Abstract
The flower heliotropism of Anemone rivularis (Ranunculaceae) was investigated on the Yulong Snow Mountain, near Lijiang in the northwest of the Yunnan province of China. We discovered that tepals in this species drive the peduncles to track the sun, and that this flower heliotropism was likely an adaptation for parental environmental effects on reproductive fitness. In brief, A. rivularis flowers retained sun-tracking behavior following removal of pistils and stamens, but lost heliotropic movement, if tepals were removed. Light is the major factor to affect floral heliotropism, the tepal-received light signal was in the blue frequency. Meanwhile, the peduncles were found to bend significantly on the top portion of both control flowers and those lacking pistils and stamens, but instead of keeping a vertical peduncle orientation in flowers with tepals removed. Furthermore, the floral temperature was steadier, and seed sizes and numbers were greater for control flowers than for flowers with tepals removed. Therefore, we conclude that the tepals trigger the flower heliotropism in A. rivularis and play an important role on not only increasing but also keeping optimal thermal condition of flower interior. We further conclude that flower heliotropism enhances the pollen viability and seed production, resulting in higher reproductive success for this alpine species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In alpine ecosystems, flower development and pollinator activity are limited by fluctuating air temperatures and other climatic variability (Körner 2003). In order to compensate for various unfavorable factors, floral traits of some alpine species affect reproductive functions via regulating the microenvironment within flowers. Flower heliotropism, a form of positive phototropism (Kevan 1972; Hart 1990), is considered to be a behavioral adaptation for efficient absorption of solar energy and adjustment of floral temperature (Kjellberg et al. 1982; Corbett et al. 1992; Kudo 1995; Totland 1996; Luzar and Gottsberger 2001; Patino et al. 2002; Galen 2006). Flower heliotropism has been demonstrated in several families, such as Ranunculaceae, Compositae, Rosaceae, Convolvulaceae, and Papaveraceae (Knutson 1981; Stanton and Galen 1989; Patino and Grace 2002).
Previous studies of flower heliotropism have primarily focused on some taxa in the arctic and subarctic regions, and in American or European alpine environments (Hocking and Sharplin 1965; Kevan 1972; Stanton and Galen 1989; Totland 1996; Wada 1998). These studies indicated that flower heliotropic movement has significant influence on floral and seed developments (Kevan 1972; Kjellberg et al. 1982; Young 1984; Wada 1998) and increases the pollination activity of insects on the flowers (Kevan 1975; Smith 1975; Knutson 1981; Molgaard 1989; Stanton and Galen 1989; Luzar and Gottsberger 2001). Furthermore, improved floral temperature may ultimately improve viability of offspring for reproductive success (Seymour 2001; Erickson and Markhart 2002; Orueta 2002; Tsukaya et al. 2002; Young et al. 2004). However, some work on flower heliotropism in the hotter tropical and sub-tropical environments indicated that exposure to solar radiation and the resulting elevated temperatures of the floral organs’ parts may have a negative effect on floral development (Patino et al. 2002). Thus, adaptations to prevent overheating were also critical for reproductive success (Lang and Begg 1979; Patino and Grace 2002).
Heliotropic movements are often mediated by changes in cell turgor pressure (Vogelmann 1984) and can occur both rapidly and reversibly (Koller 1980). Flower morphology often plays a role in directing solar energy toward gynoecia, and heliotropism aids this by orienting toward the sun to capture solar radiation with parabolic antenna petals (Kevan 1972; Knutson 1981; Stanton and Galen 1989). Conversely, convolvulaceous flowers in tropical environments are equipped to prevent overheating of the gynoecia, as their trumpet-shape corollas act like a parasol to shield radiation (Patino and Grace 2002). In addition to structural effects enabling temperature regulation in heliotropic flowers, physiological strategies can be employed. For instance, the increase in floral temperature by solar tracking could result in increased evaporative cooling at the expense of water loss, to maintain an appropriate floral microenvironment (Patino and Grace 2002; Galen 2006). Moreover, physiological analyses have been conducted on mechanisms of some heliotropic leaf movements (Koller 1986). In general, blue light reception systems in plants appear to be associated with rapid movements of plastids and/or whole organs (Britz 1979; Koller 1986). Stanton and Galen (1993) confirmed the blue light specificity of flower heliotropism in Runuculus adoneus A. Gray, for which the photosignal was received at the top of the peduncle, sequentially bending occurred in a lower region due to greater cell elongation on the shaded side of the peduncle (Sherry and Galen 1998).
