Abstract
Recent studies have shown significant impacts of past landscapes on present distributions of species, and discussed the existence of an extinction debt. Understanding of the processes building an extinction debt is fundamentally important for explaining present and future diversity patterns of species in fragmented landscapes. Few empirical studies, however, have examined the responses of different plant functional groups (PFGs) to historical landscape changes. We aimed to reveal PFG-based differences in species’ persistence by focusing on their vegetative, reproductive, and dispersal traits. We examined whether the present distributions of PFGs of grassland species in the edges of remnant woodlands established on former semi-natural grasslands are related to the past surrounding landscapes at different time periods and spatial scales. The effects of past landscapes varied significantly among the PFGs. Richness of short, early flowering forbs and tall, late-flowering, wind-dispersed forbs showed significant positive relationships with the surrounding habitat proportions more than 50 years ago (the 1950s) and at wide spatial scales (more than 1 km2). Richness of tall, late-flowering forbs with unassisted and other types of dispersal mechanisms showed positive relationships with the surrounding habitat proportions in recent times (the 1970s) and at smaller spatial scales (0.25 km2). Our results suggested that plant growth form, flowering season and dispersal ability—with additional information on seed bank persistence—can be good indicators for identifying species’ specific sensitivity to surrounding habitat loss. Trait-based approaches can be useful for understanding present and future distributions of grassland species with different persistence strategies in human-modified landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historical landscape change has strongly affected the processes of species colonization and extinction by affecting dispersal ability across landscapes (Bruun et al. 2001; Lunt and Spooner 2005). The concept of an “extinction debt” (Tilman et al. 1994) predicts that present populations of species are not in equilibrium with current fragmented landscapes, and that species will go extinct in the future after a time-lag following surrounding habitat loss. This is also called “relaxation time” (Diamond 1972). The existence of long time-lags between landscape change and community response highlights the significance of past landscapes for a better explanation of present and future species distributions. Understanding the mechanisms underlying an extinction debt is fundamentally important for providing effective biodiversity conservation strategies in human-modified landscapes (Vellend et al. 2006; Lindborg 2007).
Recent studies show significant impacts of past landscapes on the present distribution of plant species (e.g., Eriksson et al. 2002; Lindborg and Eriksson 2004; Honnay et al. 2005; Helm et al. 2006; Cousins 2006, 2009b) and discuss the existence of an extinction debt in the landscape (Cousins 2009a; Kuussaari et al. 2009). However, not all studies have provided empirical evidence of delayed responses of species to landscape changes (e.g., Krauss et al. 2004; Piessens et al. 2004; Adriaens et al. 2006), and divergent results make it difficult to draw any general conclusions about an extinction debt in landscapes.
Differences in responses to landscape changes among species are among the key factors in accurately detecting extinction debt (Kuussaari et al. 2009). Although recent studies have examined species- or trait-based responses to historical landscape changes (Kolb and Diekmann 2005; Vellend et al. 2006; Lindborg 2007; Piqueray et al. 2011), empirical evidence of these differences are still sparse. Most importantly, analysis of a single trait might not sufficiently explain a species’ persistence in fragmented landscapes compared to multiple-trait analysis. To derive a general understanding of the underlying mechanisms in building an extinction debt, it is essential to focus on combinations of these traits and their background effects on species persistence (Adriaens et al. 2006). Species that have similar trait combinations can be defined as functional groups, representing a functional role of the species in a community (Noble and Gitay 1996; Lavorel et al. 1997; Grime 2002). Thus, approaches based on plant functional group (PFG) can deepen the understanding of the background processes that allow species persistence in fragmented landscapes.
