Abstract
The higher growth rates of resprouting shoots compared with those of mature plants in resprouter woody species are supported by higher rates of photosynthesis and transpiration. In this contribution we hypothesize that species with higher resprouting vigour will show a larger enhancement of photosynthesis in resprouting shoots. We test this hypothesis by comparing gas exchange and leaf parameters between resprouting and mature plants in Erica scoparia and E. australis. These two Erica species co-occur in Mediterranean heathlands of the Strait of Gibraltar. Erica scoparia has a higher rate of post-disturbance starch recovery than E. australis, which makes it more resistant to recurrent disturbance. We tested the hypothesis that enhancement of photosynthesis and water use characteristics of resprouting shoots compared with mature plants should be more pronounced in E. scoparia. In both species, resprouts had higher efficiency in the use of light and higher maximum net photosynthesis than mature shoots. However, contrary to expectations, differences in the photosynthetic performance between resprouts and mature plant shoots were larger in E. australis. Higher root to shoot ratios in resprouting E. australis plants, determined by their slower above-ground recovery, together with stronger demand from carbon sinks might explain this result.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vegetation dynamics in Mediterranean-type ecosystems are largely driven by the recurrence of severe disturbance events (e.g. fire). Resprouting is a common response amongst Mediterranean woody plants to survive top-kill—the complete removal of photosynthetic parts after severe disturbance (Keeley 1986; Bond and Midgley 2001). Resprouts in top-killed woody plants are produced from a bank of protected meristems whose growth is fuelled by non-structural carbohydrate reserves (mainly starch) stored in the xylem tissue of below-ground organs (Bell and Pate 1996; Kozlowski 1992; Paula and Ojeda 2009).
It has long been established that, in general, the vegetative growth rates of resprouting shoots are faster than those of mature plants in resprouter woody species. The first growth flush of resprouting shoots is boosted by mobilized carbohydrate reserves (DeSouza et al. 1986; Bowen and Pate 1993; Canadell and López-Soria 1998). After that, the carbon demand to sustain vegetative regrowth is supplied by higher rates of photosynthesis and transpiration compared with mature plants (Castell et al. 1994; Wildy et al. 2004). Such enhanced photosynthetic activity of resprouts has been ascribed to an improvement in soil-to-leaf water supply, determined by a temporarily increased root:shoot ratio (Kruger and Reich 1993; Castell et al. 1994; Fleck et al. 1998; Utsumi et al. 2010). However, enhanced photosynthetic activity could also be considered a natural property of resprouting shoots, since they temporarily revert to a pre-reproductive state (Iwasa and Kubo 1997) and levels of photosynthesis and stomatal conductance in woody plants are generally higher in juvenile stages (Bond 2000; Thomas and Winner 2002; Niinemets 2010). These two complementary explanations argue for the enhanced photosynthesis and water relations of resprouting shoots.
In addition to boosting shoot growth and supporting maintenance respiration costs, a substantial fraction of the carbon photosynthesized by the resprouting shoots is allocated to refill carbohydrate (starch) reserves (Iwasa and Kubo 1997; Noble 2001; Wildy and Pate 2002). Recovery of starch reserves is critical because resprouter woody plants mobilize and use almost all their stored starch reserves to boost post-disturbance regrowth (Paula and Ojeda 2009). Without replenished starch reserves a subsequent disturbance event might compromise their resprouting capacity and, hence, their survival (Zedler et al. 1983; Bell and Pate 1996; Canadell and López-Soria 1998). Thus, there is reason to believe that selection might have favoured higher rates of photosynthesis in resprouting shoots, which is correspondingly associated with less conservative water use to retain resprouting capacity across successive disturbance events. In fact, carbohydrate levels are known to exert control on gene regulation and are implicated in changes in resource allocation patterns amongst different plant parts (Koch 1996).
Erica scoparia L. and E. australis L. (Ericaceae) are two heath species that co-occur in Mediterranean heathlands of the Strait of Gibraltar region (Ojeda et al. 2000). Both species resprout after the complete removal of their above-ground biomass, mobilising virtually all their root starch reserves to boost the first resprouting flush (Paula and Ojeda 2009). In a recent study, Paula and Ojeda (2011, this issue) found marked differences between these two species in the recovery rates of below-ground (root) starch reserves after post-disturbance regrowth. Resprouting E. scoparia plants reached pre-disturbance starch levels significantly faster than those of E. australis, which made E. scoparia more resistant to recurrent disturbance. A more marked enhancement of photosynthetic activity and water relations in resprouts of E. scoparia compared with mature plants, or a less marked enhancement in E. australis resprouts, might account for the abovementioned slower recovery rate of starch reserves in E. australis.
