Abstract
A common perception, particularly in South Africa, is that heavily and continuously grazed communal land leads to degradation and loss of plant diversity when compared to commercial rangeland farming or conservation areas. We focus on whether this applies to the Grassland Biome of South Africa and whether the opposite can occur, namely, an increase in plant species richness under heavy grazing. A study of a contrast between a communal area of the former Ciskei and a neighbouring nature reserve showed that intense utilization under communal use led to a significant increase in plant species richness. However, this increase was scale-dependent with the greatest significant difference occurring at sample plot scale (50 m2) but converging at the broader scale of the whole study site. Species that increased with heavy grazing included those from arid Karroid areas as well as some from more mesic grassland and savanna areas. The contribution of beta diversity to gamma diversity across the grazing contrast was relatively low which reflects the relatively high proportion of species shared between treatments. Total plant canopy cover declined sharply with heavy grazing. In terms of plant canopy cover, grazing favoured annual over perennial plants, prostrate over erect plants, and stoloniferous over tussock plant architecture. This pattern was not supported when expressed in terms of number of species belonging to these grazing response groups or traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a global perception that heavy livestock grazing reduces biodiversity and that biodiversity is maximized in ‘pristine or primary vegetation’ (Alkemade et al. 2009). Land degradation, defined as land-uses that lead to a persistent loss in ecosystem productivity (Scholes and Biggs 2005), is often assumed to result in a decline in biodiversity (e.g. Gisladottir and Stocking 2005). A well-known contemporary grazing-diversity model indicates a decline in diversity with heavy grazing intensity, at least outside areas of high resources (Cingolani et al. 2005). In southern Africa, ‘excessively heavy grazing’ has frequently been given as one of the causes of a decline in biodiversity (for example, Biggs et al. 2008).
There are few studies of the effect of grazing on plant species richness in the Grassland Biome and this paucity of information is not unique to South Africa. Results in North American grassland vary from finding no relationship between grazing and species richness (Bai et al. 2001) to finding a significant increase in species richness with heavy grazing (Hickman et al. 2004). The latter paradigm is overall poorly tested (O’Connor et al. 2010). In temperate Australian grassland, native species richness declined across a range of spatial scales in response to increasing grazing intensity which is in keeping with results of previous studies in that region (Dorrough et al. 2007).
Rangeland and biodiversity research in South Africa has been biased towards areas of commercial livestock ranching, and extrapolating findings to communal rangeland is particularly problematic (O’Connor et al. 2010). Communal land in South Africa is characterized by continuous grazing usually with higher stocking densities than with commercial, commodity-based ranching. In many communal lands, there is a strong disincentive for the individual to destock, and stock are often kept as assets rather than production units (Scholes 2009).
The common expectation in the South African Grassland Biome is that, based on grazing pressure, plant species richness is highest on conservation areas, lowest on communal rangeland and intermediate on commercial rangeland (O’Connor 2005). Grassland not rested from grazing will experience a greater reduction of plant diversity (O’Connor et al. 2010). A national degradation audit in South Africa in the late 1990s found that communal land tenure was the strongest predictor of vegetation and soil degradation (Vetter et al. 2006). This audit was based on the perceptions of agricultural extension officers and resource conservation technicians from the Department of Agriculture (Hoffman and Todd 2000). However, the relative impact of communal and commercial forms of land tenure in grasslands of South Africa is contentious and requires resolution (O’Connor et al. 2003).
In the southern KwaZulu-Natal Province (KZN) communal, commercial and conservation management systems had a minimal effect on species richness (O’Connor 2005). This was confirmed for other areas of the Grassland Biome in KZN. Thus, in southern KZN, stocking rates that varied from low to high had no significant effect on plant species richness (Martindale 2007). Similarly, no significant difference in plant species richness was found between grassland wetlands on the Coleford Nature Reserve and on two nearby communal areas which had stocking densities several times higher than that on the Reserve (Walters et al. 2006). No significant differences in plant species richness were found between the Cathedral Peak protected area and adjacent communal (lease) land, but differences in stocking densities between the two land-use types were uncertain (Peden 2004). By contrast, in grassland of the Eastern Cape Province (Hoare 2003), a decline in species richness was found on communal ground which was also considered to be in a significantly worse condition for livestock production than grassland under commercial farming systems (Kiguli et al. 1999). Although communal areas in a few parts of the Savanna Biome has been found to have significantly higher species richness than adjacent conservation areas, (Shackleton 2000) it appears that this has not yet been shown for the Grassland Biome.
