave, the T A0 seedlings were placed in the T HW12 chamber to act as controls in order to test putative acclimation of the T HW12 seedlings to previous heat wave exposure. T A0 seedlings were hence denoted as having no previous exposure history (N-PEH), while T HW12 seedlings were denoted as having previous exposure history (PEH). Different lowercase indices (a, b,…) indicate significant differences between all values at P = 0.05
Heat wave exposure history had no overall effect on g s and E, but the interaction with treatment period was significant (Table 2). All PEH seedlings showed increased g s and E during the heat wave, compared with values measured before the heat wave, and the effect was greatest in the C E treatment (up to +307 % for g s, P < 0.001; up to 375 % for E, P < 0.001; Figs. 2, 3). This increase did not occur in N-PEH seedlings, except for E in C A.
Mean (±SE, n = 3) stomatal conductance (g s) and intrinsic water use efficiency (WUEi) of Q. rubra seedlings grown under two [CO2] treatments: 380 (C A) and 700 (C E) μmol CO2 mol−1, and four temperature treatments: ambient (T A0), and +12 °C heat wave (T HW12). N-PEH: seedlings with no previous heat wave exposure history; PEH: seedlings with previous heat wave exposure history. Different lowercase indices (a, b,…) indicate significant differences between all values at P = 0.05
There was a significant [CO2] × history × period effect on WUEi. While all seedlings showed significantly decreased WUEi during the heat wave (Fig. 3), this effect was most pronounced for PEH seedlings in the C E treatment (−65 % compared with before the heat wave, P < 0.001). Lastly, photosynthetic efficiency parameters Φ PSII and q P were significantly affected by exposure history to +12 heat waves (Table 2). Averaged across [CO2] treatments and periods, Φ PSII and q P of N-PEH seedlings was 35 % and 33 % (respectively, both P < 0.01) higher than Φ PSII and q P of PEH seedlings (Fig. 4).
Mean (±SE, n = 3) effective PSII quantum yield (Φ PSII) and photochemical quenching (q P) of Q. rubra seedlings grown under two [CO2] treatments: 380 (C A) and 700 (C E) μmol CO2 mol−1, and four temperature treatments: ambient (T A0), and +12 °C heat wave (T HW12). N-PEH: seedlings with no previous heat wave exposure history; PEH: seedlings with previous heat wave exposure history. Different lowercase indices (a, b,…) indicate significant differences between all values at P = 0.05
Consecutive heat waves experiment
In C A T HW12, compared to values before their respective heat waves (PREJuly and PREAugust), A net showed a significant decline during the July heat wave (−61 %, P < 0.001) but not during the August heat wave (−19 %, P = 0.26). In contrast, in the C E T HW12 treatment, significant declines in A net were found in during both heat waves (−40 %, P < 0.001 during the July heat wave and −49 %, P < 0.001 during the August heat wave) (Fig. 5).
Mean (±SE, n = 4) net photosynthesis (A net) of Q. rubra seedlings grown under two [CO2] treatments: 380 (C A) and 700 (C E) μmol CO2 mol−1, and three temperature treatments: ambient (T A0), ambient +3 °C (T A3), and +12 °C heat wave (T HW12). Measurements were performed before (PRE), during (DUR), and after (POST) two heat waves in July and August 2010
There was no significant temperature effect on g s during the July heat wave (Table 3). In contrast, across [CO2] treatments and treatment periods during the August heat wave, there was a significant reduction in g s in T W12 compared with T A0 (−30 %, P < 0.05). In addition, there was a significant temperature × treatment period interaction during the August heat wave, in which g s in the different temperature treatments evolved differently over the course of the three periods. Notably, g s of the T HW12 seedlings increased during the August heat wave in both C A and C E and dropped again post-heat wave. This behaviour was not observed during the July heat wave. Seedlings in C A T HW12 showed no significant difference in g s during the July heat wave compared with pre-heat wave, but showed a significant decline post-heat wave. Seedlings in C E T A0 had higher g s during the July heat wave (+27 %, P < 0.05) (Fig. 6).
