Abstract
There has been a dramatic shift in dominance from Stipa grandis communities to S. krylovii communities in the Inner Mongolia steppe of China, in recent decades due to climate change and human activity. We examined the growth and carbohydrate allocation pattern of S. grandis and S. krylovii under controlled conditions. The experimental approach involved a drought stress treatment and a simulated defoliation (clipping) treatment of both species. Growth (above ground biomass and root biomass) and carbon allocation (concentration of leaf total phenolics and pool of total non-structural carbohydrate) variables were evaluated at the end of the experiment. Responses to drought stress differed significantly between S. grandis and S. krylovii. For S. krylovii, growth and the pool of total non-structural carbohydrate were more negatively affected by drought stress, whereas concentration of total phenolics was positively affected. Drought stress reinforced responses to defoliation, and drought stress × defoliation interaction was significant for all of the variables. There was a distinct defoliation response level for growth after drought stress between the two species. For aboveground biomass, both species responded positively to drought stress, which changed from responses equivalence to S. krylovii being superior; for root biomass, the two species responded oppositely to drought stress, which changed from S. grandis being superior to S. krylovii being superior. There was a weak and reverse defoliation response level for the carbon allocation pattern after drought stress between the two species, with S. krylovii changing from superior in defense to superior in storage. These results suggested that S. grandis utilized an avoidance strategy (investment in defense compounds) and S. krylovii utilized a tolerance strategy (investment in storage for regrowth) in response to defoliation under drought stress, supporting the idea that stress-tolerant species may become the new dominant species because of their ability to re-grow after disturbance. This provided a possible explanation for the replacement of S. grandis communities from the view point of adaptive strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both climate change and human activity have significant effects on natural processes of terrestrial ecosystems. Climate change is a major force for community shifts, especially in arid and semi-arid areas (Brown et al. 1997; Yang et al. 2011). Moreover, human activity is a major force shaping the composition and structure of plant communities throughout the world (Garnier et al. 2007; Kohyani et al. 2009; Wan et al. 2011). For example, human activities caused sandy desertification to expand in northern China, where the total desertification area exceeded 350,000 km2 by the mid-1990s (Tao and Wei 1999). In fact, the effects of climate change and human activity always interact in natural environments—thus the effect of human activity may be much more serious under conditions of climate change (Distel et al. 1996; Loeser et al. 2007). Responding to a combination of various intensities of stress and disturbance, plants have evolved toward particular strategies expressed in distinctive combinations of biological characteristics (Grime 2002; Kühner and Kleyer 2008; Sonnier et al. 2010). Research on adaptive strategies utilized by plants in response to climate change and human activity has drawn much attention from community and restoration ecologists (Walther et al. 2002; Cleland et al. 2007; Cingolani et al. 2005).
Plant species can be divided into those sensitive to physical damage (i.e., are unprotected and unable to regrow) and those that are resistant. Resistant species include plants utilizing avoidance strategies (e.g., defense) and plants utilizing tolerance strategies (Briske 1986; Belsky et al. 1993; Zheng et al. 2010, 2011). The preference of either avoidance or tolerance strategy can be explained by species’ net photosynthesis production allocation patterns. Species utilizing avoidance prefer investment of net photosynthetic production in defense compounds (e.g., total phenolics) to deter herbivores, while species utilizing tolerance prefer investment in storage compounds (e.g., total nonstructural carbohydrates; TNC), and species using tolerance strategy do not prevent disturbance but compensate for damage (Archer and Tieszen 1986; Imaji and Seiwa 2010).
In response to recent climate change and human activity, Stipa grandis communities were replaced by S. krylovii communities in the Inner Mongolia steppe of China. S. grandis and S. krylovii are two dominant species in the Inner Mongolia steppe, a semi-arid area in northern China S. grandis occupies relatively moist and fertile habitats, whereas S. krylovii occupies dry and infertile habitats. Many empirical studies have shown that dominant species determine the structure and composition of the community and maintain community productivity (Walther et al. 2002; Smith and Knapp 2003; Beierkuhnlein et al. 2011), so study of dominant species could partially explain the community replacement phenomenon. Previous studies of the replacement of the S. grandis community by the S. krylovii community have focused on comparison between S. grandis and S. krylovii for nitrogen (N) economy, leaf traits, and karyotypes in natural habitats (Wu et al. 2009; Yuan et al. 2005, 2006; Jia et al. 2010). Few studies have explained their differential responses to climate change (e.g., drought stress) and human activity (e.g., grazing)—except for adaptive responses to polyethylene glycol-induced osmotic stress in a common garden experiment between the two species (Wang et al. 2005). In the present study, we examined the growth (including aboveground biomass and root biomass) and carbohydrate allocation pattern (including C p and P TNC) of S. grandis and S. krylovii under treatments of defoliation and drought stress in controlled conditions. Specifically, we expected that (1) S. krylovii would perform better than S. grandis in response to drought stress and defoliation, and this would indicate growth superiority shift from S. grandis to S. krylovii; and (2) S. grandis would utilize avoidance strategy, and S. krylovii would utilize tolerance strategies in response to defoliation under drought stress, which would further result in the growth superiority shift between the two species.