In this study, we address the question posed above by testing the hypothesis that, when compared with mature plants, the enhancement in photosynthetic performance and water use of resprouting shoots should be more pronounced in species with a higher rate of post-disturbance starch recovery. We compare gas exchange parameters and leaf characteristics between resprouting and mature (undisturbed) plants in E. scoparia and E. australis, species that differ significantly in the post-resprouting recovery rate of starch reserves (Paula and Ojeda, 2011, this issue). This study adds to our understanding of the marked differences in resistance to recurrent disturbance between these two otherwise ecologically and taxonomically close resprouter heath species (Paula and Ojeda 2006, 2011; this issue).
Methods
Study site and experimental design
The study was conducted at the Monte Murta Forestry Station, (36º19′35′′N; 5º33′25′′W), within Los Alcornocales Natural Park, on the northern side of the Strait of Gibraltar, Spain (Fig. 1). For a description of the environment of this region, see Ojeda et al. (2000). Altitude in Monte Murta ranges from 350 to 450 m asl. Climate is mild Mediterranean, with mean annual rainfall ca. 1,300 mm. Bedrock is dominated by siliceous sandstone, which produces acid, nutrient-poor soils, particularly on ridges and upper slopes. Evergreen cork-oak (Quercus suber) woodlands with a heath-shrub understorey are dominant, except in valley bottoms and gorges, where semi-deciduous oak (Quercus canariensis) forests prevail. Sandstone mountain crests and upper slopes are covered by treeless, open heathlands. Recurrent wildfires (with an average return interval of 25–30 years) constitute the major disturbance factor in these heathlands (Ojeda et al. 1996; Ojeda et al. 2010).
The study site was located in a mature open heathland patch (i.e. more than 15 years since the last fire) on a south-exposed upper slope (ca. 400 m asl) where both E. scoparia and E. australis were abundant (38 and 25% relative cover, respectively). Ten plants of each species were randomly selected and tagged. In September 2006, at the end of summer, their aboveground biomass was removed by clipping to the ground level. All these clipped plants resprouted, and no post-resprouting mortality occurred in either species. Close to each clipped plant, a non-clipped individual (hereafter, mature plants) of the same species was chosen and tagged.
Gas exchange parameters and leaf characteristics
The response of resprouting and mature plants of the two species to changes in light intensity (photosynthetic photon flux density, PPFD) was determined in the field with a portable CIRAS-2 infrared gas analyser, equipped with a PLC6 (U) Automatic Universal Leaf Cuvette (1.7 cm2) and a LED light source (PP Systems, Ltd., Herts, UK). Net photosynthesis, A [μmol (CO2) m−2 s−1], and stomatal conductance, Gs [mol (H2O) m−2 s−1], were recorded at incrementally decreasing light intensities, from saturating PPFD (2,000 μmol m−2 s−1) to complete darkness, with temperature, humidity and CO2 levels held constant inside the cuvette (20°C, 50% and 360 ppmv, respectively). After checking stability of A values in the infrared gas analyser, time interval between measurements was set at 120 s. Since leaves of Erica species are small, terete and numerous, the upper section of a south-exposed, apical shoot of each individual was enclosed in the cuvette for measurements. In all cases, the shoot apex itself was left outside the cuvette, as only fully developed leaves were used for gas exchange measurements in both resprouting and mature plants. After each measurement, the shoot section inside the cuvette was clipped off, put in a zip-lock plastic bag and stored in a cooler. At the end of each day, all the collected shoot sections were taken to the lab and frozen at −20°C for later determination of their overall leaf area values to express net photosynthesis (A), stomatal conductance (G s) and dark respiration (R d) measurements of the shoot sections on a unit leaf area basis. To obtain the overall leaf area of each shoot section, its leaves were pulled off after defrosting, spread on a flatbed scanner and scanned together. SigmaScan Pro software (SPSS Inc., Chicago, IL) was used to measure overall leaf area, with an error <3%.
Three series of leaf gas exchange measurements (photosynthesis and stomatal conductance) were conducted across the spring of 2007, covering the first growing season of the resprouts. Each measurement series extended over 7 days from 9:00 to 13:00 h. As many tagged plant individuals as possible were analysed within this period, alternating between species and regeneration stages (resprouting vs. mature).