Species persistence in fragmented landscapes depends on the species’ clonal ability (Maurer et al. 2003; Piessens and Hermy 2006; Lindborg 2007), seed bank longevity (Piessens et al. 2004, 2005; Lindborg 2007; Tremlová and Münzbergová 2007), and species turnover rate or life span (Bossuyt and Honnay 2006; Vellend et al. 2006). Although few studies have provided empirical evidence, long-distance dispersal may be another crucial aspect for the survival of plant populations in a fragmented landscape (Bohrer et al. 2005; Adriaens et al. 2007; Lindborg 2007). Plant height also affect dispersal distances of seeds (van Dorp et al. 1996; Vittoz and Engler 2007; Thomson et al. 2011), and therefore might be important in determining species persistence against surrounding habitat loss. Here, we considered the combined effects of species vegetative, reproductive, and dispersal traits by adopting a PFG-based approach, focusing on grassland plant species in an agricultural landscape. Our approach leads to a more profound understanding of the underlying ecological mechanisms determining species’ persistence in a fragmented landscape (Verheyen et al. 2003; Hérault and Honnay 2005; Adriaens et al. 2006).
The main objective in this study was to identify differences in the relative importance of the past surrounding landscapes (i.e., important time periods and spatial scales) among functional groups of grassland species. We examined whether the present distributions of PFGs of grassland plant species in the edge vegetation of remnant woodlands established in former semi-natural grasslands areas are related to the past surrounding landscapes at different time periods and at different spatial scales. A previous study conducted in the same study area has already revealed that the impacts of past surrounding landscapes on the present distribution of grassland species could be recognized even in the linear vegetation of woodland edge, and detected existence of an extinction debt in the landscape (Koyanagi et al. 2009). Through the analyses, we aimed to reveal the PFG-based differences in species’ persistence and estimate the differences in underlying processes building an extinction debt among the PFGs in the fragmented landscape.
Methods
Study area
This study was conducted in the Tsukuba-Inashiki upland region, which lies in the eastern part of Japan’s Kanto Plain. The altitude ranges from approximately 20 to 30 m above sea level. The study area is located on a diluvial upland overlain by immature soils derived from aeolian volcanic ash or tephra, called the Kanto Loam layer. The mean annual temperature is 13.5°C (2.3°C in January and 25.2°C in August), with a mean annual precipitation of 1,236 mm (Japan Meteorological Agency 2011).
The region’s potential climax vegetation is a mixed evergreen–broadleaved woodland dominated by Quercus myrsinaefolia, Quercus glauca, and Castanopsis sieboldii (Miyawaki 1986). Two main types of semi-natural habitats for grassland species had been maintained by the traditional agricultural system; open semi-natural upland grassland and open secondary woodlands which consist mainly of Quercus acutissima, Quercus serrata, and Pinus densiflora (Kamata and Nakagoshi 1990; Koyanagi et al. 2009). The semi-natural grassland was burned in early spring and mown in summer (between the end of July and the beginning of October), and the practices were repeated almost every other year (Sprague et al. 2000). Semi-natural grassland provided fodder for domestic livestock, materials for the construction of thatched roofs, and organic fertilizer for arable land, including paddy fields (Sprague et al. 2000). The secondary oak and pine woodlands provided firewood and charcoal for domestic use and compost from fallen leaves and twigs. The understorey vegetation was also mown to produce organic fertilizer or livestock feed every summer and autumn. These traditional management practices enabled many grassland species to coexist in the understorey vegetation of the secondary oak and pine woodlands by maintaining high light availability (Fujii and Zinnai 1979; Yamamoto and Itoga 1988, Fig. 1).
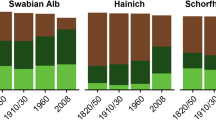
Both semi-natural grassland and secondary woodlands had been maintained under the traditional management regime for many centuries [i.e., at least since the Edo era (1603–1867)] before decrease in their areas (Sprague et al. 2000). These semi-natural habitats used to be widely distributed in the study area in the 1880s, but they decreased gradually until the 1950s by conversion to cropland as a result of agrarian land reform, and further decreased drastically by the 1960s by the rapid economic growth causing urbanization and agricultural intensification (Sakiyama and Itoga 1994; Fig. 2). As a result, nearly all semi-natural grasslands had disappeared over time. The land-use conversions since the 1970s to recent times have been moderate, but most of the remnant woodlands in this region were abandoned during that period (Yamamoto and Itoga 1988). In the present highly fragmented landscapes, only small remnant fragments are left in the past semi-natural grassland and secondary woodlands areas (Fig. 2).
