Abstract
Recent studies have highlighted the role of lianas in shaping stand dynamics both in tropical and temperate forests. However, English ivy (Hedera helix L.), one of the most widespread lianas in Europe, has received little attention. We conducted a study in the Siro Negri alluvial forest (NW Italy) to determine what factors most affected ivy distribution and investigate its interactions with the trees in the stand. We evaluated the influence of tree size, age, species, and neighborhood crowding on ivy occurrence. In addition, growth ring widths were used to explore the development pattern of climbing stems. Fifty-two percent of trees in our study plots carried ivy, a value comparable to liana incidence found in mature tropical forests. Tree characteristics and their spatial pattern significantly influenced ivy distribution. Preferred hosts were large, isolated trees, while the effect of tree age and species on ivy occurrence was marginal. Growth pattern analysis revealed that radial growth was positively related to the available space on the tree trunk for each ivy stem. We conclude that neighborhood crowding around trees and competition among climbing stems relying on the same trunk may reduce the colonization rate of ivy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lianas are woody climbing plants that begin their life as terrestrial seedlings and later ascend to the canopy using trees as structural supports (Putz and Mooney 1991). Over the past few years, their importance in forest biodiversity has been recognized (Schnitzer and Bongers 2002): They increase woody species diversity and provide food and refuge for many animal species (Emmons and Gentry 1983; Campanello et al. 2007; Page et al. 2010). Lianas play a central role in forest dynamics as they compete with tree regeneration and adult trees for light, water, and soil nutrients. In some cases, they have been found to reduce tree growth rates and fecundity (Allen et al. 2005; Ladwig and Meiners 2010a; Ichihashi and Tateno 2011). Lianas can also influence forest composition as they affect tree species differentially, modifying inter-specific relationships. Furthermore, lianas can occupy available space on the ground earlier than pioneer tree species, affecting gap colonization and forest succession (Laurence et al. 2001; Schnitzer and Bongers 2002; Toledo-Aceves and Swaine 2008).
Since lianas have such an important role in forest dynamics, research on their distribution, ecology, and relationships with other plants is critical. Several studies have investigated liana dynamics at the stand level, revealing that abiotic factors, forest structure, and stand dynamics influence their distribution (Laurence et al. 2001; Allen et al. 2005; Londré and Schnitzer 2006; Morrissey et al. 2009; Gianoli et al. 2010; Macía 2011; Nogueira et al. 2011). Few studies have taken an individual tree approach to liana-tree relationships, and there is little information on how lianas interact with their hosts, especially for temperate forests (Blick and Burns 2011; Ichihashi and Tateno 2011). As structural parasites, lianas need suitable supports to reach the canopy and maintain themselves there. As a consequence, host tree characteristics, such as size and species, influence lianas colonization patterns, growth rates, distribution, and diversity (Leicht-Young et al. 2010; Nogueira et al. 2011).
At a global scale, lianas are widespread in tropical forests, where they represent between ten and 40 % of the woody species (Schnitzer and Bongers 2002), but they are also common in several temperate ecosystems. In Europe, there are few autochthon liana species, and white vine (Clematis vitalba L.), grapevine (Vitis spp.), and English ivy (Hedera helix L.) are the most diffuse. The genus Hedera originated in the Tertiary, experiencing a postglacial recolonization from the Mediterranean regions. As ivy belongs to a largely tropical family (Araliaceae), it benefits from warm summers and is intolerant to cold temperatures (Metcalfe 2005). It favors nutrient-rich and moist soils. Unlike many of the woody species found in southern European plain forests, ivy is an evergreen species. It presents two distinct forms. At the beginning of its life, the chamephyte life form creates extensive carpets on the forest floor with prostrate, sterile stems and palmately lobed shade leaves. When suitable supports are available, the phanerophyte life form can ascend toward better light conditions. The climbing stems support reproductive shoots, with unlobed oval sun leaves (Nola 1997; Garfì and Ficarrotta 2003; Metcalfe 2005). Like other lianas, ivy is a serious concern for forest managers. Despite its contribution to biodiversity maintenance and to nutrient cycling processes (Badre et al. 1998), ivy is often regarded as a problem as it is believed to reduce tree growth rates. Moreover, outside of its natural distribution areas, ivy is an invasive species that poses a grave threat to native plants (Clarke et al. 2006; Biggerstaff and Beck 2007), and active management is required to control it.