The Grassland Biome of South Africa is an important rangeland production area and is rich in plant species diversity (O’Connor and Bredenkamp 1997). However, it is also one of the most heavily impacted, least protected (Rouget et al. 2004) and severely threatened (Neke and Du Plessis 2003) biomes in South Africa. The Biome is limited to summer rainfall areas with frequent frost in winter (Rutherford and Westfall 1994) as a result of the relatively high elevation and inland continentality (Mucina and Rutherford 2006).
This article aims to quantify and understand the impact of apparently severe land degradation on plant diversity in part of the Grassland Biome in the Eastern Cape. The study contributes to a pilot research programme of the South African National Biodiversity Institute (SANBI) on the relationships between plant diversity and land degradation in different biomes of South Africa. This arose following the South African National Spatial Biodiversity Assessment (Rouget at al 2004) that highlighted the inadequate understanding of the impact of habitat degradation on biodiversity. Although biodiversity can be viewed at various hierarchical levels of organization and complexity (such as ‘biodiversity integrity’—O’Connor and Kuyler 2009), this article addresses biodiversity at the plant species level since it appears from the foregoing that even at this simple and practical level, conclusions on the effect of grazing on plant diversity and richness in the South African Grassland Biome remain unclear. Many indices that are used to express biodiversity change remain species-based (Lamb et al. 2009). We do not prejudge the issue of potential functional redundancy of species in ecosystems.
Study area
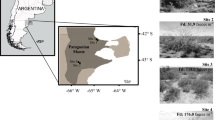
Protected areas are often used as a reference state for degradation (Scholes 2009). High contrast boundaries between communal areas and nature reserves were systematically scanned for the Eastern Cape Province, mainly using SPOT5 satellite imagery. One of the most striking contrasts was along the northern edge of the Tsolwana Nature Reserve and the adjacent communal area which is part of the former Ciskei. Field inspection confirmed that the contrast was due to different grazing intensities and possible wood removal on the communal side. There was no indication of any pre-existing environmental gradients across the fence. The communal area of the former Ciskei has been regarded as excessively overstocked resulting in severe degradation of the vegetation (Forbes and Trollope 1991). More recently, dominance of income sources from state grants has resulted in less commitment to management of these communal rangelands (Moyo et al. 2008).
The study site was situated in Queenstown Thornveld, southeast of Tarkastad on the Queenstown plains north of the Winterberge (Fig. 1) in the Tsolwana Local Municipality. Slope was gentle. Acacia karroo was the sole constituent of the emergent, low, sparse tree layer. Queenstown Thornveld thus does not have the typical multi-species tree composition of most savanna vegetation types and is clearly part of the Grassland Biome (Rutherford et al. 2006).
Rainfall is mainly in summer with an annual mean of 480 mm. Geology of the site is sedimentary rocks of the Tarkastad Subgroup (Beaufort Group, Karoo Supergroup) overlain with loamy sand or sandy loam soil. Although the reserve has some erosion gullies, these were confined to alluvial fans and viewed as part of a natural cycle (Cobban and Weaver 1993). Sampling was carried out away from these features or any degraded areas on the reserve (Fatunbi and Dube 2008).
The 8,500 ha reserve was created 30 years prior to the study and the fence line used in the study was established at that time. The transfer to the former Ciskei of the adjacent commercial land and its transformation to communal use also occurred about then (Wotshela 2004). The official 2008 animal census of the reserve indicated a stocking density of 0.13 large stock unit (LSU) equivalents per hectare (D. Peinke, personal communication). About 70% of this stocking density comprised six species. These were: black wildebeest (Connochaetes gnou, 20%), eland (Taurotragus oryx, 18%), mountain reedbuck (Redunca fulvorufula, 11%), springbok (Antidorcas marsupialis, 8%), red hartebeest (Alcelaphus buselaphus, 7%) and cape mountain zebra (Equus zebra, 7%). The communal areas are typically available to all livestock species at all times of the year (Bennett and Barrett 2007). The veterinary inspection census of 2010 for the Amaqwathi Tribal Authority indicated a stocking density of 0.67 LSU equivalents per hectare (information provided by the Nthabathemba Agricultural Office, Eastern Cape Department of Agriculture, and corrected for missing animals by adding 10%). The last-mentioned office also indicated that fluctuations in animal numbers from year to year in the communal area had been small and the results for the 2010 census would be expected to differ by less than 10% from the animal numbers in 2008. A separate census in 2010 using repeated road strip counts for that part of the Amaqwati Tribal Authority within a 5 km radius of the study area revealed a slightly lower stocking density of 0.62 LSU equivalents per hectare. This comprised sheep (Ovis aries, 78%), goats (Capra aegagrus, 12%) and cattle (Bos taurus, 10%). Stocking density was thus four to five times greater on the communal land than that on the nature reserve.