Land-use data
We investigated the distribution of potentially suitable habitats during the past 100–150 years by using 100 × 100 m grid of land-use data at four different time periods; the 1880s, 1950s, 1970s, and 1990s (Fig. 2). The details of the data sources and data creation procedures are provided in Koyanagi et al. (2009). Among the eight different land-use categories (i.e., paddy field, cropland, grassland, woodland, urban landuse, other landuse, and open water), we used grassland and woodland as the potentially suitable habitats for grassland species (Koyanagi et al. 2009).
Data sampling
From July to August in 2007, 2008, and 2009, we conducted vegetation surveys in the edge vegetation of the remnant woodlands (72 sites) located in the different 100 × 100 m grid cells (Fig. 2). The surveyed woodlands have no history of the past cultivation and have been maintained as grassland or woodland since the 1880s. The occurrence of all herbaceous, grass, and woody species was recorded along the roads within 1–2 m from the pavement edge. The total length of road surveyed at each site ranged from 15 to 54 m, depending on the length of the woodland edge.
Species classification
We focused mainly on the differences in vegetative, reproductive, and dispersal traits of the target grassland species. Semi-natural grassland vegetation in the study area belongs to the Pleioblastus chino–Miscanthus sinensis phytosociological community (Miyawaki 1986, 1994). Thus, we defined characteristic species of this community type as typical grassland species. Information provided by Sasaki (1973) and Okuda (1997) explaining semi-natural grassland vegetation and their characteristic species was also used to identify target grassland species. On the basis of the differences in plant growth form, plant height, flowering season and dispersal mode, we identified five PFGs of grassland species (Fig. 3): FS, short early flowering forbs; FTW, tall late-flowering forbs with wind dispersal; FTU, tall late-flowering forbs with unassisted and other types of dispersal mechanisms; GS, grasses and sedges; and SV, shrubs and woody vines. We gathered all the trait information from existing literatures (Miyawaki 1994; Satake et al. 1998; Natural History Museum and Institute, Chiba 2003). Although the data was incomplete for PFG classification, we also gathered information on seed bank persistence and density for some of the target grassland species from our recently published study (Koyanagi et al. 2011).
Data analysis
The past and present landscapes were analyzed with regard to the proportions of potentially suitable habitats (i.e., grassland and woodland) around the sampling sites at five different spatial scales (3 × 3, 5 × 5, 7 × 7, 11 × 11, and 15 × 15 grid cells; about 0.09, 0.25, 0.49, 1.21, and 2.25 km2, respectively) at four different time periods (the 1880s, 1960s, 1980s, and 2000s). These five spatial scales were chosen to include the range at which the surrounding habitats might show relationships with the present grassland species distributions (see Koyanagi et al. 2009). We calculated the proportions of grassland and woodland cells surrounding the central cell that contained the sampling site within the five different spatial scales (i.e., 3 × 3, 5 × 5, 7 × 7, 11 × 11, and 15 × 15 grid cells) at each time period, and tested them as variables to explain the present distribution of grassland species at each site.
First of all, we assessed the impacts of surveyed length of woodland edge vegetation on the richness of all species and grassland species using a generalized linear model (GLM) with a logarithmic link function that followed a Poisson distribution. Second, we evaluated the spatial autocorrelation among the sampled sites using Moran’s I correlograms (Sawada 1999). The value of Moran’s I generally varies between +1 and -1. Positive autocorrelation in the data translates into positive values of Moran’s I; negative autocorrelation produces negative values. Absence of autocorrelation results in a value close to zero (Legendre and Legendre 1998). We used the Moran’s I metric to calculate autocorrelation at several different distance units in all the response and explanatory variables (response variables: richness values of all species, grassland species and five PFGs; explanatory variables: habitat proportions at each time period and spatial scale). Analysis of spatial autocorrelation was performed with Spatial Analysis for Macroecology (SAM) version 4.0 (Rangel et al. 2010).