Ivy home range extends from southern Scandinavia to the Mediterranean area. However, few studies have been conducted on ivy dynamics, mainly located in the Rhine valley, France (Trèmolières et al. 1988; Schnitzler 1995; Badre et al. 1998; Schnitzler and Heuzé 2006; Heuzé et al. 2009). Research on other species can be only partially transposed to ivy, as morphological and ecological features vary between liana species (Gianoli et al. 2010). Such studies indicated that tree attributes and forest stand structure influence liana distribution in different ecosystems (Campanello et al. 2007; Madeira et al. 2009; Blick and Burns 2011; Nogueira et al. 2011). On the other hand, drivers of liana development following host ascension have been rarely considered (Brandes et al. 2011). We conducted a study in the Siro Negri forest, one of the very few fragments in northern Italy of alluvial forest, a preferential habitat for ivy (Schnitzler and Heuzé 2006). The following hypotheses on the relationships between ivy and trees have been tested: (i) Ivy occurrence within a temperate alluvial forest is influenced by size, age, and species of trees. (ii) Tree distribution around potential host trees affects ivy occurrence, e.g., isolated trees host more ivy than trees in crowded areas. (iii) Growth of climbing ivy stems is influenced by host tree characteristics and limited by reduced available space on the tree trunk.
Methods
Study site
The study was conducted within the Siro Negri reserve, on the southern side of the Ticino Regional Park, the province of Pavia, northern Italy (45°12′N, 9°03′E, 65 m a.s.l.). Mean annual rainfall is about 800 mm, with most precipitation in spring and autumn, and the average annual temperature is 13.6 °C (Pavia weather station, 10 km from the study site). The reserve covers about 9 ha and is one of very few relicts of the original alluvial forest in northern Italy. One of the borders corresponds to the Ticino river bank. Floods occur sporadically during spring or autumn (every 5–10 years during the last 40 years), with water level up to 1.50-m height in the forest during exceptional events. Groundwater level is around −4.50 m in winter, while during summer, it reaches −3.50 m due to irrigation of corn and rice fields in the surroundings (Sartori unpublished).
In the forested area, described as a Polygonato multiflori-Quercetum roboris association (Sartori 1984), the main tree species are Acer campestre L., Carpinus betulus L., Crataegus monogyna Jacq., Populus alba L., Populus nigra L., Quercus robur L., Robinia pseudacacia L., and Ulmus minor Mill. The forest accommodates trees of over 100 years. The last important human disturbances coincide with the two world wars, when there was an elevated demand for firewood (1910–1920 and 1940–1960, Motta et al. 2009). No logging has been carried out since the establishment of the reserve in 1970.
Field sampling
Two permanent plots (50 × 50 m) were established during 2005 at the southeastern border of the reserve (Motta et al. 2009). Two other permanent plots (60 × 60 m) were established at less than 100 m of distance in the inner part of the reserve during 2009. Trees with diameter at breast height (DBH) ≥7.5 cm were identified, labeled with numbered plastic tags, and mapped. All trees in the reserve were mapped between 2009 and 2011.
During 2011, tagged trees in the four permanent plots (total area equal to 1.22 ha) were resurveyed, and a census of ivy was carried out. For each tree, we measured the diameter of living ivy stems ≥0.5 cm at 1.3 m from the base. All the stems were measured without distinguishing between ramets and genets, because it was difficult to identify their origin, and their dynamics and effects on trees are comparable (Gerwing et al. 2006; Schnitzer et al. 2006; Campanello et al. 2007). The degree of ivy occupancy on trees was classified according to a five class system: 0, ivy absent or below 1.3 m on the tree trunk; 1, ivy present on the lower half of the trunk; 2, ivy reaching the second half of the trunk, but not the crown; 3, ivy on the lower part of the principal branches; 4, ivy covering the whole crown.