Mean (±SE, n = 4) stomatal conductance (g s) and transpiration (E) of Q. rubra seedlings grown under two [CO2] treatments: 380 (C A) and 700 (C E) μmol CO2 mol−1, and three temperature treatments: ambient (T A0), ambient +3 °C (T A3), and +12 °C heat wave (T HW12). Measurements were performed before (PRE), during (DUR), and after (POST) two heat waves in July and August 2010
A significant treatment period effect and treatment period × temperature interaction was present for E in both heat waves (Table 3). During the July heat wave, E significantly increased in all treatments, with the exception of C E T A3, where the increase was not significant. The strongest increase was found in the T HW12 seedlings. Accordingly, during the August heat wave, the highest E was also found in T HW12 seedlings (Fig. 6).
During the July heat wave, no significant differences were found for Φ PSII (Table 3), although seedlings in C A T HW12 showed a reduction in Φ PSII of −22 % (P < 0.05) compared with PREJuly and did not recover during the 5 days after the heat wave (−27 %, P < 0.05). During the August measurements, significant [CO2], temperature and treatment period effects were found (Table 3). Here, seedlings in C E T HW12 showed a smaller reduction in Φ PSII (−22 %, P < 0.01) than C A T HW12 (−29 %, P = 0.08), which persisted after the heat wave (−17 %, P < 0.05), though values were still higher (+149 %, P < 0.001) in C E T HW12 than in C A T HW12 (Fig. 7).
Mean (±SE, n = 4) effective PSII quantum yield (Φ PSII) and light-acclimated PSII efficiency (F v′/F m′) of Q. rubra seedlings grown under two [CO2] treatments: 380 (C A) and 700 (C E) μmol CO2 mol−1, and three temperature treatments: ambient (T A0), ambient +3 °C (T A3), and +12 °C heat wave (T HW12). Measurements were performed before (PRE), during (DUR), and after (POST) two heat waves in July and August 2010
A significant [CO2] × temperature interaction affected F v′/F m′ during the July measurements. Averaged across the treatment period, no significant differences were found between the temperature treatments at ambient [CO2]. However, F v′/F m′ was significantly higher in C E T HW12 compared with C E T A0 (+17 %, P < 0.05). During the August measurements, there was a significant treatment period × temperature interaction on F v′/F m′ (Table 3). Compared with pre-heat wave values, F v′/F m′ in C A T A0 and C A T A3 was significantly higher during the August heat wave, while C A T HW12 and C E T HW12 declined significantly (−29 %, P < 0.001 and −33 %, P < 0.001; respectively). After the heat wave, C A T HW12 recovered completely while C E T HW12 was still significantly lower (−13 %, P < 0.05) compared with pre-heat wave values (Fig. 7).
During both July and August, there was a significant effect of elevated [CO2] on A net, g s, and E (Table 3). Averaged across all temperature treatments and periods within a monthly measurement, A net was 67 % (P < 0.001) and 30 % (P < 0.01) higher in the elevated [CO2] treatment compared to ambient [CO2] in July and August, respectively. A stronger effect of elevated [CO2] on g s was found in August (−50 %, P < 0.001) than in July (−26 %, P < 0.01). A stronger [CO2] effect on E was also observed in August (−45 %, P < 0.001) compared with July (−22 %, P < 0.01). Elevated [CO2] had a significant effect on F v′/F m′ across all treatment periods, but only in August on Φ PSII (Table 3). F v′/F m′ was significantly higher in elevated [CO2] in both months (July: +14 %, P < 0.01; August: +11 %, P < 0.05). While there was no effect of elevated [CO2] on Φ PSII in July heat wave, it was significantly higher (+144 %, P < 0.001) in August compared with ambient [CO2].