Materials and methods
Species
S. grandis and S. krylovii, two perennial C3 tussock grasses, are the most widely distributed grasses and dominate the landscape of the vast semi-arid area of the Inner Mongolia steppe, China. Both species start to expand their leaves in mid-May, and their aboveground parts die completely from autumn to next early spring. S. grandis can grow to almost 1 m tall at the peak time of the growing season (late August), whereas S. krylovii is slightly shorter. S. grandis is the dominant species of the steppe in central Asia, and the S. grandis community is the main climatic climax of the typical temperate steppe area. The S. krylovii community is on the west side of S. grandis community and is the zonal formation of the typical steppe and part of desert steppe.
Experimental design
The experiment was carried out at the experimental site of Nankai University (39.10° N, 117.16° E) in Tianjin, China. The mean annual precipitation and temperature were 550–680 mm and 12.3 °C, respectively, with most rainfall during summer and highest temperatures in July and early August. In addition, photosynthetically available radiation varied between 600 and 1,800 μmol m−2 s−1.
Seeds were collected from communities dominated by S. grandis and S. krylovii in the Inner Mongolia steppe in autumn 2007. The seeds were sown in plastic pots that were filled with vermiculite on January 20, 2010. Seedlings were watered to ensure optimum growing conditions, thus avoiding serious mortality. On June 12, 2010, 60 healthy seedlings (mean tiller number of 8.53 ± 0.43 and 8.98 ± 0.47 per plant for S. grandis and S. krylovii, respectively) were chosen from each species and planted with one seedling per pot (20 × 21 cm). Soil fertility in the pots was similar and soil organic matter, availability of N, and phosphorus were 40, 3, and 0.6 g kg−1, respectively. All plots were placed in a shed outdoors with a transparent ceiling to allow sunlight, but not rainfall, to pass through.
We used a three-way factorial design with species and two environmental treatments which included two levels of drought stress (i.e., no drought and drought stress) and two levels of defoliation (i.e., no defoliation and defoliation twice). No-drought (normally watered) plants received 300 mL water every 3 days, while plants under drought stress received 75 mL water every 5 days. Defoliation was simulated by clipping the aboveground parts with scissors to a height of 5 cm, and clipping was performed at the end of July and at the end of August, 2010. Clipped biomass was not added to the final aboveground biomass. Each treatment was performed with 15 replicates, and all pots were randomly positioned. During the experiment, shading, fertility stress, and light stress were avoided, and regular weeding and insect control were conducted. The experiment ran for 143 days and was terminated on December 6, 2010.
Measurements and data analysis
On December 5, 2010, 4–5 green leaves per plant were clipped and freeze-dried for determination of total phenolics. On December 6, 2010, each plant was excavated and washed carefully, and then transported to the laboratory in a polyethylene box with ice in it. In the laboratory, plants were separated into roots, green leaves, and brown leaves; then the respective biomass was recorded after 0.5 h at 105 °C and then 72 h at 80 °C in an oven. The dried roots were used for determination of TNC.
The total phenolic concentration of leaves (C p) was determined by visible spectrophotometry (Waterman and Mole 1994), and C p was used to evaluate a plant’s investment in defense. The concentration of total TNC (C TNC) in roots was determined using an enzymatic hydrolysis method with the modification of substituting Teles’ reagent with dinitrosalicylic acid (Silveira et al. 1978). Underground organs are the major storage region for carbohydrate reserves (White 1973), so the pool of TNC in roots (P TNC) was used to evaluate plant investment in storage. In the present study, P TNC was a product of C TNC and dry root biomass.