Dendrochronological analysis
An increment core from all the trees with DBH ≥10 cm and from a subsample of trees with DBH <10 cm was taken at 50 cm from the tree base. In addition, an increment core was taken at 50 cm from the tree base from 60 randomly chosen ivy stems in the plots. The stems were cored from side to side to get a core with two radii in order to account for possible asymmetrical growth of ivy stems. In the laboratory, all the cores were fixed to wooden supports and prepared with a razor to obtain an optimal surface resolution.
Cores from trees were used to estimate tree age. For cores that did not intersect the pith, their position was estimated by means of a pith locator, and the number of missing rings was estimated (Motta and Nola 2001). When it was not possible to estimate missing rings, the core was discarded.
Ring widths of ivy cores were measured to the nearest 0.01 mm using a LINTAB device and CATRAS software (Aniol 1983). Cores with rotten wood or other irregularities were discarded. Cores were also discarded when the two series of radii for each did not match (visual comparison). Series from the two radii of each of the remaining 43 cores (28.3 % discarded) were averaged to estimate annual stem growth rate.
Data analysis
The relationships between ivy and trees were explored on an individual tree basis. Descriptors of ivy, computed for each host tree, were number of stems, diameter sum, basal area, diameter of the largest stem, and the degree of ivy occupancy. Tree features were diameter, age (when available), and species. Also, to analyze the effects of neighborhood crowding on ivy-tree interactions, we calculated the number and the sum of diameters of trees in a circle of 10 m around the tree and the neighborhood ratio calculated as the ratio between the sum of neighbors’ diameters and the subject tree diameter (Daniels et al. 1986; Castagneri et al. 2008). A radius of 10 m was selected because the largest trees in the stand have a crown radius >8 m; thus, they compete with trees at a distance of more than 8 m.
Firstly, we ran all the variables in a Redundancy analysis (RDA) to order ivy attributes in relation to tree and neighborhood characteristics. RDA was done on the Canoco 4.0 software (Ter Braak and Smilauer 1998) and the statistical significance of the relations between variables was tested by a Monte Carlo test with 10,000 permutations. Next, to refine the assessment of ivy-tree interaction, we performed two types of models: (1) binary logistic regression models to evaluate the characteristics that determined the presence/absence of ivy on a tree, with stepwise entry of predictor variables (tree characteristics and neighborhood measures); (2) general linear models (GLMs) to determine which factors influenced ivy abundance on host trees, again, via stepwise entry of predictor variables (tree characteristics and neighborhood measures). In this case, response variables were number of ivy stems on the tree, sum of stem diameters, and their basal area. When continuous variables were not normally distributed, a transformation was applied to improve linearity of relationships. Variable collinearity and distribution of the residual were checked for each model. Finally, we analyzed ivy growth rate in relation to tree characteristics, neighborhood crowding, and ivy abundance on tree trunk. Individual growth rate, which was the response variable, was calculated as mean radial increment during the last 5 and 10 years, obtained from the ivy cores. All models were computed using SPSS 17.0 (SPSS Inc. Chicago IL).
Results
Ivy distribution
Ivy density in the Bosco Negri alluvial forest was 657, 346, and 225 stems ha−1 considering stems of ≥0.5, 1, and 2.5 cm, respectively (diameter thresholds commonly used in liana censuses, Gerwing et al. 2006). The basal area of stems ≥0.5 cm was 5609 cm2 ha−1 (Table 1). Of the surveyed trees (n = 374), 52 % hosted one or more ivy stems, although ivy was found only on the tree trunk in many cases, and just 17 % of the trees had ivy at crown level (classes of occupancy 3 and 4). Other liana species (Clematis vitalba L., Vitis spp.) occurred sporadically (0–2 individuals per plot).
A few differences were observed between the four plots. Plot 3, characterized by low tree density and large diameters, had the highest percentage of trees hosting ivy, the largest number of ivy stems larger than 0.5 cm, and the largest ivy basal area (Table 1). Plots 1 and 2, located at the forest border, had a much higher tree density. The percentage of trees hosting ivy, the density of stems ≥1 and 2.5 cm, and ivy basal area were rather similar to those observed in Plot 4.