We realize that with this sampling approach diversity maxima may lie between extremes according to the Intermediate Disturbance Hypothesis (Roxburgh et al. 2004).
Methods
In this study, the nature reserve side of the fence is referred to as LU (Low Utilization) and the communal side is termed HU (High Utilization). This terminology is intended to fit with the broader pilot study where both grazing and firewood extraction co-occur.
Sampling was done in early March 2008 towards the end of the rainy season. Twelve sample plots of 50 m2 (10 × 5 m) were randomly placed on each side of the fence at distances varying between 25 and 35 m from the fence to avoid possible disturbance effects near the fence. The canopy cover of each plant species was recorded as well as the total canopy cover of each plot. Every effort was made to record all species in plots including inspections within and under all shrub canopies and within tussocks to avoid problems of overlooking (Archaux et al. 2009). The same observer estimated cover in all plots to reduce possible variation in the estimates between different observers (Kercher et al. 2003). Plots were recorded alternately on each side of the fence to minimize bias and any observer exhaustion effect (Bergstedt et al. 2009). Species were classed according to plant traits, namely by life form (annual, perennial), growth form (forb, geophyte, graminoid, shrub, tree), habit (erect, prostrate) and architecture (leafy stem, rosette, stoloniferous, tussock). Plant specimens were identified by the National Herbarium of SANBI. The Gulliver form (Bond and van Wilgen 1996) and mature or emergent form of A. karroo were recorded separately. Our sampling took advantage of a natural field experiment in which the consequential logistic problems in avoiding pseudo-replication (Hurlbert 2004) were insurmountable (Hargrove and Pickering 1992). See Rutherford and Powrie (2010) for discussion on issues of pseudo-replication in fence-line contrast studies.
Soil degradation may negatively influence biodiversity (Bohensky et al. 2004). To investigate this, six soil samples were taken to a depth of 50 mm from each side and analysed for pH, conductivity, resistance, carbon and textural classes by the Provincial Department of Agriculture of the Western Cape (Elsenberg) according to methods as given by The Non-affiliated Soil Analysis Work Committee (1990). Surface coarse fragment cover was estimated. Water absorption was determined using a pipette and timing (in s) for moist, undisturbed soil to absorb a drop of water (Land Type Survey Staff 1991). Ten drops at six stations on each side of the fence were used.
Plant canopy cover and soil parameter values were analysed using two-tailed t tests after square root transformation [(x + 1)1/2] of the data (Krebs 1989). Where appropriate we applied the step-up false discovery rate correction which is less restrictive and statistically more powerful than using the family-wise error rate which includes the Bonferroni correction (García 2004). Results for plant cover were compared with those of the independent Indicator Species Analysis of Dufrêne and Legendre (1997) according to McCune and Grace (2002). Ordination of plots used the non-metric multidimensional scaling (NMS) (PC-Ord) after a number of runs from different starting configurations to avoid the dangers of local minima. The Shannon-Wiener index of diversity (H’) was calculated as in (Krebs 1989) but using ln base e. The values of this index were also converted to their number equivalents which expresses a species-neutral diversity (Jost 2009). Alpha diversity (α) was calculated for LU and HU and gamma diversity (γ) was calculated for the whole study site by pooling the observations. Independent beta diversity (β) across the contrast was calculated assuming the additive partitioning of diversity where γ = μ(α) + β and μ(α) is mean alpha diversity (Legendre et al. 2005; Jost 2007). Species accumulation curves were derived using sample-based rarefaction (Mao Tau expected richness function in EstimateS 8.0) as described by Colwell et al. (2004). Evenness was calculated as the ratio of diversity index to maximum possible diversity index for the given number of species (Krebs 1989).