Discussion
Acclimation after repeated heat waves at monthly intervals
Our first hypothesis was that seedlings with a history of heat wave exposure (PEH seedlings) would have higher net photosynthesis (A net) and effective PSII quantum yield (Φ PSII) than seedlings with no history of heat wave exposure (N-PEH seedlings), when exposed to a +12 °C heat wave. This hypothesis was not supported by our results, as average A net and Φ PSII were equal or higher for the N-PEH seedlings than for the PEH seedlings during all treatment periods. Part of this difference in A net response may have been due to the significantly lower stomatal conductance (g s) of the PEH seedlings during all but one treatment period, as stomatal closure may limit diffusion of atmospheric CO2 into the leaf, leading to reduced carboxylase activity (Farquhar et al. 1980). In a successive heat wave experiment on arctic species, Marchand et al. (2006) also found that g s of individuals previously exposed to heat waves was lower outside the heat waves than g s of control individuals. Higher g s of N-PEH seedlings compared with PEH seedlings during the heat wave might be explained by a greater need for the N-PEH seedlings to evaporatively cool their leaf tissue. Plants with a history of heat exposure often apply biochemical methods to deal with an additional heat event (Vierling 1991) and are able to withstand higher ambient temperatures without increasing g s to cool evaporatively (Fischer et al. 1970). At 35 °C (PREAugust), PEH seedlings therefore might not have been experiencing heat stress, while N-PEH seedlings were. In addition to being higher, g s of N-PEH seedlings lacked some or all of the dynamic behaviour of g s observed in the PEH seedlings. We suggest that the dynamic stomatal behaviour of the PEH seedlings reflects acclimation of water strategy in response to the T HW12 treatment. Reduced g s during periods of relatively low temperatures allowed these seedlings to reduce water loss and store water, perhaps against potential high temperatures in the future when soil water might be more limited, a response that prioritises survival over carbon gain.
Our second hypothesis, that elevated [CO2] would mitigate differences in A net and Φ PSII between N-PEH and PEH seedlings, was supported during and after the heat wave. Previous studies have shown that stimulation of photosynthesis by elevated [CO2] increases under higher temperatures (Sage and Kubien 2007), which could explain our results during the heat wave. The reason for the dissimilar response between our results before and after the heat wave is less clear, but might mean that the PEH seedlings suffered lasting damage during the July heat wave that reduced A net. It would furthermore seem that A net and Φ PSII of N-PEH seedlings did not recover to their PRE values in the C E treatment, while they did in the C A treatment. It is possible that the concurrent decrease in g s of the C E N-PEH seedlings impeded evaporative cooling, and therefore contributed to this reduced photosynthetic activity.
Photosynthetic responses to consecutive heat waves
The severe heat wave had a significant impact on A net during July in both [CO2] treatments. However, this response was less distinct during the August heat wave in the C A treatment, which could suggest thermal acclimation to the very high July heat wave temperature (Sage and Kubien 2007; Yamori et al. 2014). This finding further supports our third hypothesis that A net differences between T A0 and TW12 would be smaller during the August heat wave than during the July heat wave, possibly because of photosynthetic acclimation. Thermal acclimation to higher temperatures in tree species has previously been reported in Pinus taeda (Teskey and Will 1999), Quercus species (Gunderson et al. 2010) and three tropical tree species (Cheesman and Winter 2013). However, because a constant moderate temperature increase was applied in these studies rather than a severe heat stress event, we suggest that a shift in temperature optimum does not completely explain the smaller decrease in A net after the August heat wave. Surprisingly, no increased thermotolerance of A net was found under elevated [CO2] in the T HW12 treatment. On the contrary, the August heat wave seemed to have had a greater negative effect on A net of the T HW12 seedlings under elevated [CO2] than under ambient [CO2]. It is possible that the reduced A net of the C E T HW12 seedlings was caused by the lower g s values during the August heat wave.
Acclimation to high temperatures has also been associated with acclimation of Rubisco activase activity, expression of heat-shock proteins, and thermal acclimation of respiration (Yamori et al. 2014). At ambient [CO2], the reduction in A net coincided with decreased Φ PSII during and after both heat waves in the T HW12 treatments, and also coincided with decreased light-acclimated PSII efficiency (F v′/F m′) during the August heat wave. Counter-intuitively, at elevated [CO2] both F v′/F m′ and Φ PSII exhibited an equal or more severe decline in the August heat wave compared with the July heat wave. As suggested in Ameye et al. (2012), the decline and partial recovery of Φ PSII can be associated with reversible thermal deactivation of Rubisco activase (Haldimann and Feller 2004). However, no signs of acclimation in fluorescence parameters were found during the second (August) heat wave and acclimation of Rubisco activase would result in only small changes in A net, suggesting that these was not the underlying mechanisms for thermal acclimation of A net (Cavanagh and Kubien 2014).