Similar to Suding et al. (2003), we quantified the effect of each treatment (drought or defoliation) with a natural-log-transformed response ratio: lnRRtolerance = ln(Performance stress condition/Performance non-stress condition). lnRRDr and lnRRDe indicated the effect of drought stress and defoliation, respectively. Values of lnRR are symmetric around 0, so that positive values indicate a positive effect of the treatment and negative values indicate a negative effect.
Response to drought stress and defoliation between the two species was analyzed using one-way ANOVA. Response to drought stress was assessed by comparing the lnRRDr of all variables without defoliation, and response to defoliation was assessed by comparing all variables with and without drought stress, respectively. The overall effect of drought stress and defoliation was analyzed using general linear models (GLM), with lnRRDe as dependent variables, and drought stress and species as fixed factors. A significant main effect of species indicated a distinct response level to defoliation. A significant interaction between species and drought indicated a change of response level between no-drought and drought treatments. A significant main effect of drought stress indicated a difference in response between no-drought and drought stress. All statistical analyses were conducted using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL).
Results
All variables were negatively affected by defoliation and drought stress except for C p. There was an significant interaction between defoliation and drought stress for all variables, and this interaction had different effects between the two species for all the variables except for P TNC. Aboveground biomass and carbon-based compounds were significantly different between the two species (Table 1).
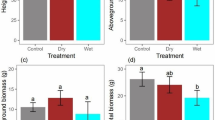
The lnRRDr of S. grandis and that of S. krylovii were negative for aboveground biomass, root biomass and P TNC, indicating that water was an important limiting resource for both species (Fig. 1). However, S. grandis and S. krylovii differed significantly in their responses to drought stress (P < 0.05). The growth (aboveground biomass and root biomass) and the P TNC of S. krylovii were much more negatively affected than those of S. grandis by drought stress. The C p of S. grandis was negatively affected by drought stress, while that of S. krylovii was positively affected by drought stress.
The lnRRDe of S. grandis and S. krylovii differed for no-drought and drought treatments, indicating the importance of defoliation for growth of both species and the interaction between drought stress and defoliation (Figs. 2, 3). Root biomass of S. krylovii was more negatively affected by defoliation, and its investment in defense (C p) was facilitated by defoliation under no-drought treatment. Growth of S. krylovii was positively affected by defoliation, and its investment in defense (C p) was negatively affected by defoliation under drought stress. In contrast, growth of S. grandis was negatively affected by defoliation, and its investment in defense was facilitated by defoliation under drought stress.
Drought stress had significant effects on lnRRDe of S. grandis and S. krylovii for both growth and carbon allocation patterns (Table 2; Fig. 4). When each species was exposed to a normal water regime and a drought-stress regime, there was a distinct response level between the two species biomass production, which changed from response equivalence (aboveground biomass) or S. grandis being superior (root biomass) to S. krylovii being superior; a weak level was formed for the lnRRDe of C p, changing from S. krylovii being superior to S. grandis being superior. Response of P TNC to defoliation showed a reverse pattern (Fig. 4).
Discussion
The interaction of drought stress with grazing has been widely studied, and drought stress was thought to reinforce the negative effect of grazing (Heitschmidt et al. 1999, 2005; Teague et al. 2004). Loeser et al. (2007) proposed that episodic drought interacted with grazing, leading to infrequent but biologically important shifts in plant communities. The present study showed that drought stress reinforced species’ responses to defoliation. Responses to drought stress or defoliation in relatively moist areas did not change the growth superiority of S. grandis (Figs. 1, 2); however, the defoliation response level changed, and S. krylovii showed superior growth when each species was exposed to a normal water regime and a drought stress regime (Table 2; Figs. 3, 4). This indicated that different response strategies to disturbance were used by S. grandis and S. krylovii. Good regrowth ability after damage implies utilization of tolerance strategies of a species and is widely defined as compensatory growth (Strauss and Agrawal 1999; Fornoni 2011). Compensatory growth could lessen the effect of damage and is an alternative or supplement to plant defenses, enabling the plant to tolerate disturbance (Meijden et al. 1988; Lehtila and Syrjanen 1995). The superior growth response ratio (lnRRDe) of S. krylovii indicated its utilization of a tolerance strategy.