Results
Total numbers of species on LU and on HU were similar and no significant difference in diversity indices nor in species evenness was found between the treatments (Table 1). However, mean species richness expressed as mean number of species per sample plot (or species density sensu Gotelli and Colwell 2001) was significantly higher on HU. With increasing spatial scale, mean species richness remained greater on HU than on LU with the difference becoming increasingly narrower (Fig. 2). The ordination of the 24 plots in species space (Fig. 3) showed a clear separation of LU and HU plots. The final stress was 13.4 with the proportion of the variance given by the first two axes corresponding to an r2 of 0.79 (8.2 and 0.90 respectively for three axes). Plots were more tightly clustered on HU than on LU. The number of species shared between LU and HU was well above 50% (60 out of 104). Mean alpha species diversity of 11.18 was the major contributor to the total gamma diversity of 13.27 with beta species diversity contributing only 2.09.
Mean total canopy cover declined significantly from LU to HU (Table 1). Species number did not always follow canopy cover according to plant trait. Thus, whereas cover of perennials and erect plants declined significantly on HU, their respective species complement increased significantly on HU. Conversely, both cover and species number increased significantly on HU for prostrate plants. Cover of stoloniferous and annual plants increased markedly on HU. Although there was no increase in cover of forbs on HU, there was a significant increase in number of forb species with heavy grazing. Cover of the strongly correlated graminoid and tussock groups declined significantly on HU.
Cover of Cymbopogon pospischilii and Digitaria eriantha declined from a relatively high base by at least an order of magnitude on HU (Table 2). There were far more species that were indicated to increase significantly in cover on HU. Species with the most substantial and significant increases in cover on HU were Tragus koelerioides, Aristida congesta subsp. congesta and Pentzia globosa. Cover of other species that increased significantly on HU and that had a large increase in frequency of occurrence in the sample plots included Lotononis calycina, Blepharis integrifolia var. integrifolia and Nenax microphylla. There were no apparent local extinctions indicated on HU of any of the more frequent species. Cover of Gazania krebsiana subsp. serrulata, Lithospermum cinereum and Portulaca quadrifida was significantly greater on HU and were all apparently absent on LU.
The Indicator Analysis of the 50 most frequent species confirmed most of the seventeen significant species differences given in Table 2. Only A. karroo and Lepidium pinnatum were rejected whereas the differences for Helichrysum rosum var. rosum and Melica decumbens were indicated as significant at P < 0.05
Eight species in Table 2 appeared to be relatively tolerant of both treatments with each experiencing less than a 25% change in both cover and frequency from LU to HU. Of these, those with a frequency of more than 50% on both LU and HU were Asparagus burchellii, Galenia sp., Hibiscus pusillus, Lessertia cf. brachystachya and Trichodiadema pomeridianum. Those with lower frequency were Sutera halimifolia, Euphorbia inaequilatera and Gladiolus permeabilis subsp. edulis.
Frequency of A. karroo in sample plots did not track its decline in cover on HU (Table 1). The frequency of the low Gulliver form to the mature or emergent form of A. karroo was eight to six on LU but ten to zero on HU, respectively. These Gullivers were heavily grazed down on HU.
Soil texture was mainly sandy loam with some loamy sand on LU and mostly loamy sand on HU. The fraction of fine sand declined significantly on HU (Table 3). Cyanobacterial soil crusts were common on LU whereas the crusts on HU were physical and uncommon. Absorption of water drops on the biological soil crusts on LU was slow, ranging from 4 to 150 s. Absorption was virtually instantaneous off the crusts on LU and on and off crusts on HU. Cover of coarse fragments averaged about 1% on LU and 3% on HU.
Discussion and conclusions
The greater species richness found with grazing on HU was significant at sample plot scale but at broader study-site scale this difference tended to disappear as evidenced by the near-equal total species numbers for the site and the converging rarefaction curves. This implies a greater degree of sharing of species between HU and LU which is supported by the tighter clustering in the ordination of plots on HU. If variability is a reliable metric of response to disturbance (Fraterrigo and Rusak 2008), the reduced compositional variation coupled with the significantly higher mean species richness per plot with heavy grazing may be interpreted as reflecting greater habitat differentiation (Jackson and Sax 2010) not between sample plots but rather within the scale of the plot. It is also possible that presumed reduced competition between plants on HU may allow greater species richness. Competition is of minor importance in consumer-controlled ecosystems (Bond and Keeley 2005). The lack of adequate fuel load and hence absence of fire on HU may also allow fire-sensitive species to enter the system. Whether a ‘fire threshold’ has been surpassed (Briske et al. 2005) with sustained high grazing pressure on HU will depend on the reversibility of the system after removal of the level of grazing pressure.