Finally, we evaluated the relative importance of the surrounding habitat proportions at different time periods and spatial scales in explaining the present richness of all grassland species and different PFGs of grassland species. We performed the GLM and model selection based on both AICc (Akaike’s information criterion corrected for small sample sizes) and AICc differences (ΔAICc) using a logarithmic link function that followed a Poisson distribution. In this procedure, the explanatory parameters of the surrounding habitat proportions in each time period at the same spatial scales were included one at a time. To evaluate the relative importance of the explanatory parameters included in the models at explaining the present richness values, we calculated the Akaike weight (w i ) of each model as an indicator of the strength of evidence that the selected best model is convincingly the best (Burnham and Anderson 2002). Then, we calculated the sum of Akaike weights (∑w i ) of all models that include a particular parameter, and the sum value can be the evidence of importance of that parameter. To predict influence of each parameter (i.e., positive or negative) we used model averaging by weighting the estimate values by the Akaike weights (Burnham and Anderson 2002). All statistical procedures were performed with R version 2.12.2 (R Development Core Team 2011).
Results
We recorded a total of 246 species at the 72 sample points, including 38 typical grassland species. The average richness of all species and grassland species was 33.4 and 5.21 per site, respectively. All grassland species recorded in this study were classified into the five different PFGs (Appendix 1—Supplementary material). The numbers of all species recorded in the edge vegetation were significantly positively correlated with the length of surveyed vegetation along the roads (P < 0.001), while richness of grassland species showed no relationships with surveyed edge lengths (P = 0.513). Considering the response variables, no significant spatial autocorrelations were found in the richness of grassland species and groups FTW at any distance units (Appendix 2—Supplementary material). For the other response variables (i.e., richness of all species, and groups FS, FTU, GS, and SV), significant spatial autocorrelations were only found at very few of the distance units. For surrounding habitat proportions, spatial autocorrelations were not found at most of the distance units at any time periods except 1950s at smaller spatial scales (i.e., 3 × 3 and 5 × 5) (Appendix 3—Supplementary material). At larger spatial scales (i.e., 11 × 11 and 15 × 15 grid scale), both positive and negative spatial autocorrelations (i.e., P < 0.05) were found at most of the distance units in the 1880s and 1950.
The model-selection procedure suggested that four models could be considered as plausible (i.e., ΔAICc < 4, Appendix 4—Supplementary material) to explain the present richness of all grassland species. Not the present but the past habitat proportions in the 1950s showed the largest ∑w i at a 11 × 11 grid scale, and had strong positive effect on the present grassland species richness (Table 1). All the plausible models for explaining each PFG’s richness are shown in Appendix 4—Supplementary material. The relative importance of the surrounding habitat proportions varied among the groups (Table 1, Fig. 4). Among the five PFGs, only groups FS and FTW showed distinct relationships with the surrounding habitat proportions at the past, while groups GS and SV showed relatively weak relationships at all time periods and spatial scales (Table 1). The present richness of group FS was positively correlated with the surrounding habitat proportion in the 1950s at the 15 × 15 grid scale (Fig. 4). The richness of group FTW was positively correlated with those in the 1950s and 1970s at the 11 × 11 grid scale (Table 1), although the overall effect of surrounding habitat proportions in the 1950s was stronger than those in the 1970s (Fig. 4a). The richness of group FTU showed significant relationships with the surrounding habitat proportions in the 1950s and 1970s at the 5 × 5 grid scale, although the effects were not as distinct as those of groups FS and FTW (Table 1, Fig. 4). The overall effect of surrounding habitat proportions in the 1970s was stronger than those in the 1950s (Fig. 4a).
Results of generalized linear models, which investigated the relationships between the richness of all grassland species and of five different PFGs and explanatory variables using a logarithmic link function that followed a Poisson distribution. The investigated a time periods and b spatial scales are shown separately. Each value shows the sum of Akaike weights (w i ) for each explanatory variable (∑w i ), which reflects the relative importance of each variable (the larger the ∑w i the more important a variable i is, relative to other variables). The sums of Akaike weights were shown as positive and negative values for those variables that have positive and negative effects, respectively, on the response variables. The PFGs are explained in Fig. 3
Discussion
The relative effects of the past surrounding suitable habitat (i.e., surrounding habitat proportions) varied markedly according to PFGs. Our results suggested that combinations of growth form, plant height, and dispersal mode can be good indicators for identifying species’-specific sensitivity to surrounding habitat loss. As different species respond differently to historical landscape changes, the relative importance of the past landscapes could vary among species with different functional traits. Our results provide useful information for understanding different persistence strategies that determine plants’ ability to cope with surrounding habitat loss.