In arid and semi-arid areas, species’ tolerance to climate change and human activity plays an important role in plant distribution and abundance (Crawley 1990; Burt-Smith et al. 2003; Del-Val and Crawley 2005; Williamson and Wardle 2007). Previous studies have shown that species dominant in drier and nutrient-poor sites were generally more stress tolerant than species dominant in mesic and fertile environments (Mahmoud and Grime 1976; Wilson and Keddy 1986). In the present study, we showed that S. krylovii was stress tolerant and that its tolerance to defoliation ensured its dominance in response to climate change and human activity. Consistent with our conclusion, Corcket et al. (2003) found that survival of dominant species from a relatively drier habitat (Bromus erectus) was not affected by drought stress, while the survival of dominant species from a relatively moister habitat (Brachypodium pinnatum) was significantly decreased in response to drought stress.
We observed a trade-off between defense and tolerance investment. Concentration of defense compounds in leaves usually indicates plant quality for herbivores and pathogens (Koricheva 1999) and is useful in evaluating a plant’s defense ability against herbivores (Imaji and Seiwa 2010). Carbon storage plays a particularly important role in plant regrowth after a period of inactivity or in recovery after disturbance (Chapin et al. 1990; Heilmeier and Monson 1994), and TNC level provides an estimate of the amount of energy available for plant growth (Marquis et al. 1997). In response to defoliation, S. grandis increased investment in defense compounds and decreased investment in storage; however, S. krylovii showed the opposite preference of investment when each species was exposed to a normally watered treatment and a drought stress treatment (Fig. 4). Increased tolerance of a plant involves a pre-existing high level of carbon storage in roots for allocation to aboveground reproduction (Strauss and Agrawal 1999). Considering the significant difference in regrowth between the two species, relatively higher investment in TNC was acceptable for S. krylovii after defoliation under drought stress. The biased allocation of net photosynthesis production by species can be explained by optimal growth strategy (i.e., carbon acquisition strategy) in relation to the relative resource availability of habitats (Coley et al. 1985). Species originating from relatively mesic zones are thought to have higher growth advantages (e.g., higher resource acquisition ability and higher aboveground production) than species originating from relatively xeric zones (Grime 2002; Corcket et al. 2003). In the present study, different strategies between S. grandis and S. krylovii were related to their primary habitats. S. grandis is found in a relatively moist and fertile habitat, and use of a defense strategy enabled it to avoid herbivores, grow quickly and get a dominant position. The habitat of S. krylovii is relatively dry and infertile, so the use of a tolerance strategy ensured its ability to survive and recover from damage or poor conditions. Other researchers showed similar results. For example, Imaji and Seiwa (2010) found that shade-intolerant Castanea sp. preferentially invested more carbon in growth rather than defense because severe competition occurred for light in gaps. While shade-tolerant Quercus sp. preferentially invested more carbon in defense than in storage because damage from herbivores and pathogens was common in its habitat.
Climate change and human activity are the main driving forces of replacement between the S. grandis and S. krylovii communities. Compared with avoidance strategy, utilization of tolerance strategy in response to defoliation under drought stress facilitated growth of S. krylovii. Considering the importance of the dominant species to community structure, different response strategies to defoliation under drought stress partially determined the dominant position of S. krylovii in the degraded S. grandis communities. Studies on replacement phenomenon showed that the identity of the dominant species resulted in differences in productivity between disturbed and undisturbed areas (Altesor et al. 2005; Castro and Freitas 2009). However, the S. grandis steppe displayed better productivity and higher quality than the area occupied by S. krylovii. Therefore, bearing in mind the dry conditions of the Inner Mongolia steppe and the fact that the communities dominated by S. grandis do not support extensive grazing during drought while S. krylovii is rather robust to grazing during drought, to protect the S. grandis communities from being replaced by S. krylovii communities which are less productive and lower in quality for forage, grazing pressure should be maintained at a reasonable intensity in the former ecosystem.