The relatively large number of species shared between LU and HU contrasts with the less than 50% shared across a major grazing pressure contrast in Succulent Karoo (Rutherford and Powrie 2010). This together with the low contribution of beta diversity to gamma diversity in grassland may indicate a probability of lower levels of local species extinction in grassland according to grazing treatment.
The increaser-decreaser pattern of response to grazing is an entrenched paradigm for range management in South Africa (for example, Foran et al. 1978; Tainton et al. 1980; Heard et al. 1986; Hardy et al. 1999) but is in need of review in the light of accumulating contradictory evidence (O’Connor et al. 2003). Classification of increaser and decreaser species was found in a continent-wide review in Australia to be inconsistent and often required context (Vesk and Westoby 2001). However, in the current study, the response to grazing of a number of species in Table 2 generally confirms that typically published elsewhere although several increaser–decreaser species classification schemes were found to be difficult to interpret especially with only two points of reference along the grazing gradient.
One apparent anomalous response to grazing was the highly significant increase in cover and frequency of the shrub, N. microphylla, on HU given its high palatability or grazing index rating (Le Roux et al. 1994; du Toit 2000). However, it is also regarded as highly resistant to grazing (Le Roux et al. 1994) and might decline on LU due to greater competition from perennial grass species. N. microphylla has been recorded to respond positively to protection from grazing in less grassy areas (O’Connor and Roux 1995).
Although some of the significantly increasing species on HU had a predominantly drier karroid distribution (e.g. Amphiglossa triflora and Aptosimum procumbens), other increasers were typically from the more mesic northeast grasslands and savannas (e.g. G. krebsiana subsp. serrulata and Lotononis calycina). Therefore, despite the study site’s position in a more arid part of the Grassland Biome, less than 100 km from the main body of the Nama-Karoo Biome, heavy grazing did not promote only karroid elements. However, species such as P. globosa which is widespread in the Nama-Karoo and the more arid parts of the Grassland Biome, increased significantly and by a much greater magnitude on HU.
The heavy impact of goat grazing on A. karroo agrees with earlier observations in the Eastern Cape (Hester et al. 2006). A. karroo is particularly resilient to browsing by goats. Even basal ring-barking, while killing the canopy, results in vigorous coppicing from the base of the stem (Scogings and Macanda 2005). The high ratio of Gullivers to emergent/mature forms of this species found with heavy grazing in the current study confirms its resilience and possibly also reflects a degree of wood removal on the communal side.
The responses of plant traits to grazing conform to the expectations from a world synthesis (Díaz et al. 2007) at least as reflected by their canopy cover changes. Thus, grazing favoured annual over perennial plants, prostrate over erect plants, and stoloniferous over tussock architecture. It is interesting that in terms of species numbers none of these conclusions is supported.
It is instructive to note that although the communal Sterkspruit District has since the 1920 s been rated repeatedly as one of the most severely degraded districts in South Africa, it appears resilient with no decline in livestock numbers (Vetter 2009). Other communal rangeland systems have shown similar persistence despite predictions of imminent collapse (Mace 1991; Harrison and Shackleton 1999; Sullivan and Rohde 2002; Vetter 2009) and many regard communal tenure as a viable system (Scogings et al. 1999). This study confirms some other studies in the Grassland Biome that show that plant species richness need not decline under heavy communal grazing, and can even increase significantly at plot scale, the latter as shown by this study. The retention of a relatively large pool of rarer species on the communal ground may lend support to the proposition that minor species may increase resilience in ecosystem functioning under perturbations that favour them over the past dominants (Walker et al. 1999; Lyons et al. 2005).