A number of studies have reported that an increase in temperature had a positive effect on photosynthetic performance and growth (Sage and Kubien 2007; Saxe et al. 2001; Way and Oren 2010). We observed a number of negative effects of short-term extreme heat events on gas exchange physiology, including reduced A net, Φ PSII, and F v′/F m′. Filewod and Thomas (2014) showed that 3 days of record high temperatures (31–33 °C) in May had a lasting impact on photosynthetic potential of Acer saccharum during the rest of the growing season. Besides effects on photosynthesis, plant reproduction may become impaired. Abeli et al. (2012) observed that, following the 2003 heat wave in Europe, flower production in Alopecurus alpinus and Vicia cusnae was significantly decreased compared with previous years.
It has already been reported for Arabidopsis that pre-exposure to moderate heat stress resulted in a higher accumulation of different molecular chaperones such as small heat-shock proteins and HSP70s, as well as reactive oxygen species and redox response enzymes, such as ascorbate peroxidase, which resulted in a higher survival rate after a more severe heat stress treatment (Mittler et al. 2012). In addition, inducible photoprotective mechanisms were shown for different Mediterranean and Atlantic species after the European heat wave of 2003, with the accumulation of the antioxidant tocopherol and xanthophyll cycle pigments (Garcia-Plazaola et al. 2008) which eventually led to increased thermotolerance (Zsofi et al. 2009). The observed decrease in F v′/F m′ during the heat waves in this experiment may therefore be an indication of greater photoprotection through increased activation of the xanthophyll cycle.
The rise in A net in response to long-term exposure to elevated [CO2] observed in this study is consistent with the literature. Such positive effects have previously been demonstrated for Quercus rubra (Anderson and Tomlinson 1998; Cavender-Bares et al. 2000; Kubiske and Pregitzer 1996). Growth at elevated [CO2] has been shown to mitigate heat stress effects on A net (Sage and Kubien 2007), and such mitigation was found during the July heat wave (Ameye et al. 2012), but was absent during the August heat wave (Fig. 4), thus partly confirming our fourth hypothesis that elevated [CO2] would mitigate differences in A net and Φ PSII among the temperature treatments during both heat waves. Similar results have also been reported by Hamilton et al. (2008), who investigated the interactive effects of temperature and [CO2] on pre-heat-stressed plants. They found for the C3 species Chenopodium album and Pisum sativum that elevated [CO2] mitigated the heat (+15 °C) stress effects on photosynthesis of plants with no previous +15 °C exposure. However, plants pre-exposed to temperatures 15 °C above growth temperature did not experience any mitigating effect. It is notable that the A net response to a +12 °C August heat wave is different in Fig. 1 than in Fig. 4. The likely explanation lies in the difference in measurement time between the two experiments. The acclimation experiment measurements (Fig. 1) were performed around 1200 hours, while the consecutive heat wave experiment measurements (Fig. 4) were performed around 1500 hours. It is known that gas exchange and chlorophyll a fluorescence values can show a strong diurnal profile, with photosynthetic activity generally being highest in the morning. We, therefore, argue that at 1200 hours, temperatures were not yet high enough to elicit the same response as they did at 1500 hours (Fig. 1b shows the average diurnal temperature profile).
Stomatal responses to successive heat waves
We found no temperature effect on g s in July, suggesting that the seedlings did not close their stomata to preserve water content in leaves, which was dissimilar to what we saw in August. Since these seedlings had access to an adequate water supply, this apparent lack of response may reflect a strategy of the plants to evaporatively cool their leaves through transpiration. This idea is largely supported by the fact that transpiration rose to values between +66 and +112 % in the plants exposed to the July and August heat waves. The absence of a temperature effect on g s was also reported for two Eucalyptus species, which were subjected to a +4 °C treatment, and were watered daily (Ghannoum et al. 2010).
In conclusion, we observed that severe heat waves reduced A net in both July and August compared to pre-heat wave values, and that average A net was significantly lower in the heat wave treatment than in the ambient temperature treatments during the heat wave periods. Our results suggest that Q. rubra seedlings subjected to consecutive heat waves may moderate stomatal conductance outside the heat wave as a means to conserve water at lower temperatures. Thus, following an extreme and lengthy heat wave, a decrease in forest productivity, such as the one reported after the 2003 European heat wave (Ciais et al. 2005), may reflect a mechanism to conserve water that prioritizes survival over carbon acquisition. Since heat waves will have a different impact on photosynthetic performance than long-term smaller changes in average temperature, a better understanding of photosynthetic performance both during and after heat waves is of paramount importance if we hope to better understand how plants will respond to changes in climate.