Abbreviations
- C p :
-
Concentration of total phenolics
- TNC:
-
Total nonstructural carbohydrates
- C TNC :
-
Concentration of total nonstructural carbohydrates
- P TNC :
-
Pool of total nonstructural carbohydrates
References
Altesor A, Oesterheld M, Leoni E, Lezama F, Rodríguez C (2005) Effect of grazing on community structure and productivity of a Uruguayan grassland. Plant Ecol 179:83–91. doi:10.1007/s11258-004-5800-5
Archer SR, Tieszen LL (1986) Plant response to defoliation: hierarchical considerations. NATO Adv Sci Inst Ser 108:45–59
Beierkuhnlein C, Thiel D, Jentsch A, Willner E, Kreyling J (2011) Ecotypes of European grass species respond differently to warming and extreme drought. J Ecol 99:703–713. doi:10.1111/j.1365-2745.2011.01809.x
Belsky AJ, Carson WP, Jensen CL, Fox GA (1993) Overcompensation by plants: herbivore optimization or red herring? Evol Ecol 7:109–121
Briske DD (1986) Plant response to defoliation: morphological considerations and allocation priorities. Rangelands: a resource under siege. Cambridge University Press, Cambridge
Brown JH, Valone TJ, Curtin CG (1997) Reorganization of an arid ecosystem in response to recent climate change. Proc Natl Acad Sci 94:9729–9733
Burt-Smith G, Grime J, Tilman D (2003) Seedling resistance to herbivory as a predictor of relative abundance in a synthesised prairie community. Oikos, pp 345–353
Castro H, Freitas H (2009) Above-ground biomass and productivity in the Montado: from herbaceous to shrub dominated communities. J Arid Environ 73:506–511. doi:10.1016/j.jaridenv.2008.12.009
Chapin FS III, Schulze E-D, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447
Cingolani AM, Posse G, Collantes MB (2005) Plant functional traits, herbivore selectivity and response to sheep grazing in Patagolian steppe grassland. J Appl Ecol 42:50–59. doi:10.1111/j.1365-2664.2004.00978.x
Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD (2007) Shift plant phenology in response to global change. Trends Ecol Evol 22:357–365
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Corcket E, Liancourt P, Callaway RM, Michalet R (2003) The relative importance of competition for two dominant grass species as affected by environmental manipulations in the field. Ecoscience 10:186–194
Crawley MJ (1990) Rabbit grazing, plant competition and seedling recruitment in acid grassland. J Appl Ecol 27:803–820
Del-Val E, Crawley MJ (2005) Are grazing increaser species better tolerators than decreasers? An experimental assessment of defoliation tolerance in eight British grassland species. J Ecol 93:1005–1016
Distel RA, Peláez DV, Bóo RM, Mayor MD, Elía OR (1996) Growth of Prosopis caldenia seedlings in the field as related to grazing history of the site and in a greenhouse as related to different levels of competition from Stipa tenuis. J Arid Environ 32:251–257
Fornoni J (2011) Ecological and evolutionary implications of plant tolerance to herbivory. Funct Ecol 25:399–407. doi:10.1111/j.1365-2435.2010.01805.x
Garnier E, Lavorel S, Ansquer P et al (2007) Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: a standardized methodology and lessons from an application to 11 European sites. Ann Bot 99:967–985. doi:10.1093/aob/mcl215
Grime JP (2002) Plant strategies, vegetation processes, and ecosystem properties. Wiley, Chichester
Heilmeier H, Monson RK (1994) Carbon and nitrogen storage in herbaceous plants. A whole plant perspective on carbon-nitrogen interactions. SPB Academic Publisher, The Hague
Heitschmidt R, Haferkamp M, Karl M, Hild A (1999) Drought and grazing: I. Effects on quantity of forage produced. J Range Manag 52:440–446
Heitschmidt R, Klement K, Haferkamp M (2005) Interactive effects of drought and grazing on northern Great Plains rangelands. Rangel Ecol Manag 58:11–19
Imaji A, Seiwa K (2010) Carbon allocation to defense, storage, and growth in seedlings of two temperate broad-leave tree species. Oecologia 162:273–281. doi:10.1007/s00442-009-1453-3
Jia MQ, Gao YB, Yan Y (2010) Leaf traits of Stipa krylovii Roshev. and S. grandis P. Smirn. in middle and eastern Inner Mongolia steppe. J Tianjin Norm Univ (Natural Science Edition) 30:57–63
Kohyani PT, Bossuyt B, Bonte D, Hoffmann M (2009) Differential herbivory tolerance of dominant and subordinate plant species along gradients of nutrient availability and competition. Plant Ecol 201:611–619. doi:10.1007/s11258-008-9515-x
Koricheva J (1999) Interpreting phenotypic variation in plant allelochemistry: problems with the use of concentrations. Oecologia 119:467–473