Ivy-tree relationships
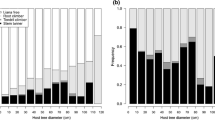
Relationships between ivy and trees’ attributes were statistically significant (p < 0.001) in the RDA analysis, explaining 43.8 % of variance (Fig. 1). As age information was not available for all the trees, a second RDA was performed on a subset of 207 samples. The relationships described by this model were equivalent to the previous one, and explained 44.1 % of variance (model not shown). All the ivy descriptors were positively associated to each other. With regards to ivy-host interactions, while tree diameter (TR_d) and age (TR_age) were positively related to ivy descriptors, neighborhood crowding (expressed as neighbors number (NE_n), sum of diameters (NE_s_d), and neighborhood ratio (NE_ra) were negatively related to ivy abundance. In terms of species, RDA showed that Q. robur trees were big and isolated, hosting large amounts of ivy, whereas A. campestre and C. monogyna trees were small with many neighbors and hosted little ivy. The other species were in an intermediate position. The inclusion of plot as a factor did not significantly improve RDA (the variance explained by the model was 44.3 %; model not shown).
Redundancy analysis ordination biplot. Full-line arrows represent the biplot scores of tree variables: TR_d tree diameter; NE_n number of neighbors in a circle of 10 m around the tree; NE_s_d sum of diameters of neighbors; NE_ra neighborhood ratio. Dotted arrows represent the biplot scores of ivy descriptors: HE_n number of ivy stems on the tree; HE_s_d sum of diameters; HE_ba basal area; HE_d_M diameter of the biggest stem; HE_cl class of occupancy. The dots represent tree species: Acer, Crataegus, Quercus, Robinia, Ulmus, and Others
RDA provided a general pattern of ivy-host relationships. The logistic regression model showed that the presence of ivy was positively related to big tree diameters and negatively related to the number of neighboring trees (Table 2), indicating that larger isolated trees were more likely to host ivy than the others. The model correctly predicted the presence/absence of ivy on 69.5 % of trees. Tree species increased the Nagelkerke R 2 from 0.276 to 0.303, but it was not significant at p = 0.05. Tree age was positively related to ivy, while the sum of diameters of neighbors and neighborhood ratio correlated negatively; but these factors were discarded as redundant in the stepwise regression. Including plot as a factor did not improve the model.
The abundance of ivy on host trees (n = 194) was influenced by the same factors that determined its presence. Tree diameter was the most important feature, positively influencing ivy abundance, while the number of neighbors had a negative effect (Table 3). Tree species emerged as a significant factor only in predicting ivy stem number, with C. monogyna hosting fewer stems that the other species (p = 0.004). The best model performed similarly in predicting the number of ivy stems on the tree and their sum of diameters, while it explained a lower percentage of variance for ivy basal area.
Ivy growth patterns
The largest sampled ivy stem had a diameter of 14 cm at 50 cm from the base and 53 counted rings, while the oldest one had 69 rings and a diameter of 9.5 cm. Five stems had an estimated age >50 years. The mean annual radial growth rate was 1.05 (±0.54) mm over the last 5 years and 1.03 (±0.47) mm over the last 10 years. Relationships between host tree characteristics and ivy growth were rather weak, and only host tree age was positively related to the rate of growth over the last 10 years (Pearson’s r = 0.36, p < 0.05, n = 34). However, ivy growth was related to the surface available on the tree trunk for each ivy stem, calculated as the ratio between the trunk circumference and the sum of ivy stems’ diameters. Available space was positively correlated to the growth rate both over the last 5 years (Pearson’s r = 0.39, p < 0.01, n = 43) and the last 10 years (r = 0.40, p < 0.01, n = 43) (Fig. 2).