References
Alkemade R, van Oorschot M, Miles L, Nellemann C, Bakkenes M, ten Brink B (2009) GLOBIO3: a framework to investigate options for reducing global terrestrial biodiversity loss. Ecosystems 12:374–390
Archaux F, Camaret S, Dupouey J-L, Ulrich E, Corcket E, Bourjot L, Brêthes A, Chevalier R, Dobremez J-F, Dumas Y, Dumé G, Forêt M, Forgeard F, Lebret Gallet M, Picard J-F, Richard F, Savoie J-M, Seytre L, Timbal J, Touffet J (2009) Can we reliably estimate species richness with large plots? An assessment through calibration training. Plant Ecol 203:303–315
Bai Y, Abouguendia Z, Redmann RE (2001) Relationship between plant species diversity and grassland condition. J Range Manag 54:177–183
Bennett J, Barrett H (2007) Rangeland as a common property resource: contrasting insights from communal areas of central Eastern Cape Province, South Africa. Hum Ecol 35:97–112
Bergstedt J, Westerberg L, Milberg P (2009) In the eye of the beholder: bias and stochastic variation in cover estimates. Plant Ecol 204:271–283
Biggs R, Simons H, Bakkenes M, Scholes RJ, Eickhout B, van Vuuren D, Alkemade R (2008) Scenarios of biodiversity loss in southern Africa in the 21st century. Global Environ Change 18:296–309
Bohensky E, Reyers B, Van Jaarsveld AS, Fabricius C (eds) (2004) Ecosystem services in the Gariep basin: a basin-scale component of the Southern African Millennium Ecosystem Assessment. SUN PReSS, Stellenbosch
Bond WJ, Keeley JE (2005) Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol Evol 20:387–394
Bond WJ, van Wilgen BW (1996) Fire and Plants. Chapman & Hall, London
Briske DD, Fuhlendorf SD, Smeins FE (2005) State-and-transition models, thresholds, and rangeland health: a synthesis of ecological concepts and perspectives. Rangeland Ecol Manag 58:1–10
Cingolani AM, Noy-Meir I, Díaz S (2005) Grazing effects on rangeland diversity: a synthesis of contemporary models. Ecol Appl 15:757–773
Cobban DA, Weaver AvB (1993) A preliminary investigation of the gully features in the Tsolwana Game Reserve, Ciskei, southern Africa. S Afr Geogr J 75:14–21
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Díaz S, Lavorel S, McIntyre S, Falczuk V, Casanoves F, Milchunas DG, Skarpe C, Rusch G, Sternberg M, Noy-Meir I, Landsberg J, Zhang W, Clark H, Campbell BD (2007) Plant trait responses to grazing–a global synthesis. Glob Change Biol 13:313–341
Dorrough JW, Ash JE, Bruce S, McIntyre S (2007) From plant neighbourhood to landscape scales: how grazing modifies native and exotic plant species richness in grassland. Plant Ecol 191:185–198
Du Toit PCV (2000) Estimating grazing index values for plants from arid regions. J Range Manag 53:529–536
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Fatunbi AO, Dube S (2008) Land degradation evaluation in a game reserve in Eastern Cape of South Africa: soil properties and vegetation cover. Sci Res Essay 3:111–119
Foran BD, Tainton NM, Booysen PdeV (1978) The development of a method for assessing veld. Proc Grassl Soc South Afr 13:27–33
Forbes RG, Trollope WSW (1991) Veld management in the communal areas of Ciskei. J Grassl Soc South Afr 8:147–152
Fraterrigo JM, Rusak JA (2008) Disturbance-driven changes in the variability of ecological patterns and processes. Ecol Lett 11:756–770
García LV (2004) Escaping the Bonferroni iron claw in ecological studies. Oikos 105:657–663
Gisladottir G, Stocking M (2005) Land degradation control and its global environmental benefits. Land Degrad Dev 16:99–112
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Hardy MB, Hurt CR, Bosch OJH (1999) Veld condition assessment. In: Tainton NM (ed) Veld management in South Africa. University of Natal Press, Pietermaritzburg, pp 194–207
Hargrove WW, Pickering J (1992) Pseudoreplication: a sine qua non for regional ecology. Landsc Ecol 6:251–258
Harrison YA, Shackleton CM (1999) Resilience of South African communal grazing lands after the removal of high grazing pressure. Land Degrad Dev 10:225–239
Heard CAH, Tainton NM, Clayton J, Hardy MB (1986) A comparison of five methods for assessing veld condition in the Natal midlands. J Grassl Soc South Afr 3:70–76