The alpine areas of the Himalayas harbor the richest alpine flora in the world (Wu and Wu 1998) and comprise a major biodiversity hotspot (Myers et al. 2000), but no study of the mechanism and functional adaptation of an instance of flower heliotropism in this area has been documented until now. It is important to fill this gap in our knowledge of flower heliotropic movement with regard to its reproductive function and adaptive function. In this study, we investigated a species of Ranunculaceae, Anemone rivularis Buch.-Ham., endemic to the alpine Himalayas, which displays flower heliotropic behavior that is quite different from previous reports from other taxa. We documented its diurnal patterns of A. rivularis floral heliotropic movement, identified its physiological mechanisms, and determined its effect on floral temperature, parental functions, and reproductive adaptation. The aims of this study were to determine the cues behind its flower heliotropic behavior, to investigate key steps in the adaptive mechanisms of floral heliotropism, and to shed light on the ecological adaptation of this alpine plant in the Himalayas.
Materials and methods
Study species
Anemone rivularis is a perennial herb endemic to the Himalaya Mountains regions. The plants are in bloom from May to July, each with an average of 3–10 flowers (Fig. 1a). These hermaphroditic flowers bear 6–10 tepals (i.e., perianth organs that cannot be distinguished as sepals vs. petals), which are white and shiny on the adaxial surface and purplish on the abaxial surface (Fig. 1b–d). Each flower is on a separate peduncle that is subtended by several bracts and the inner whorls of gynoecia surrounded by several whorls of anthers (Fig. 1b–d). The flowers remain open for about 8 days, while anthers begin to dehisce from the outermost whorls inward on the third day. The ovaries are dark green, containing one ovule each, and give rise to achenes. The single-seeded fruits are hard and dry, and all unfertilized ovaries remain in the fruit until seed maturation.
Study site
During the summer of 2007 and 2008, the experiments of A. rivularis were carried out on Yulong Snow Mountain, near Lijiang, northwestern Yunnan of China. The study site was located in an open alpine meadow at an elevation of 3,600 m (29°21′16″N, 111°12′33″E), where A. rivularis was the most abundant blooming species from May to July (Fig. 1a). Other plants in this meadow included Berberis pruinosa Franch., Taraxacum acumeriopoduun (D. Don) DC, Potentilla lancinata Cardot, Gentiana rigescens Franch. ex Hemsl., and Rosa spinosissima L. In addition, several individuals of A. rivularis grow on an open slope at the Kunming Botanical Garden, China (25°08′42″N, 102°44′31″E, elevation 1,940 m).
Diurnal floral movement
We characterized A. rivularis flower heliotropism using three treatments (i.e., control flowers, flowers with tepals removed, and flowers with pistils and stamens removed) during clear and mostly calm days in 2007 and 2008. Measurements included the angular deviation of the flower from the sun, the flower deviation from horizontal, and the compass orientation of each flower. The deviation angle of flowers from the sun was measured with a clinometer following Stanton and Galen (1989) and Totland (1996). It consisted of a shallow funnel (radius of 50 mm) with a pointer at the center, and the length of this shadow was used to calculate angular deviation from the sun. An inclinometer was placed parallel to the plane of tepals to record angular deviation of the flower from horizontal east (0°). Finally, the orientation was measured using a compass, with magnetic north recorded as 0°.
Bending site and cell length of peduncles
In order to determine which portion of the peduncle is responsible for floral movement, the whole peduncle was artificially divided into three portions: the top, 1–3 cm below the flower; the middle, 4–6 cm lower; and the bottom, 7–10 cm lower. Fifty newly opening flowers were randomly selected to ascertain the peduncle curve apex as the flowers tilted toward the sun at 0800 on June 1, 2007.
A cell length analysis was conducted according to Sherry and Galen (1998), to determine whether the heliotropic response involves different growth at the cellular level (i.e., whether bending involved differential growth rates on the two sides of the peduncle in different treatments). We collected 10 stems each of control flowers (intact flowers), flowers with tepals removed, and flowers with pistils and stamens removed, and fixed the tissue in FAA. The tepals, pistils, and stamens were removed at their bases using scissors. Samples were dehydrated in an alcohol series, infiltrated for 5 days in molten paraffin, and embedded in paraffin (EG-1160, LEICA, Germany). Samples were then sectioned in 5 μm thick slices with a microtome blade (RM-2135, LEICA, Germany) and stained with safranin-fast green. The average epidermal cell lengths along the shaded side (outer side, OS) and sunlit side (inner side, IS) were measured by light microscopy (BX51, OLYMPUS, Japan). Under 200× magnifications, the lengths of 20 cells were measured in each of five microscope fields, and statistical analysis was performed on the ratio of the average shaded side cell length over the average sunlit-side cell length. Student’s t test was used to test differences in the ratio of relative shaded-side to sunlit-side cell length among different flower treatments.