Although vegetation of woodland edge (i.e., road verge) are not equivalent to that of semi-natural grassland vegetation (Tikka et al. 2000), historical impacts of surrounding habitat proportions could be recognized as well as the impacts of the present environmental variables (Koyanagi et al. 2009). For example, woodland type, road width, and steepness of verges have significantly affected the present distribution of grassland species in the edge vegetation (Koyanagi et al. 2009). The vegetation of road verges have been managed by more frequent mowing (i.e., twice or three times during summer) compared to semi-natural grassland, which makes differences in species compositions and grassland species richness between these habitats (Tikka et al. 2000). The effects of the present local sites conditions, however, are not so strong to eliminate the effects of suitable surrounding habitat both at the present and past (Koyanagi et al. 2009).
The present richness of all grassland species was correlated positively with the past habitat proportions, especially in the 1950s at the 11 × 11 grid scale, and negatively in the 1990s, the recent time period. These results could support the existence of an extinction debt (Cousins 2009a; Kuussaari et al. 2009), i.e., a significant time-lag of grassland species’ responses to landscape changes as shown in the previous study (Koyanagi et al. 2009). The most drastic loss of potential habitats for grassland species (i.e., semi-natural grassland and open secondary woodland) occurred between the 1950s and the 1970s (periods of rapid economic growth) in the study area. Almost half of the traditional semi-natural habitat and open secondary woodland areas disappeared during those periods with land-use conversion for building developments and intensive agriculture (Sakiyama and Itoga 1994, Fig. 2). The negative relationships between the present richness of grassland species and the surrounding habitat proportions of the recent years (i.e., the 1990s) might be because of the recent loss of potential habitats in surrounding areas by rapid urban development. Although the proportions of surrounding suitable habitat in the recent time periods decreased drastically in some woodland sites, edge vegetation of these woodlands still retain pools of grassland species.
In functional group level, the present richness of group FS (short early flowering forbs) showed significant relationships with the surrounding habitat proportions in the 1950s, whereas that of group FTW (tall, late-flowering, and wind-dispersed forbs) showed significant relationships with those both in the 1950s and 1970s. Group FTU (tall, late-flowering forbs with unassisted and other types of dispersal mechanisms) showed more distinct relationship with surrounding habitat proportions in the 1970s than 1950s, although the effects of surrounding landscapes were less significant for explaining the richness of this group. We found that the important spatial scales of surrounding landscapes were also different among the functional groups FS, FTW, and FTU. These results offer empirical support for the notion that differences in a groups’ capacity for persistence depend on the combinations of vegetative, reproductive, and dispersal traits. Although dispersal ability was not thoroughly justified (i.e., wind dispersal does not guarantee long-distance dispersal; see Soons et al. 2005) in this study, group FTW species could persist longer than species with unassisted or less-dispersed species of group FTU. The effective spatial scales could also be related to dispersal distances, favoring species with widely dispersed seeds, which would have a seed supply from a wider surrounding area (Malanson and Cairns 1997; Bohrer et al. 2005). This can be the reason for group FTW species to be affected by the surrounding habitat proportions at the larger spatial scale. The reproductive characteristics can be the key factors for group FS species to be affected by the past surrounding habitat proportions at larger spatial scale. The longer time-lag of group FS might be due to their longer persistence of seeds in the soil (Appendix 1—Supplementary material). A recent seed bank analysis (Koyanagi et al. 2011) revealed that half of all FS species produce long-term persistent seeds (e.g., Lysimachia japonica f. subsessilis, Viola grypoceras, Ixeris dentate, and Potentilla freyniana), whereas seed densities of FTU and FTW species are quite low or they produce transient seeds (Appendix 1—Supplementary material). Dispersal disadvantages of group FS species (all are unassisted or have other dispersal mechanisms except for I. dentata, a wind-dispersed species; Appendix 1—Supplementary material) might be mitigated by the longer persistence of their seeds in the soil, allowing their persistence in the fragmented landscape (Lindborg 2007). Group FS species complete their flowering and seed production before summer, which makes it possible to transfer their seeds along the roads (i.e., spread wider areas) when mowing management is conducted. Although the underlying mechanisms are still indistinct, human-induced dispersal might have played some role for group FS species to be affected by the surrounding habitat proportions at the larger spatial scale.