Hester AJ, Scogings PF, Trollope WSW (2006) Long-term impacts of goat browsing on bush-clump dynamics in a semi-arid subtropical savanna. Plant Ecol 18:277–290
Hickman KR, Hartnett DC, Cochran RC, Owensby CE (2004) Grazing management effects on plant species diversity in tallgrass prairie. J Range Manag 57:58–65
Hoare DB (2003) Biodiversity and performance of grassland ecosystems in communal and commercial farming systems in South Africa. In Anon Biodiversity and the ecosystem approach in agriculture, forestry and fisheries. FAO, Rome, pp 10–27
Hoffman MT, Todd S (2000) A national review of land degradation in South Africa: the influence of biophysical and socio-economic factors. J South Afr Stud 26:743–758
Hurlbert SH (2004) On misinterpretations of pseudoreplication and related matters: a reply to Oksanen. Oikos 104:591–597
Jackson ST, Sax DF (2010) Balancing biodiversity in a changing environment: extinction debt, immigration credit and species turnover. Trends Ecol Evol 25:153–160
Jost L (2007) Partitioning diversity into independent alpha and beta components. Ecology 88:2427–2439
Jost L (2009) Mismeasuring biological diversity: response to Hoffmann and Hoffmann (2008). Ecol Econ 68:925–928
Kercher SM, Frieswyk CB, Zedler JB (2003) Effects of sampling teams and estimation methods on the assessment of plant cover. J Veg Sci 14:899–906
Kiguli LN, Palmer AR, Avis AM (1999) A description of rangeland on commercial and communal land, Peddie district, South Africa. Afr J Range Forage Sci 16:89–95
Krebs CJ (1989) Ecological methodology. HarperCollins, New York
Lamb EG, Bayne E, Holloway G, Schieck J, Boutin S, Herbers J, Haughland DL (2009) Indices for monitoring biodiversity change: are some more effective than others? Ecol Indicat 9:432–444
Land Type Survey Staff (1991) A procedure for describing soil profiles. SIRI Report No GB/A/91/67. Soil and Irrigation Research Institute, Pretoria
le Roux PM, Kotzé CD, Nel GP, Glen HF (1994) Bossieveld: grazing plants of the Karoo and karoo-like areas. Department of Agriculture, Pretoria
Legendre P, Borcard D, Peres-Neto PR (2005) Analyzing beta diversity: partitioning the spatial variation of community compositional data. Ecol Monogr 75:435–450
Lyons KG, Brigham CA, Traut BH, Schwartz MW (2005) Rare species and ecosystem functioning. Conserv Biol 19:1019–1024
Mace R (1991) Overgrazing overstated. Nat 349:280–281
Martindale G (2007) Influence of livestock grazing on plant diversity of Highveld Sourveld grassland in KwaZulu-Natal. MSC thesis, School of Animal, Plant and Environmental Sciences, University of Witwatersrand, Johannesburg
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Moyo B, Dube S, Lesoli M, Masika PJ (2008) Communal area grazing strategies: institutions and traditional practices. Afr J Range Forage Sci 25:47–54
Mucina L, Rutherford MC (eds) (2006) The vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria
Neke KS, Du Plessis MA (2003) The threat of transformation: quantifying the vulnerability of grasslands in South Africa. Conserv Biol 18:466–477
O’Connor TG (2005) Influence of land use on plant community composition and diversity in Highland Sourveld grassland in the southern Drakensberg, South Africa. J Appl Ecol 42:975–988
O’Connor TG, Bredenkamp GJ (1997) Grassland. In: Cowling RM, Richardson DM, Pierce SM (eds) Vegetation of southern Africa. Cambridge University Press, Cambridge, pp 215–257
O’Connor TG, Kuyler P (2009) Impact of land use on the biodiversity integrity of the moist sub-biome of the grassland biome, South Africa. J Environ Manag 90:384–395
O’Connor TG, Roux PW (1995) Vegetation changes (1949–71) in a semi-arid, grassy dwarf shrubland in the Karoo, South Africa: influence of rainfall variability and grazing by sheep. J Appl Ecol 32:612–626
O’Connor TG, Morris CD, Marriott DJ (2003) Change in land use and botanical composition of KwaZulu-Natal’s grasslands over the past fifty years: Acocks’ sites revisited. S Afr J Bot 69:105–115
O’Connor TG, Kuyler P, Kirkman KP, Corcoran B (2010) Which grazing management practices are most appropriate for maintaining biodiversity in South African grassland? Afr J Range Forage Sci 27:67–76