Floral and ambient temperature
In order to assess whether solar-tracking movement influenced floral temperature, differently treated flowers, i.e., control flowers and flowers with tepals removed, were used to record floral temperature in the study site during 2007 and 2008. The temperatures of 10 flowers were measured at 1-h intervals at evenly spaced points within flowers for 24 h, using copper-constantan thermocouple thermometers (CENTER-309, TES, Taiwan), each with a 0.5-mm diameter microprobe inserted in the center of a flower. Ambient air temperatures were concurrently measured at flower level using an automatic recording thermometer sensor (TES-1361, TES, Taiwan). The significance of floral temperature differences between flowers with and without tepals was tested with Student’s t test for each treatment, as well as for the differences between the floral temperatures and the ambient air temperature.
Pollen viability and seed production
In order to determine the effect of temperature on pollen viability, the method was following Patino and Grace (2002). A total of 30 flowers were collected from the Kunming Botanical Garden in 2008. In the laboratory, anthers from these flowers were removed and placed, respectively, into five vials containing 1 ml of 2.5% sucrose solution. Five vials with pollen were placed in a constant temperature bath. The experimental temperature treatments in water baths were set at 1, 6, 18, 28, and 33°C, respectively, and we used 18°C ambient temperature as a control. After the vials in each temperature bath reached the treating temperature, we took out one vial every 60 min. The next day, each vial was placed in 0.5% MTT buffer for 1 h. Then we placed 1 μl of water-pollen solution on a microscope slide to estimate pollen viability (BX51, OLYMPUS, Japan), counting red-stained pollen grains as living and non-stained or white pollen grains as dead. Finally, the percentages of pollen viability at each temperature were calculated.
In order to assess the effect of maternal solar-tracking on pollen performance, the number of pollen grains germinating and pollen tubes reaching midway down the style were measured for control flowers (n = 10) and flowers with tepals removed (n = 10) following Galen and Stanton (2003). Hand-pollination was performed on all flowers with pollens form intact flowers. After 8 h and again after 24 h, 10 pistils were collected from each flower and placed in vials containing fixative (3:1, ethanol:acetic acid). Pistils were rinsed, cleared, stained with aniline blue, and visualized under a fluorescence microscope (Axioplan2, ZEISS, Germany). We counted the number of pollen grains germinating on the stigma after 8 h and the number reaching the midway of the pistils after 24 h. In order to determine whether sun-tracking affects pollen germination and pollen tube growth, Student’s t test was used to test for the effect of treatments on the number of pollen grains germinating or pollen tubes reaching the midpoint of pistils, respectively.
We performed bagging experiments on 25 intact flowers, and kept 25 intact flowers and 25 tepal-removed flowers unbagged in 2007 and 2008. Bagging was carried out prior to anthesis using clear polypropylene netting to prevent insect visitation, and the netting was removed after anthesis. The unbagged flowers were exposed throughout anthesis to naturally occurring pollinators. In addition, artificial supplemental pollination was conducted on 25 intact flowers and 25 tepal-removed flowers in 2008. Cross-pollinating flowers were hand-pollinated with fresh pollen from other intact flowers when stigmas were mature, and the anthers were removed before dehiscing. Before the seeds started to disperse, we determined the seed set in each flower under different treatments by counting aborted and mature achenes (seed set = mature seeds/[mature seeds + aborted seeds]). Concurrently, we measured seed length and width with a digital vernier caliper to calculate seed size. One-way ANOVA was used to compare data from the different treatments and the control.
The effects of light and temperature on flower heliotropism
We conducted the temperature and light treatments for flowers with peduncles, to investigate whether flower heliotropism in A. rivularis shows light sensitivity and to determine the responses of flowers to light and temperature. Flower buds of A. rivularis that had just started to open were cut from the plant at 1600 h at the Kunming Botanical Garden, along with 6 cm of stems, and placed in distilled water in darkness at 20°C as a pretreatment until the third day. Then they were exposed to various experimental conditions, and the peduncle angle from horizontal plane was measured at 2-h intervals for 2 days (straight up = 90°). All measurements were made on 4–5 flowers from each treatment. First, some pretreated flowers were separately transferred to temperature-controlled cabinets at 10/10, 30/30, or 10/30°C (DT: day temperature/NT: night temperature); besides, some transferred to light-controlled chambers for 24-h light, 24-h dark, 2-h light, 2-h dark, 6-h light, or 6-h dark conditions. Day and night periods were controlled by turning the lights on and off. Day light was provided by white fluorescent tubes (300 μmol m−2 s−1), and the relative humidity monitored with a hygrometer was about 50–60%. In addition, some pretreated flowers were treated with the blue and/or red light in special light boxes, which were made from blue- or red-transmitting acrylic filters with blue or red fluorescent light tubes. The data were compared via one-way ANOVA.