The first series of measurements was made in March (early spring), 6 months after clipping, when all resprouting plants had presumably used most of their root starch reserves (Paula and Ojeda 2009). Six resprouting and five mature plants of E. scoparia and six resprouting and six mature plants of E. australis were analysed in the first series. The second series was conducted in the first week of May (middle spring), and a total of seven plants of each regeneration stage (i.e. resprout and mature) and each species were analysed, except for mature E. australis, of which only six individuals could be measured. Finally, the third series was conducted in the first week of June (late spring). Six plants per regeneration stage were measured in E. scoparia in this last series, and only five per regeneration stage in E. australis.
To describe the photosynthetic response of each individual to variations in irradiance, light-response curves were obtained by fitting the actual photosynthesis-irradiance data (A and PPFD) to the hyperbolic tangent model (Chalker 1980):
where Tanh is the hyperbolic tangent and A max, α and R d are three key photosynthetic parameters to be estimated by the model based on the actual data recorded in the field. A max is the maximum net photosynthesis or photosynthetic capacity at saturating light levels; α is the initial slope of the curve and indicates the efficiency in light utilization; R d corresponds to the rate of respiration at PPFD = 0 (i.e. dark respiration).
Finally, intrinsic water use efficiency (WUEi) was calculated for each individual shoot section as the quotient between net photosynthesis and stomatal conductance (A/G s) under saturating light conditions. To get WUEi of each individual, we averaged its A and G s values at the three highest PPFD levels (between 2,000 and 1,500 μmol quanta m−2 s−1).
Leaf fresh and dry weights were calculated only from the shoot sections collected in May (middle spring). While defrosting, leaves of each shoot section were weighed together to the nearest 0.1 mg to obtain the overall leaf fresh weight. Then, after being scanned for quantifying the overall leaf area (see above), leaves were oven-dried (60°C) to constant mass and weighed again so as to obtain the dry weight. The specific leaf area (SLA, cm2 g−1) of each shoot section was then calculated as the quotient between its overall leaf area and dry weight. Finally, leaf water content (LWC; g g−1) was calculated for each shoot section as the difference between fresh and dry leaf weights standardised by dry leaf weight.
Data analyses
A max, α, G s and WUEi (log-transformed) were compared between regeneration stages (i.e. resprout vs. mature) across measurement series (i.e. spring period) and species by means of three-way ANOVAs. Dark respiration (R d) did not follow a normal distribution because many plants showed nil R d. (i.e. below the sensitivity of the infrared gas analyser). Therefore, we treated this as a binary variable (zero for plants without respiration, one otherwise) and used a generalized linear model (GLM) with a binomial error distribution and logit link function to test for differences in the probability of R d between species, regeneration stages and measurements. Model fitting and estimation of dispersion was conducted by analysis of deviance (McCullagh and Nelder 1989). LWC and SLA (log-transformed) of shoot sections were compared between regeneration stages across species by means of two-way ANOVAs conducted separately for each variable.
Results
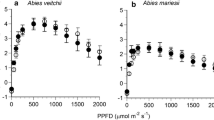
The photosynthetic response to changes in light intensity was highly variable in resprouting individuals of the two Erica species but, on average, showed higher photosynthetic performance than mature plants (Table 1, Fig. 2). Overall, resprouts had significantly higher light use efficiencies (i.e. higher α) and higher values of maximum net photosynthesis (i.e. higher A max) than mature plants across the three spring measurement series (non-significant stage × period interaction; Table 2). While difference in light use efficiency between resprouts and mature plants was more marked in E. australis than in E. scoparia (significant species × stage interaction; Table 2; P < 0.001 for E. australis and P = 0.192 for E. scoparia in the Tukey’s HSD post hoc test), between species difference was only marginally significant for maximum net photosynthesis (P < 0.1; Table 2). In both species, dark respiration was highest in early and, to a lesser extent, middle spring, being nil for most plants in late spring (Table 1). Probability of dark respiration (R d) was higher in resprouting shoots than in mature plants for both species, but these differences were more marked in E. australis (significant species × stage interaction; Table 3).
Average leaf area based response curves of net photosynthesis (A) to increasing light intensity (PPFD) in shoot sections of mature plants (solid line, filled circles) and resprouts (broken line, open circles) of Erica australis and E. scoparia in early (A, B), middle (C, D) and late spring (E, F). Whiskers represent standard errors
In both species, resprouting shoots showed higher values of stomatal conductance (G s) than mature plants (Table 1), although differences between regeneration stages were more marked in E. australis than in E. scoparia, as evidenced by the significant species × stage interaction (Table 2). Since differences between resprouts and mature plants in G s were higher than in A max, resprouting plants showed an overall lower WUEi than mature plants (Table 2; Fig. 3). Between-stage differences were similar between species across the three spring measurement series, as evidenced by the lack of significant interactions (Table 2).