Peden MI (2004) The impact of communal land-use on the biodiversity of a conserved grassland at Cathedral Peak, uKhahlamba-Drakensberg Park, South Africa. Masters in Environmental and Development Faculty of Science and Agriculture. University of KwaZulu-Natal, Pietermaritzburg
Rouget M, Reyers B, Jonas Z, Desmet P, Driver A, Maze K, Egoh B, Cowling RM, Mucina L, Rutherford MC (2004) South African national spatial biodiversity assessment: technical report vol 1: terrestrial component. South African National Biodiversity Institute, Pretoria. http://www.sanbi.org/biodiversity/NSBA%202004%20Vol%201%20Terrestrial%20Component.pdf. Accessed 12 Aug 2009
Roxburgh SH, Shea K, Wilson JB (2004) The intermediate disturbance hypothesis: patch dynamics and mechanisms of species coexistence. Ecology 85:359–371
Rutherford MC, Powrie LW (2010) Severely degraded rangeland: implications for plant diversity from a case study in Succulent Karoo, South Africa. J Arid Environ 74:692–701
Rutherford MC, Westfall RH (1994) Biomes of southern Africa: an objective categorization. Mem Bot Surv S Afr 63:1–94
Rutherford MC, Mucina L, Powrie LW (2006) Biomes and bioregions of southern Africa. In: Mucina L, Rutherford MC (eds) The vegetation of South Africa Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria, pp 30–51
Scholes RJ (2009) Syndromes of dryland degradation in southern Africa. Afr J Range Forage Sci 26:113–125
Scholes RJ, Biggs R (2005) A biodiversity intactness index. Nature 424:45–49
Scogings P, Macanda M (2005) Acacia karroo responses to early dormant season defoliation and debarking by goats in a semi-arid subtropical savanna. Plant Ecol 179:193–206
Scogings P, De Bruyn T, Vetter S (1999) Grazing into the future: policy making for South African communal rangelands. Dev South Afr 16:403–414
Shackleton CM (2000) Comparison of plant diversity in protected and communal lands in the Bushbuckridge lowveld savanna, South Africa. Biol Conserv 94:273–285
Sullivan S, Rohde R (2002) On non-equilibrium in arid and semi-arid grazing systems. J Biogeogr 29:1595–1618
Tainton NM, Edwards PJ, Mentis MT (1980) A revised method for assessing veld condition. Proc Grassl Soc South Afr 15:37–42
The Non-affiliated Soil Analysis Work Committee (1990) Handbook of standard soil testing methods for advisory purposes. Soil Society of South Africa, Pretoria
Vesk PA, Westoby M (2001) Predicting plant species’ responses to grazing. J Appl Ecol 38:897–909
Vetter S (2009) Drought, change and resilience in South Africa’s arid and semi-arid rangelands. S Afr J Sci 105:29–33
Vetter S, Goqwana WM, Bond WJ, Trollope WW (2006) Effects of land tenure, geology and topography on vegetation and soils of two grassland types in South Africa. Afr J Range Forage Sci 23:13–27
Walker B, Kinzig A, Langridge J (1999) Plant attribute diversity, resilience, and ecosystem function: The nature and significance of dominant and minor species. Ecosystems 2:95–113
Walters DJJ, Kotze DC, O’Connor TG (2006) Impact of land use on vegetation composition, diversity, and selected soil properties of wetlands in the southern Drakensberg mountains, South Africa. Wetl Ecol Manag 14:329–348
Wotshela L (2004) Territorial manipulation in apartheid South Africa: resettlement, tribal politics and the making of the northern Ciskei, 1975–1990. J South Afr Stud 30:317–337
Acknowledgements
We thank: The late Chief Nimrod N Hinana, head of the tribal authority for Amaqwati, for permission to work on the communal land; the Eastern Cape Parks Board for permission to work on the Tsolwana Nature Reserve; Johan van der Nest and Sizwe Mkhulise, former and current reserve managers respectively, for logistic assistance; Avumile Mbanjwa for help with field sampling; Jiholo Mthuthuzeli for historical information.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rutherford, M.C., Powrie, L.W. Can heavy grazing on communal land elevate plant species richness levels in the Grassland Biome of South Africa?. Plant Ecol 212, 1407–1418 (2011). https://doi.org/10.1007/s11258-011-9916-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-011-9916-0