Relationship between trunk circumference available to ivy stem (i.e., the ratio between host trunk circumference and sum of ivy stem diameters) and its mean ring width over the last 5 years. Both axes are log-scaled. The straight line is the linear regression fitted to the data, with confidence intervals (dotted lines)
Discussion
Ivy abundance
An increase in the abundance and distribution of lianas has been observed both in tropical and in temperate forests over recent decades, possibly due to the increase in atmospheric CO2, warmer temperatures, and increased forest fragmentation caused by disturbances and land use changes (Allen et al. 2005; Londré and Schnitzer 2006; Van der Heijden and Phillips 2008). It is therefore important to carry out quantitative surveys and monitor temporal variations in liana incidence in different forest ecosystems (Ladwig and Meiners 2010b; Schnitzer and Bongers 2011). It is usually assumed that lianas have a higher incidence in tropical than in temperate ecosystems (Schnitzer and Bongers 2002; Leicht-Young et al. 2010). However, the percentage of trees carrying ivy in the Siro Negri alluvial forest (45–79 %) was similar to those observed in many mature tropical and subtropical forests (Putz 1984; Campanello et al. 2007; Nesheim and Økland 2007), while the number of stems and basal area ha−1 was lower than that generally found in tropical forests (Schnitzer and Bongers 2002; Schnitzer et al. 2006; Van der Heijden and Phillips 2008; Madeira et al. 2009; Gianoli et al. 2010). Studies in two temperate alluvial forests in the Rhine valley, France (Schnitzler and Heuzé 2006), found 61.9 and 29.4 English ivy stems ha−1, both being much lower than the numbers observed in the Siro Negri forest. The observed ivy abundance could be related to mild climate, high water availability from the close river, and reduced stand size. Liana occurrence is positively related to forest fragmentation (Londré and Schnitzer 2006); thus, the forest analyzed herein could have more ivy than larger alluvial forests with similar stand and site features.
In addition to macro-ecological factors, stand density and tree size distribution can also influence the quantity of lianas (Van der Heijden and Phillips 2008; Madeira et al. 2009). Our survey was carried out in four plots very close to each other, but differing in tree density and diameter distribution. One of the plots (Plot 3) had a lower tree density compared to the others, but the number of ivy stems and basal area were similar or slightly larger than in the other plots. Within the same forest, patches with few but suitable supports can bear similar or larger amount of ivy than patches with many less suitable trees.
Host preference
Lianas are structural parasites that require suitable supports to access the canopy. A few recent studies, conducted both in tropical and temperate forests, indicate that the availability of suitable host trees may have more influence than climate and soil properties on vine and liana distribution (Nesheim and Økland 2007; Van der Heijden and Phillips 2008; Leicht-Young et al. 2010). In our study, we scrutinized several tree attributes, some of which have rarely been considered in previous research.
In the temperate alluvial forest of Siro Negri, larger trees were more likely to host ivy than smaller ones, and the largest host trees had the most ivy. A similar pattern has been observed in several studies on lianas conducted in various ecosystems. However, different liana species exhibit different host size preferences, depending on their climbing mechanism. Stem twiners and tendril climbers better ascend small supports, and thus are more abundant on small diameter trees. On the other hand, root climbers such as ivy adhere to trunks with adventitious roots rather than wrapping around them and prefer to ascend trees of larger diameters and rough bark (Putz 1984; Allen et al. 1997; Schnitzler and Heuzé 2006; Yuan et al. 2009; Madeira et al. 2009; Morrissey et al. 2009; Leicht-Young et al. 2010; Blick and Burns 2011; Nogueira et al. 2011).
It has been suggested that large trees have more lianas because they are, generally, older than smaller ones, meaning more colonization time for lianas (Campanello et al. 2007; Nesheim and Økland 2007; Ladwig and Meiners 2010a). However, tree age is not usually measured directly. In our study, the tree age of a subset of trees (55 % of the samples) was assessed by tree-ring analysis. This attribute turned out to be of secondary importance in ivy colonization, suggesting that large trees hosted more ivy than smaller ones mainly because they provided a larger surface that could accommodate many stems.
The majority of studies analyzing liana-tree interactions have found evident species associations. Trees with rougher bark host more root climbers because they provide a good surface to ascend. Moreover, crown morphology also seems to affect liana-tree relationships, and trees with high stature, small leaves, and a light crown host more lianas due to better light conditions (Nesheim and Økland 2007; Morrissey et al. 2009). In the Siro Negri forest, Q. robur appeared to be preferred, and almost all individuals of this species hosted ivy. However, thorough analysis revealed that ivy climbed Q. robur trees because they were large and had few neighbors, whereas the effect of species per se was negligible: When variations in size between species were excluded, different tree species were found to be colonized to a similar degree by ivy. This finding, though, cannot be generalized as the species composition in the Siro Negri forest is limited to deciduous broadleaf species, all characterized by relatively rough bark in the adult phase; Schnitzler and Heuzé (2006) observed that ivy does in fact show species preference in alluvial forests in northeastern France.