Intrinsic water use efficiency (WUEi) at saturating light levels (see text) in mature plants (closed circles) and resprouts (open circles) of Erica scoparia and E. australis in the three spring periods. Symbols represent mean values and whiskers ±SD. WUEi increased along the study period, but only in E. scoparia (P = 0.041, Tukey’s HSD post hoc test comparing early and late spring values)
Leaves of E. scoparia had higher SLA and LWC values than those of E. australis and the two leaf parameters were significantly higher in resprouts than in mature plants (Table 4; Fig. 4). Differences between resprouts and mature plants were similar in the two species, as denoted by the lack of species × stage interaction (Table 4).
Specific leaf area (SLA), maximum net photosynthesis (A max), leaf water content (LWC) and stomatal conductance (G s) in leaves sampled in middle spring for mature plants and resprouts of Erica scoparia (closed symbols) and E. australis (open symbols). Symbols represent median values, whereas the lower and upper whiskers indicate the 0.25 and the 0.75 quantile, respectively
Discussion
In both Erica species, resprouting plants had higher light use efficiency (α) and higher maximum net photosynthesis (A max) than mature plants, commensurate with the reported enhancement of the photosynthetic parameters in resprouts compared with mature plants for other woody resprouter species (e.g. DeSouza et al. 1986; Castell et al. 1994; Fleck et al. 1998). A temporarily increased root:shoot ratio in resprouting plants is potentially responsible for their higher gas exchange rates by raising soil-to-leaf hydraulic conductivity (Kruger and Reich 1993).
It is well established that a high hydraulic supply increases leaf water status and hence stomatal conductance (Buckley 2005). In this sense, our results show significantly higher values for both leaf water content (LWC) and stomatal conductance (G s) in resprouting plants (Fig. 4). High G s values improve photosynthetic performance by increasing CO2 availability in the leaves (Reich et al. 1999). Since differences in G s between regeneration stages were more marked than differences in A max in both species, leaves of resprouts had lower intrinsic water use efficiency (WUEi) values than leaves of mature plants. Therefore, the higher photosynthetic performance of resprouting plants is associated with less conservative water use.
As well as high G s, our results show that low sclerophylly (i.e. high SLA) is associated with the increased photosynthetic performance of resprouting plants (see Fig. 4). Resprouting shoots revert to juvenile, pre-reproductive stages (Iwasa and Kubo 1997), characterized by high SLA compared with mature plants (Thomas and Winner 2002). High SLA enhances photosynthesis by increasing the light-capture area per mass and shortening CO2 diffusion paths from the stomata to the chloroplasts (Parkhurst 1994; Reich et al. 1999; Thomas and Winner 2002).
Fitted values of dark respiration (R d) were higher (more negative) in resprouting plants and the difference between resprouting and mature plants was more marked at the beginning of the growing season, as indicated by the significant stage × period interaction (Table 3). Higher R d may denote higher metabolic rates of the resprouts during their first regrowth pulse. But towards the end of the first growing season (late spring), R d values of the resprouts of the two species dropped to almost nil and became equal to those of mature plants. By contrast, the differences in photosynthetic parameters (α, A max) between resprouts and mature plants referred to above were maintained across the growing season (from early to late spring). This suggests that the enhanced photosynthesis of resprouts supports the vegetative regrowth in very early stages, whereas in the later stages, even within the first growing season, the metabolic demand decreases and some of the fixed carbon would be diverted to replenishing depleted below-ground starch reserves (Iwasa and Kubo 1997).
When comparing the two species, contrary to our initial hypothesis, differences in the photosynthetic performance between regeneration stages were greater in E. australis than in E. scoparia, even though the rate of post-disturbance starch recovery is markedly lower in E. australis (Paula and Ojeda 2011, this issue). We propose two possible explanations for this somewhat counterintuitive result.