References
Abeli T, Rossi G, Gentili R, Gandini M, Mondoni A, Cristofanelli P (2012) Effect of the extreme summer heat waves on isolated populations of two orophitic plants in the north Apennines (Italy). Nord J Bot 30:109–115
Ameye M, Wertin TM, Bauweraerts I, McGuire MA, Teskey RO, Steppe K (2012) The effect of induced heat waves on Pinus taeda and Quercus rubra seedlings in ambient and elevated CO2 atmospheres. New Phytol 196:448–461
Anderson LJ, Cipollini D (2013) Gas exchange, growth, and defense responses of invasive Alliaria petiolata (Brassicaceae) and native Geum vernum (Rosaceae) to elevated atmospheric CO2 and warm spring temperatures. Am J Bot 100:1544–1554
Anderson PD, Tomlinson PT (1998) Ontogeny affects response of northern red oak seedlings to elevated CO2 and water stress—I. Carbon assimilation and biomass production. New Phytol 140:477–491
Bauweraerts I, Wertin TM, Ameye M, McGuire MA, Teskey RO, Steppe K (2013) The effect of heat waves, elevated [CO2] and low soil water availability on northern red oak (Quercus rubra L.) seedlings. Glob Chang Biol 19:517–528
Bauweraerts I, Ameye M, Wertin TM, McGuire MA, Teskey RO, Steppe K (2014) Water availability is the decisive factor for the growth of two tree species in the occurrence of consecutive heat waves. Agric For Meteorol 189–190:19–29
Berry JA, Björkman O (1980) Photosynthetic response and adoption to temperature in higher plants. Ann Rev Plant Biol 31:491–543
Boyette MD, Bilderback TE (1996) A small backyard greenhouse for the home gardener. North Carolina Cooperative Extension Service, Rayleigh, pp 1–4
Cavanagh AP, Kubien DS (2014) Can phenotypic plasticity in Rubisco performance contribute to photosynthetic acclimation? Photosynth Res 119:203–214
Cavender-Bares J, Potts M, Zacharias E, Bazzaz FA (2000) Consequences of CO2 and light interactions for leaf phenology, growth, and senescence in Quercus rubra. Glob Chang Biol 6:877–887
Cheesman AW, Winter K (2013) Growth response and acclimation of CO2 exchange characteristics to elevated temperatures in tropical tree seedlings. J Exp Bot 64:3817–3828
Ciais P, Reichstein M, Viovy N et al (2005) Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533
Coumou D, Rahmstorf S (2012) A decade of weather extremes. Nat Clim Chang 2:491–496
Coumou D, Robinson A (2013) Historic and future increase in the global land area affected by monthly heat extremes. Environ Res Lett 8:034018
Diffenbaugh NS, Ashfaq M (2010) Intensification of hot extremes in the United States. Geophys Res Lett 37:1–14
Farquhar GD, von Caemmerer S, Berry JA (1980) Abiochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Filewod B, Thomas SC (2014) Impacts of a spring heat wave on canopy processes in a northern hardwood forest. Glob Chang Biol 20:360–371
Fischer RA, Hsiao TC, Hagan RM (1970) After-effect of Water Stress on Stomatal Opening Potential: I. Techniques and magnitudes. J Exp Bot 21:371–385
Gaffen DJ, Ross RJ (1998) Increased summertime heat stress in the US. Nature 396:529–530
Garcia-Plazaola JI, Esteban R, Hormaetxe K, Fernandez-Marin B, Becerril JM (2008) Photoprotective responses of Mediterranean and Atlantic trees to the extreme heat-wave of summer 2003 in Southwestern Europe. Trees 22:385–392
Ghannoum O, Phillips NG, Sears MA, Logan BA, Lewis JD, Conroy JP, Tissue DT (2010) Photosynthetic responses of two eucalypts to industrial-age changes in atmospheric [CO2] and temperature. Plant Cell Environ 33:1671–1681
Gunderson CA, O’Hara KH, Campion CM, Walker AV, Edwards NT (2010) Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Glob Chang Biol 16:2272–2286
Haldimann P, Feller U (2004) Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ 27:1169–1183
Hamerlynck EP, Huxman TE, Loik ME, Smith SD (2000) Effects of extreme high temperature, drought and elevated CO2 on photosynthesis of the Mojave Desert evergreen shrub, Larrea tridentata. Plant Ecol 148:183–193