Using information on tree position in the stand, we analyzed the influence of neighboring trees on the likelihood of a tree to carry ivy, to our knowledge an aspect never considered in previous studies. Isolated trees, i.e., trees with few neighbors, were more likely to host ivy, and isolated host trees had more ivy stems than host trees in crowded areas. As tree size and density are usually inversely related, we checked whether the effect of neighborhood was discernible from that of tree diameter. In the analyzed stand, the two parameters were slightly negatively correlated (r = −0.24). However, the neighborhood ratio, expressed as the ratio between tree diameter and the sum of the neighbor’s diameters, had a stronger (negative) relation with ivy descriptors than tree diameter alone. The stepwise inclusion of neighborhood variables also enhanced the performance of all the regression models. We concluded that neighborhood crowding directly influenced ivy colonization. This is possibly because isolated tree trunks received more light than the others, making them more attractive to light-demanding ascendant ivy stems. This finding provides an interesting contribution to research on how forest structure affects lianas. Several authors report an increase in liana density in disturbed areas and forest gaps, but the causes of this pattern have not yet received much attention (Ladwig and Meiners 2010b; Gianoli et al. 2010). We observed that smaller numbers of neighbors directly increased both tree likelihood of being colonized and ivy abundance on host trees. It is likely that the incidence of light-demanding root climbers increases when a disturbance reduces the number of neighbors around surviving trees.
Growth patterns
Very little information exists on the longevity of lianas and on what affects their growth patterns (Brandes et al. 2011). In comparison to other liana species, however, relatively numerous dendrochronological studies have been conducted on ivy, possibly because tree rings are more evident than in many tropical liana species. The mean annual growth rate for ivy found in the Bosco Negri (mean ring width in the last 5 years equal to 1.05 ± 0.54 mm) and maximum age (69 years) were roughly comparable to those reported by other authors. Nola (1997) measured a mean annual radial increment of 0.94 mm (±0.34) and a maximum age of 68 years in southern Italy. Garfì and Ficarrotta (2003) found a mean ring width of 0.86 mm (±0.25) and maximum age of 47 years in Sicily. Heuzé et al. (2009) reported a mean growth rate between 0.50 mm (±0.42) and 2.06 mm (±0.67) and maximum age of 66 years in northeastern France.
Growth pattern analysis can provide interesting indications on liana dynamics. Unlike ivy distribution, we found that ivy growth was scarcely influenced by host tree characteristics and neighborhood crowding. We did, however, find a slight but significant relationship between stem growth and trunk surface available for each stem. We hypothesize that when many ivy stems rely on the same tree, they compete. Positive interaction between liana stems has been reported, but this was related to mechanical facilitation in the colonization of trees, as older stems provided support to new ones for reaching the upper canopy (Campanello et al. 2007). We are not aware of any other findings on competition between lianas, and further analyses should be conducted on other species to support our finding.
Conclusions
Ivy is an important component of temperate forests, but especially outside its natural distribution, it is often regarded as a threat to other woody plants. Management of this species should be driven by better knowledge of its ecology and natural interactions with other plants. As a structural parasite, its distribution depends strongly on the availability of suitable host trees. Light-demanding climbing ivy stems ascend trees to reach better light conditions. Indeed, we observed that preferred hosts were large, isolated trees that probably receive more light than others. Tree species and tree age appeared weakly related to ivy distribution.
Natural and human-induced large disturbances may create enhanced light conditions in the forest, which can lead to massive colonization of ivy. On the contrary, our analysis shows that the climbing form of ivy can be limited by neighborhood crowding around potential host trees, i.e., by natural forest closure in the absence of disturbances. Competition between ivy stems relying on the same trunk could further limit the vertical colonization of host trees.