First, the enhanced photosynthetic performance of resprouting plants could be viewed, not as an adaptive response for enhancing the recovery of starch reserves after disturbance, but simply as an outcome of their root to shoot relationship (see above). It has long been established that species with large, deep root systems have high stomatal conductance and low water use efficiency, whereas species with shallow roots tend to have a conservative water use strategy (e.g. Davis and Monney 1986). There is no available data on root depth for these two Erica species, but their habitat preferences (E. australis for shallow rocky soils on sandstone ridges and crests, and E. scoparia for deeper soils on middle slopes; Ojeda et al. 2000) do not suggest the existence of a deeper root system in E. australis. Therefore, differences in root depth do not seem to explain the greater enhancement of photosynthetic performance in resprouting E. australis plants. Nevertheless, root to shoot relationships could still contribute to explaining our results, since the comparatively low above-ground growth of resprouting E. australis plants (Paula and Ojeda 2006, 2009) causes it to have a higher root:shoot ratio than E. scoparia.
A second explanation concerns the higher demands for assimilated carbon in E. australis than in E. scoparia. Shoots of E. australis bear numerous glandular trichomes, whereas those of E. scoparia are glabrous (Bayer 1996). Leaves of E. australis are also significantly tougher (lower SLA; see Table 4) and accumulate more tannins (Ramos et al. 1999) than those of E. scoparia, probably to avoid herbivory (Paula and Ojeda 2011, this issue). Furthermore, the xylem of woody roots in E. australis has a higher proportion of parenchymatic rays than that of E. scoparia (Paula 2004). Leaf toughness, glandular trichomes and tannin accumulation are all very expensive in terms of carbon and energy (Koricheva 2002; Strauss et al. 2002; Wright and Cannon 2001) and maintenance of parenchymatic rays, associated with carbon storage, also requires energy (Pate et al. 1990). Thus, resprouting plants of Erica australis, with lower regrowth compared with E. scoparia (Paula and Ojeda 2006, 2009), need higher assimilation rates per leaf area unit to support their higher carbon demands.
In conclusion, the faster rate of starch re-storage of E. scoparia compared with E. australis reported by Paula and Ojeda (2011, this issue) cannot be explained by a greater enhancement of photosynthesis in resprouting plants of E. scoparia. We invoke both differences in root:shoot ratios and demand from carbon sinks to explain our finding that E. australis shows a greater increase in photosynthetic performance during resprouting than E. scoparia.
References
Bayer E (1996) Síntesis genérica de Erica. In: Castroviejo S, Aedo C, Gómez CC, Laínz M, Montserrat P, Morales R, Muñóz GF, Nieto FG, Rico E, Talavera S, Villar L (eds) Flora Ibérica. Plantas vasculares de la península Ibérica e islas Baleares. Real Jardín Botánico, Consejo Superior de Investigaciones Científicas, Madrid, pp 485–506
Bell TL, Pate JS (1996) Growth and fire response of selected epacridaceae of south-western Australia. Aust J Bot 44:509–526
Bond BJ (2000) Age-related changes in photosynthesis of woody plants. Trends Plant Sci 5:349–353
Bond WJ, Midgley JJ (2001) Ecology of sprouting in woody plants: the persistence niche. Trends Ecol Evol 16:45–51
Bowen BJ, Pate JS (1993) The significance of root starch in postfire shoot recovery of the resprouter Stirlingia latifolia R. Br. (Proteaceae). Ann Bot 72:7–16
Buckley TN (2005) The control of stomata by water balance. New Phytol 168:275–292
Canadell J, López-Soria L (1998) Lignotuber reserves support regrowth following clipping of two mediterranean shrubs. Funct Ecol 12:31–38
Castell C, Terradas J, Tenhunen JD (1994) Water relations, gas exchange, and growth of resprouts and mature plant shoots of Arbutus unedo L. and Quercus ilex L. Oecologia 98:201–211
Chalker BE (1980) Modeling light saturation curves for photosynthesis: an exponential function. J Theor Biol 84:205–215
Davis SD, Mooney HA (1986) Water use patterns of four co-occurring chaparral shrubs. Oecologia 70:172–177
DeSouza J, Silka PA, Davis SD (1986) Comparative physiology of burned and unburned Rhus laurina after chaparral wildfire. Oecologia 71:63–68
Fleck I, Hogan KP, Llorens L, Abadía A, Aranda X (1998) Photosynthesis and photoprotection in Quercus ilex resprouts after fire. Tree Physiol 18:607