Kühner A, Kleyer M (2008) A parsimonious combination of functional traits predicting plant response to disturbance and soil fertility. J Veg Sci 19:681–692. doi:10.3170/2008-8-18436
Lehtila K, Syrjanen K (1995) Compensatory responses of two Melampyrum species after damage. Funct Ecol 9:511–517
Loeser MRR, Sisk TD, Crews TE (2007) Impact of grazing intensity during drought in an Arizona grassland. Conserv Biol 21:87–97
Mahmoud A, Grime JP (1976) An analysis of competitive ability in three perennial grasses. New Phytol 77:431–435
Marquis RJ, Newell EA, Villegas AC (1997) Non-structural carbohydrate accumulation and use in an understorey rain-forest shrub and relevance for the impact of leaf herbivory. Funct Ecol 11:636–643
Meijden E, Wijn M, Verkaar HJ (1988) Defence and regrowth, alternative plant strategies in the struggle against herbivores. Oikos 51:355–363
Silveira AJd, Teles FFF, Stull JW (1978) A rapid technique for total nonstructural carbohydrate determination of plant tissue. J Agric Food Chem 26:770–772
Smith MD, Knapp AK (2003) Dominant species maintain ecosystem function with non-random species loss. Ecol Lett 6:509–517
Sonnier G, Shipley B, Navas M (2010) Quantifying relationships between traits and explicitly measured gradients of stress and disturbance in early successional plant communities. J Veg Sci 21:1014–1024. doi:10.1111/j.1654-1103.2010.01210.x
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Suding KN, Goldberg DE, Hartman KM (2003) Relationships among species traits: separating levels of response and identifying linkages to abundance. Ecology 84:1–16
Tao W, Wei W (1999) Landuse and sandy desertification in Northern China. J Nat Resour 14:355–358
Teague W, Dowhower S, Waggoner J (2004) Drought and grazing patch dynamics under different grazing management. J Arid Environ 58:97–117
Walther G-R, Posromentin E, Convey P et al (2002) Ecological responses to recent climate change. Nature 416:389–395
Wan HW, Bai YF, Schönbach P, Gierus M, Taube F (2011) Effects of grazing management system on plant community structure and functioning in a semiarid steppe: scaling from species to community. Plant Soil 340:215–226. doi:10.1007/s11104-010-0661-2
Wang JL, Gao YB, Bai Y, Zhao NX (2005) A comparison of adaptive responses to PEG osmotic stress between Stipa grandis and S. krylovii. Acta Sicientiarum Naturalium Universitatis Nankaiensis 38:127–131
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. Blackwell Scientific Publications, Oxford
White LM (1973) Carbohydrate reserves of grasses: a review. J Range Manag 26:13–18
Williamson WM, Wardle DA (2007) The soil microbial community response when plants are subjected to water stress and defoliation disturbance. Appl Soil Ecol 37:139–149
Wilson SD, Keddy PA (1986) Species competitive ability and position along a natural stress/disturbance gradient. Ecology 67:1236–1242
Wu JB, Chen CB, Bao XY, Song WQ, Zhao NX, Gao YB (2009) Chromosome Numbers and Karyotypes of Stipa baicalensis, Stipa grandis and Stipa krylovii in Inner-mongolia Steppe. Bull Bot Res 29:534–538
Yang HJ, Wu MY, Liu WX et al (2011) Community structure and composition in response to climate change in a temperate steppe. Glob Change Biol 17:452–465. doi:10.1111/j.1365-2486.2010.02253.x
Yuan Z, Li L, Han X, Wan S, Zhang W (2005) Variation in nitrogen economy of two Stipa species in the semiarid region of northern China. J Arid Environ 61:13–25
Yuan ZY, Li Lh, Han G et al (2006) Nitrogen response efficiency increased monotonically with decreasing soil resource availability: a case study from a semiarid grassland in northern China. Oecologia 148(4):564–572
Zheng SX, Ren HY, Lan ZC et al (2010) Effects of grazing on leaf traits and ecosystem functioning in Inner Mongolia grasslands: scaling from species to community. Biogeosciences 7:1117–1132
Zheng SX, Lan ZC, Li WH et al (2011) Differential responses of plant functional trait to grazing between two contrasting dominant C3 and C4 species in a typical steppe of Inner Mongolia, China. Plant Soil 340:141–155. doi:10.1007/s11104-010-0369-3
Acknowledgments
This study was financially supported by National Natural Science Foundation of China (30800132 and 30900191).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, LP., Zhao, NX., Zhang, LH. et al. Responses of two dominant plant species to drought stress and defoliation in the Inner Mongolia Steppe of China. Plant Ecol 214, 221–229 (2013). https://doi.org/10.1007/s11258-012-0161-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-012-0161-y