The reason for the weak relationships of group GS (i.e., less sensitivity to surrounding habitat loss) might be the low habitat specificity of these species, especially the dominant M. sinensis and Imperata cylindrica, which are characteristic species of the grassland community in the region. These species can disperse widely and recolonize in open and disturbed habitats even after cultivation or land reclamation, and thus could easily recolonize habitats within a fragmented landscape (Fischer and Stöcklin 1997; Helm et al. 2006; Kuussaari et al. 2009). The weak relationships of group SV might be due to their wider dispersal range by birds, especially the woody vine species Rubus parvifolius, the most frequently recorded species in the study area. The longer lifespan of group SV than of herbaceous species could also explain its lower sensitivity to surrounding landscape changes (Bossuyt and Honnay 2006; García 2008).
This study provides useful information for species conservation in fragmented landscapes. The fact that it takes a long time for an extinction debt to be paid off indicates that even after major habitat loss dating back to the 1950s in the study area, there is still an opportunity to prevent further local extinctions of grassland species by habitat restoration (Kuussaari et al. 2009). Among the functional groups we identified, species of group FTU (e.g., Sanguisorba officinalis, Thalictrum minus var. hypoleucum, Lysimachia clethroides; Table 1) might be the most sensitive to surrounding landscape changes. The other species groups, especially group FTW (e.g., Eupatorium chinensis var. oppositifolium, Aster scaber, Cirsium japonicum, Cirsium oligophyllum; Table 1) and group FS (e.g., Potentilla sprengeliana, Potentilla freyniana), tend to coexist with the species of group FTU in remnant habitats because of their longer persistence to landscape change. The remnant forested sites that retain pools of group FTU species should be given higher priority for future restoration of grassland communities.
Although the precise time scale over which time-lags are likely to operate in the target species groups was difficult to establish using our approach (Kuussaari et al. 2009), we were able to provide empirical evidence of differences in relative lengths of time-lag among species that have different functional trait combinations. Our approach could be useful for identifying general trends in trait-based responses to historical landscape changes and for understanding not only present, but also future distributions of grassland species with different persistence strategies in human-modified landscapes.
References
Adriaens D, Honnay O, Hermy M (2006) No evidence of a plant extinction debt in highly fragmented calcareous grasslands in Belgium. Biol Conserv 133:212–224
Adriaens D, Honnay O, Hermy M (2007) Does seed retention potential affect the distribution of plant species in highly fragmented calcareous grasslands? Ecography 30:505–514
Bohrer G, Nathan R, Volis S (2005) Effects of long-distance dispersal for metapopulation survival and genetic structure at ecological time and spatial scales. J Ecol 93:1029–1040
Bossuyt B, Honnay O (2006) Interactions between plant life span, seed dispersal capacity and fecundity determine metapopulation viability in a dynamic landscape. Landscape Ecol 21:1195–1205
Bruun HH, Fritzboger B, Rindel PO, Hansen UL (2001) Plant species richness in grasslands: the relative importance of contemporary environment and land-use history since the Iron Age. Ecography 24:569–578
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Cousins SAO (2006) Plant species richness in midfield islets and road verges—the effect of landscape fragmentation. Biol Conserv 127:500–509
Cousins SAO (2009a) Extinction debt in fragmented grasslands: paid or not? J Veg Sci 20:3–7
Cousins SAO (2009b) Landscape history and soil properties affect grassland decline and plant species richness in rural landscapes. Biol Conserv 142:2752–2758
Diamond JM (1972) Biogeographic kinetics—estimation of relaxation times for avifaunas of Southwest Pacific Islands. Proc Natl Acad Sci USA 69:3199–3203