Hamilton EW, Heckathorn SA, Joshi P, Wang D, Barua D (2008) Interactive effects of elevated CO2 and growth temperature on the tolerance of photosynthesis to acute heat stress in C-3 and C-4 Species. J Integr Plant Biol 50:1375–1387
Huerta C, Freire M, Cardemil L (2013) Expression of hsp70, hsp100 and ubiquitin in Aloe barbadensis Miller under direct heat stress and under temperature acclimation conditions. Plant Cell Rep 32:293–307
IPCC (2013) Climate change 2013: The physical science basis. Intergovermental panel on climate change. Cambridge University Press, Cambridge
Karoly DJ (2009) The recent bushfires and extreme heat wave in southeast Australia. Bull Aust Meteorol Oceanogr Soc 22:10–13
Kubiske ME, Pregitzer KS (1996) Effects of elevated CO2 and light availability on the photosynthetic light response of trees of contrasting shade tolerance. Tree Physiol 16:351–358
Li H, Wang XM, Chen L et al (2013) Growth temperature-induced changes in biomass accumulation, photosynthesis and glutathione redox homeostasis as influenced by hydrogen peroxide in cucumber. Plant Physiol Biochem 71:1–10
Little EL (1971) Atlas of United States trees: volume 1. Conifers and important hardwoods. United States Government Printing Office, Washington, DC
Lu C, Zhang J (1999) Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J Exp Bot 50:1199–1206
Luo L, Zhang Y (2012) Did we see the 2011 summer heat wave coming? Geophys Res Lett 39:L09708
Lynn BH, Healy R, Druyan LM (2007) An analysis of the potential for extreme temperature change based on observations and model simulations. J Clim 20:1539–1554
Marchand FL, Kockelbergh F, van de Vijver B, Beyens L, Nijs I (2006) Are heat and cold resistance of arctic species affected by successive extreme temperature events? New Phytol 170:291–300
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37:118–125
Rahmstorf S, Coumou D (2011) Increase of extreme events in a warming world. PNAS 108:17905–17909
Reichstein M, Bahn M, Ciais P et al (2013) Climate extremes and the carbon cycle. Nature 500:287–295
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30:1086–1106
Saxe H, Cannell MGR, Johnsen B, Ryan MG, Vourlitis G (2001) Tree and forest functioning in response to global warming. New Phytol 149:369–399
Teskey RO, Will RE (1999) Acclimation of loblolly pine (Pinus taeda) seedlings to high temperatures. Tree Physiol 19:519–525
Vierling E (1991) The roles of heat shock proteins in plants. Ann Rev Plant Physiol Mol Biol 42:579–620
Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688
Way DA, Yamori W (2014) Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth Res 119:89–100
Wertin TM, McGuire MA, Teskey RO (2010) The influence of elevated temperature, elevated atmospheric CO2 concentration and water stress on net photosynthesis of loblolly pine (Pinus taeda L.) at northern, central and southern sites in its native range. Glob Chang Biol 16:2089–2103
Yamori W, Hikosaka K, Way DA (2014) Temperature response of photosynthesis in C-3, C-4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth Res 119:101–117
Zahid M, Rasul G (2012) Changing trends of thermal extremes in Pakistan. Clim Chang 113:883–896
Zsofi Z, Varadi G, Balo B, Marschall M, Nagy Z, Dulai S (2009) Heat acclimation of grapevine leaf photosynthesis: mezzo- and macroclimatic aspects. Funct Plant Biol 36:310–322
Acknowledgments
We thank the CWO, Ghent University, for a grant allowing the participation of IB and MA. This work was supported by a Grant to ROT from the United States Department of Energy NICCR Program (Grant: 07-SC-NICCR-1060).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Abeli, Rodolfo Gentili and Anne Jäkäläniemi.
Ingvar Bauweraerts and Maarten Ameye have contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Bauweraerts, I., Ameye, M., Wertin, T.M. et al. Acclimation effects of heat waves and elevated [CO2] on gas exchange and chlorophyll fluorescence of northern red oak (Quercus rubra L.) seedlings. Plant Ecol 215, 733–746 (2014). https://doi.org/10.1007/s11258-014-0352-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-014-0352-9