Iwasa Y, Kubo T (1997) Optimal size of storage for recovery after unpredictable disturbances. Evol Ecol 11:41–65
Keeley JE (1986) Resilience of mediterranean shrub communities to fires. In: Dell B, Hopkins AMJ, Lamont BB (eds) Resilience in Mediterranean-type ecosystems. Dr. W. Junk, Dordrecht, pp 95–112
Koch KE (1996) Carbohydrate-modulated gene expression in plants. Ann Rev Plant Biol 47:509–540
Koricheva J (2002) Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83:176–190
Kozlowski TT (1992) Carbohydrate sources and sinks in woody-plants. Bot Rev 58:107–222
Kruger EL, Reich PB (1993) Coppicing affects growth, root: shoot relations and ecophysiology of potted Quercus rubra seedlings. Physiol Plant 89:751–760
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman and Hall, London
Niinemets Ü (2010) Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. For Ecol Manag 260:1623–1639
Noble JC (2001) Lignotubers and meristem dependence in mallee (Eucalyptus spp.) coppicing after fire. Aust J Bot 49:31–41
Ojeda F, Marañón T, Arroyo J (1996) Postfire regeneration of a mediterranean heathland in southern Spain. Int J Wildl Fire 6:191–198
Ojeda F, Arroyo J, Marañón T (2000) Ecological distribution of four co-occurring Mediterranean heath species. Ecography 23:148–159
Ojeda F, Pausas JG, Verdú M (2010) Soil shapes community structure through fire. Oecologia 163:1–7
Parkhurst DF (1994) Diffusion of CO2 and other gases inside leaves. New Phytol 126:449–479
Pate JS, Froend RH, Bowen BJ, Hansen A, Kuo J (1990) Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of SW. Australia. Ann Bot 65:585–601
Paula S (2004) Análisis funcional de la capacidad de regeneración de tres especies de brezos rebrotadores (Erica australis, E. scoparia y E. arborea) tras perturbaciones recurrentes. Departamento de Biología. Universidad de Cádiz, Puerto Real, p 218
Paula S, Ojeda F (2006) Resistance of three co-occurring resprouter Erica species to highly frequent disturbance. Plant Ecol 183:329–336
Paula S, Ojeda F (2009) Belowground starch consumption after recurrent severe disturbance in three resprouter species of the genus Erica. Botany 87:253–259
Paula S, Ojeda F (2011) Response to recurrent disturbance of two co-occurring, resprouter heath species: the cost of withstanding herbivores. Plant Ecol. doi:10.1007/s11258-011-9927-x (special issue of Plant Ecology—Sprouting Ecology)
Ramos G, Frutos P, Fernández GM, Lavín P, Mantecón AR (1999) Valor nutritivo de especies arbustivas de puertos de montaña: efecto del contenido en taninos condensados. XXXI Jornadas de Estudio AIDA (VIII Jornadas sobre Producción Animal) ITEA. Asociación Interprofesional para el Desarrollo Agrario (AIDA), Zaragoza, pp 529–531
Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80:1955–1969
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17:278–285
Thomas SC, Winner WE (2002) Photosynthetic differences between saplings and adult trees: an integration of field results by meta-analysis. Tree Physiol 22:117–127
Utsumi Y, Bobich EG, Ewers FW (2010) Photosynthetic, hydraulic and biomechanical responses of Juglans californica shoots to wildfire. Oecologia 164:331–338
Wildy DT, Pate JS (2002) Quantifying above- and below-ground growth responses of the western Australian oil mallee, Eucalyptus kochii subsp. plenissima, to contrasting decapitation regimes. Ann Bot 90:185–197
Wildy DT, Pate JS, Sefcik LT (2004) Water-use efficiency of a mallee eucalypt growing naturally and in short-rotation coppice cultivation. Plant Soil 262:111–128
Wright IJ, Cannon K (2001) Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Funct Ecol 15:351–359
Zedler PH, Gautier CR, McMaster GS (1983) Vegetation change in response to extreme events—the effect of a short interval between fires in California chaparral and coastal scrub. Ecology 64:809–818
Acknowledgments
The authors thank Marco Antonio Tena, former director of Los Alcornocales Natural Park, for kindly providing facilities for field work; Mike Scott, Mike Lawes and two anonymous reviewers for assisting with the English grammar and also providing useful comments on the manuscript. This study was funded by research projects BOS2002-00609 and AGL2005-07440-C02-02/FOR from the Spanish Ministerio de Educación y Ciencia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goorman, R., Bartual, A., Paula, S. et al. Enhancement of photosynthesis in post-disturbance resprouts of two co-occurring Mediterranean Erica species. Plant Ecol 212, 2023–2033 (2011). https://doi.org/10.1007/s11258-011-9967-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-011-9967-2