Eriksson O, Cousins SAO, Bruun HH (2002) Land-use history and fragmentation of traditionally managed grasslands in Scandinavia. J Veg Sci 13:743–748
Fischer M, Stöcklin J (1997) Local extinctions of plants in remnants of extensively used calcareous grasslands 1950–1985. Conserv Biol 11:727–737
Fujii E, Zinnai I (1979) Studies on the relationship between the management of floor layers and the succession of Pinus plain forests in Kanto region. J Jpn Forest Soc 61:76–82 (in Japanese with English abstract)
García MB (2008) Life history and population size variability in a relict plant. Different routes towards long-term persistence. Divers Distrib 14:106–113
Grime JP (2002) Plant strategies, vegetation processes, and ecosystem properties, 2nd edn. Wiley, Chichester
Helm A, Hanski I, Pärtel M (2006) Slow response of plant species richness to habitat loss and fragmentation. Ecol Lett 9:72–77
Hérault B, Honnay O (2005) The relative importance of local, regional and historical factors determining the distribution of plants in fragmented riverine forests: an emergent group approach. J Biogeogr 32:2069–2081
Honnay O, Jacquemyn H, Bossuyt B et al (2005) Forest fragmentation effects on patch occupancy and population viability of herbaceous plant species. New Phytol 166:723–736
Japan Meteorological Agency (2011) Monthly data in an average year (1971–2000) at the Tsukuba Meteorological Station. http://www.data.jma.go.jp/jma/indexe.html. Accessed April 2011
Kamata M, Nakagoshi N (1990) Patterns and processes of secondary vegetation at a farm village in southwestern Japan. Jpn J Ecol 40:137–150 (in Japanese with English abstract)
Kolb A, Diekmann M (2005) Effects of life-history traits on responses of plant species to forest fragmentation. Conserv Biol 19:929–938
Koyanagi T, Kusumoto Y, Yamamoto S et al (2009) Historical impacts on linear habitats: the present distribution of grassland species in forest-edge vegetation. Biol Conserv 142:1674–1684
Koyanagi T, Kusumoto Y, Yamamoto S et al (2011) Potential for restoration of grassland plant species on an abandoned forested Miscanthus grassland using the soil seed bank as a seed source. Jpn J Conserv Ecol 16:85–97 (in Japanese with English abstract)
Krauss J, Klein AM, Steffan-Dewenter I et al (2004) Effects of habitat area, isolation, and landscape diversity on plant species richness of calcareous grasslands. Biodivers Conserv 13:1427–1439
Kuussaari M, Bommarco R, Heikkinen RK et al (2009) Extinction debt: a challenge for biodiversity conservation. Trends Ecol Evol 24:564–571
Lavorel S, McIntyre S, Landsberg J et al (1997) Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends Ecol Evol 12:474–478
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier Science, Amsterdam
Lindborg R (2007) Evaluating the distribution of plant life-history traits in relation to current and historical landscape configurations. J Ecol 95:555–564
Lindborg R, Eriksson O (2004) Historical landscape connectivity affects present plant species diversity. Ecology 85:1840–1845
Lunt ID, Spooner PG (2005) Using historical ecology to understand patterns of biodiversity in fragmented agricultural landscapes. J Biogeogr 32:1859–1873
Malanson GP, Cairns DM (1997) Effects of dispersal, population delays, and forest fragmentation on tree migration rates. Plant Ecol 131:67–79
Maurer K, Durka W, Stocklin J (2003) Frequency of plant species in remnants of calcareous grassland and their dispersal and persistence characteristics. Basic Appl Ecol 4:307–316
Miyawaki A (ed) (1986) Vegetation of Japan vol. 7 Kanto. Shibundo, Tokyo
Miyawaki A (ed) (1994) Handbook of Japanese vegetation, 2nd edn. Shibundo, Tokyo
Natural History Museum and Institute, Chiba (2003) Vegetation of Chiba. Chiba pref, Chiba
Noble IR, Gitay H (1996) A functional classification for predicting the dynamics of landscapes. J Veg Sci 7:329–336
Okuda S (1997) Wild plants of Japan. Shogakkan, Tokyo (in Japanese)
Piessens K, Hermy M (2006) Does the heathland flora in north-western Belgium show an extinction debt? Biol Conserv 132:382–394