References
Allen BP, Pauley EF, Sharitz RR (1997) Hurricane impacts on liana populations in an old-growth southeastern bottomland forest. J Torr Bot Soc 124:34–42
Allen BP, Sharitz RR, Goebel PC (2005) Twelve years post-hurricane liana dynamic in and old-growth southeastern floodplain forest. For Ecol Manag 218:259–269
Aniol RW (1983) Tree-ring analysis using CATRAS. Dendrochronologia 1:45–53
Badre B, Nobelis P, Trèmolières M (1998) Quantitative study and modelling of the litter decomposition in a European alluvial forest. Is there an influence of overstorey tree species on the decomposition of ivy litter (Hedera helix L.)? Acta Oecol 19:491–500
Biggerstaff MS, Beck CW (2007) Effects of English ivy (Hedera helix) on seed bank formation and germination. Am Midl Nat 15:250–257
Blick RAJ, Burns KC (2011) Liana co-occurrence patterns in a temperate rainforest. J Veg Sci 22:868–877
Brandes AFN, Lisi CS, Barros CF (2011) Dendrochronology of lianas of the Leguminosae family from the Atlantic forest, Brazil. Trees 24:1045–1060
Campanello PI, Garibaldi JF, Gatti MG, Goldstein G (2007) Lianas in a subtropical Atlantic forest: host preference and tree growth. For Ecol Manag 242:250–259
Castagneri D, Vacchiano G, Lingua E, Motta R (2008) Analysis of intraspecific competition in two subalpine Norway spruce (Picea abies (L.) Karst.) stands in Paneveggio (Trento, Italy). For Ecol Manag 255:651–659
Clarke MM, Reichard SH, Hamilton CW (2006) Prevalence of different horticultural taxa of ivy (Hedera spp., Araliaceae) in invading populations. Biol Conserv 8:149–157
Daniels RF, Burkhart HE, Clason TR (1986) A comparison of competition measures for predicting growth of loblolly pine trees. Can J For Res 16:1230–1237
Emmons LH, Gentry AH (1983) Tropical forest structure and the distribution of gliding and prehensile tailed vertebrates. Am Nat 121:513–524
Garfì G, Ficarrotta S (2003) Influence of ivy (Hedera helix L.) on the growth of downy oak (Quercus pubescens sl) in the Monte Carcaci nature reserve (central-western Sicily). Ecol Mediterr 29:5–14
Gerwing JJ, Schnitzer SA, Burnham RJ, Bongres F, Chave J, DeWalt SJ et al (2006) A standard protocol for liana censuses. Biotropica 38:256–261
Gianoli E, Saldana A, Jimènez-Castillo M, Valladares F (2010) Distribution and abundance of vines along the light gradient in a southern temperate rain forest. J Veg Sci 21:66–73
Heuzé P, Dupoueyb J-L, Schnitzler A (2009) Radial growth response of Hedera helix to hydrological changes and climatic variability in the Rhine floodplain. River Res Appl 25:393–404
Ichihashi R, Tateno M (2011) Strategies to balance between light acquisition and the risk of falls of four temperate liana species: to overtop host canopies or not? J Ecol 99:1071–1080
Ladwig LM, Meiners SJ (2010a) Liana host preference and implications for deciduous forest regeneration. J Torr Bot Soc 137:103–112
Ladwig LM, Meiners SJ (2010b) Spatiotemporal dynamics of lianas during 50 years of succession to temperate forest. Ecology 91:671–680
Laurence WF, Pérez-Salicrup D, Delamônica P, Fearnside PM, D’Angelo S, Jerozolinski A, Pohl L, Lovejoy TE (2001) Rain forest fragmentation and the structure of Amazonian liana communities. Ecology 82(1):105–116
Leicht-Young SA, Pavlovic NB, Frohnapple KJ, Grundel R (2010) Liana habitat and host references in northern temperate forests. For Ecol Manag 260:1467–1477
Londré RA, Schnitzer SA (2006) The distribution of lianas and their change in abundance in temperate forests over the past 45 years. Ecology 87:2973–2978
Macía MJ (2011) Spatial distribution and floristic composition of trees and lianas in different forest types of an Amazonian rainforest. Plant Ecol 212:1159–1177
Madeira BG, Espírito-Santo MM, D’Angelo Neto S, Nunes YRF, Arturo Sánchez Azofeifa G, Wilson Fernandes G, Quesada M (2009) Changes in tree and liana communities along a successional gradient in a tropical dry forest in south-eastern Brazil. Plant Ecol 201:291–304
Metcalfe DJ (2005) Biological flora of the British Isles Hedera helix L. J Ecol 93:632–645
Morrissey RC, Gauthier MM, Kershaw JA, Jacobs DF, Seifert JR, Fischer BC (2009) Grapevine (Vitis spp.) dynamics in association with manual tending, physiography, and host tree associations in temperate deciduous forests. For Ecol Manag 257:1839–1846
Motta R, Nola P (2001) Growth trends and dynamics in sub-alpine forest stands in the Varaita Valley (Piedmont, Italy) and their relationships with human activities and global change. J Veg Sci 12:219–230
Motta R, Nola P, Berretti R (2009) The rise and fall of the black locust (Robinia pseudoacacia L.) in the Siro Negri forest reserve (Lombardy, Italy): lessons learned and future uncertainties. Ann Sci 66:410
Nesheim I, Økland RH (2007) Do vine species in neotropical forests see the forest or the trees? J Veg Sci 18:395–404
Nogueira A, Costa FRC, Castilho CV (2011) Liana abundance patterns: the role of ecological filters during development. Biotropica 43:442–449
Nola P (1997) Interactions between Fagus sylvatica L. and Hedera helix L. a dendroecological approach. Dendrochronologia 15:23–37
Page NV, Qureshi Q, Rawat GS, Kushalappa CG (2010) Plant diversity in sacred forest fragments of Western Ghats: a comparative study of four life forms. Plant Ecol 206:237–250
Putz FE (1984) The natural history of lianas on Barro Colorado Island, Panama. Ecology 65:1713–1724
Putz FE, Mooney HA (1991) The biology of vines. Cambridge University Press, Cambridge
Sartori F (1984) Les forêts alluviales de la basse vallée du Tessin (Italie du nord). In: Cramer J (ed) Colloques phytosocologiques, la végétation des forêts alluviales, pp 201–216
Schnitzer SA, Bongers F (2002) The ecology of lianas and their role in forests. Trends Ecol Evol 17:223–230
Schnitzer SA, Bongers F (2011) Increasing liana abundance and biomass in tropical forests: emerging patterns and putative mechanisms. Ecol Lett 14:397–406
Schnitzer SA, DeWalt SJ, Chave J (2006) Censusing and measuring lianas: a quantitative comparison of the common methods. Biotropica 38:581–591
Schnitzler A (1995) Community ecology of arboreal lianas in gallery forest of the Rhine valley, France. Acta Oecol 16:219–236
Schnitzler A, Heuzé P (2006) Ivy (Hedera helix L.) dynamics in riverine forests: effects of river regulation and forest disturbance. For Ecol Manag 236:12–17
Ter Braak CJF, Smilauer P (1998) CANOCO 4. Centre for Biometry, Wageningen
Toledo-Aceves T, Swaine MD (2008) Above- and below-ground competition between the liana Acacia kamerunensis and tree seedlings in contrasting light environments. Plant Ecol 196:233–244
Trèmolières M, Carbiener R, Exinger A, Turlot JC (1988) Un exemple d’interaction non compètitive entre espèces ligneuses: le cas du lierre arborescent (Hedera helix L.) dans la foret alluviale. Acta Oecol 9:187–209
Van der Heijden GMF, Phillips OL (2008) What controls liana success in Neotropical forests? Glob Ecol Biogeogr 17:372–383
Yuan CM, Liu WY, Tang CQ, Li XS (2009) Species composition, diversity, and abundance of lianas in different secondary and primary forests in a subtropical mountainous area, SW China. Ecol Res 24:1361–1370
Acknowledgments
This study was funded by the Siro Negri National Forest Reserve and by the Ministero dell’ Ambiente e della tutela del Territorio e del Mare. The authors thank F. Sartori, the Reserve Director, L. Gianoli and M. Granata of the University of Pavia, R. Berretti, A. Bottero, F. Meloni, G. Vacchiano, and R. Motta of the University of Turin for field assistance, providing data from previous surveys, and helpful comments on this manuscript, and C. Archibald for revising the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castagneri, D., Garbarino, M. & Nola, P. Host preference and growth patterns of ivy (Hedera helix L.) in a temperate alluvial forest. Plant Ecol 214, 1–9 (2013). https://doi.org/10.1007/s11258-012-0130-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-012-0130-5