Piessens K, Honnay O, Nackaerts K et al (2004) Plant species richness and composition of heathland relics in north-western Belgium: evidence for a rescue-effect? J Biogeogr 31:1683–1692
Piessens K, Honnay O, Hermy M (2005) The role of fragment area and isolation in the conservation of heathland species. Biol Conserv 122:61–69
Piqueray J, Bisteau E, Cristofoli S et al. (2011) Plant species extinction debt in a temperate biodiversity hotspot: community, species and functional traits approaches. Biol Conserv 144:1619–1629
R Development Core Team (2011) An introduction to R. Notes on R: a programming environment for data analysis and graphics version 2.12.2. Vienna, Austria
Rangel TFLVB, Diniz-Filho JAF, Bini LM (2010) SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33:46–50
Sakiyama N, Itoga R (1994) On the change of grasslands into Pine forests in the Inashiki upland, Ibaraki prefecture. Environ Res Tsukuba 15:29–44 (in Japanese with English abstract)
Sasaki Y (1973) Plant sociology. Kyoritsu shuppan kabushiki-gaisha, Tokyo (in Japanese)
Satake Y, Ohi J, Kitamura S, Watari T, Tominari T (eds) (1998) Japanese wild plants. Heibonsha, Tokyo (in Japanese)
Sawada M (1999) ROOKCASE: an Excel 97/2000 Visual Basic (VB) add-in for exploring global and local spatial autocorrelation. Bull Ecol Soc Am 80:231–234
Soons MB, Messelink JH, Jongejans E et al. (2005) Habitat fragmentation reduces grassland connectivity for both short-distance and long-distance wind-dispersed forbs. J Ecol 93:1214–1225
Sprague DS, Goro T, Moriyama H (2000) GIS analysis using the rapid survey map of traditional agricultural land use in the early Meiji Era. J Jpn Inst Landsc Archit 63:771–774 (in Japanese with English abstract)
Thomson FJ, Moles AT, Auld TD, Kingsford RT (2011) Seed dispersal distance is more strongly correlated with plant height than with seed mass. J Ecol. doi:10.1111/j.1365-2745.2011.01867.x
Tikka PM, Koski PS, Kivela RA et al (2000) Can grassland plant communities be preserved on road and railway verges? Appl Veg Sci 3:25–32
Tilman D, May RM, Lehman CL et al (1994) Habitat destruction and the extinction debt. Nature 371:65–66
Tremlová K, Münzbergová Z (2007) Importance of species traits for species distribution in fragmented landscapes. Ecology 88:965–977
van Dorp D, van den Hoek WPM, Daleboudt C (1996) Seed dispersal capacity of six perennial grassland species measured in a wind tunnel at varying wind speed and height. Can J Bot 74:1956–1963
Vellend M, Verheyen K, Jacquemyn H et al (2006) Extinction debt of forest plants persists for more than a century following habitat fragmentation. Ecology 87:542–548
Verheyen K, Honnay O, Motzkin G et al (2003) Response of forest plant species to land-use change: a life-history trait-based approach. J Ecol 91:563–577
Vittoz P, Engler R (2007) Seed dispersal distances: a typology based on dispersal modes and plant traits. Bot Helv 117:109–124
Yamamoto S, Itoga R (1988) Forest forms and the distribution of Pinus densiflora plain forest in southwest part of Ibaraki prefecture. Landsc Res Jpn 51:150–155 (in Japanese with English abstract)
Acknowledgments
We thank the members of previous laboratory of the first author, especially A. Hoshino, Y. Kitagawa, and K. Aragane, for their comments on the earlier analysis of our data, as well as for their support during the field survey. We are grateful to S. A. O. Cousins for valuable comments and suggestions on the earlier draft. We also thank T. Amano, I. Washitani, and T. Furukawa for their helpful comments and advices. A. Auffret kindly edited English and gave us informative comments to improve our manuscript. This study was carried out under a JSPS research fellowship to T. Koyanagi, with additional support from the twenty-first century COE Program, Biodiversity and Ecosystem Restoration.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koyanagi, T., Kusumoto, Y., Yamamoto, S. et al. Grassland plant functional groups exhibit distinct time-lags in response to historical landscape change. Plant Ecol 213, 327–338 (2012). https://doi.org/10.1007/s11258-011-9979-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-011-9979-y