Results
Diurnal movement of flowers
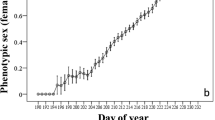
In general, the control flowers of A. rivularis opened at 0800 and closed at 1600, and most flowers faced the sun with peduncles orienting upright during the middle of the day (Fig. 1c). The accuracy of floral solar-tracking changed over the course of the day (Fig. 2). Between 0900 and 1500, most flowers oriented within 20° of directly facing the sun. The flower deviation from the sun significantly increased after 1600 (>30°), when tracking accuracy appeared to decline and peduncles started orienting downward. The compass direction of the flowers appeared to be oriented randomly at that time, as individual plants could most efficiently absorb sun radiation from different orientations.
Diurnal changes of the flower angle to horizontal east were variable from among different floral treatments. Control flowers and the flowers with pistils and stamens removed traversed an arc of ~60° over the course of a day, but the flowers with tepals removed traversed <20° during the daytime (Fig. 3). From 0900 to 1500, both control flowers and flowers with pistils and stamens removed tracked the sun closely, while flowers with tepals removed did not follow the sun’s movements but instead of keeping a vertical peduncle orientation (Fig. 1e). Additionally, field observations showed that tracking behavior appeared to stop completely when tepals fell off naturally (data not shown).
Bending site and cell elongation of peduncles
While the control flowers and the flowers with pistils and stamens removed closely tracked the sun between 0900 and 1500, bending could be observed along the whole length of the peduncle, although most significantly in the top portion of the peduncles (Fig. 1d). Conversely, all flowers with tepals removed showed no peduncle bending throughout the day.
The curving of peduncles corresponded to differences in cell length on the shaded side (outer side, OS) and sunlit side (inner side, IS) (Fig. 4). Average cell lengths on the IS and OS of the peduncles among the three treatments were found to be different (Table 1). That is, most peduncles of control flowers and flowers with pistils and stamens removed displayed significantly longer epidermal cells occurring on the OS than on the IS (t = 21.17, P < 0.01 for control flowers; t = 18.79, P < 0.01 for flowers with pistils and stamens removed). In contrast, the cell lengths of peduncles on the two sides of flowers with tepals removed were not significantly different (t = −1.35, P = 0.18).
Near-median longitudinal sections taken below the receptacle of control peduncles (a, b, c), peduncles for which pistils and stamens had been removed (d, e, f), and peduncles for which tepals had been removed (g, h, i). Part figures a, d, g show the vertical structure of peduncles for the three treatments (Bars = 0.1 mm) and b, c, e, f, h, i show the cell length of the outer side (OS) or the inner side (IS) of peduncles for the three treatments, respectively (Bars = 0.4 mm). The average cell lengths of outer side in b and f are longer than the average cell lengths of the inner side in c and e. There was no difference in cell lengths between outer side and inner side in h and i
Effects of sun-tracking on floral microclimate
In the study site, ambient air temperatures ranged from 6 to 28°C during observation periods. Generally, in 2008 control flowers and flowers with tepals removed exhibited different temperature divergence during mid-day and mid-night (1200: t = −7.95, P < 0.01 in 2007, t = −6.79, P < 0.01 in 2008; 0000: t = 3.81, P < 0.01 in 2007, t = 2.05, P < 0.05 in 2008). Unmanipulated flowers reached the maximum temperature during the sunniest period, with temperatures lower than the ambient temperature; conversely, the flowers with tepals removed were always hotter than the ambient temperature at that time (Fig. 5). During the night, the temperatures of control flowers were slightly higher than that of flowers with tepals removed, as the closed tepals of control flowers better maintained the microenvironment. Moreover, the temperatures within control flowers fluctuated less 15°C during the whole day, while the tepals removed flowers fluctuated over 20°C. Thus, the temperatures of control flowers were clearly steadier than those of the flowers with tepals removed.
Pollen viability and seed production
Temperature differences greatly influenced pollen-grain viability (Fig. 6). All pollen grains died after 5 h at 1, 6, 28, and 33°C. More than 80% of pollen grains died after 3 h at 1 and 33°C. Ambient temperature ca. 18°C did not have a large influence on pollen viability, indicating this is in the optimal floral temperature range for pollen growth in the field. Clearly, pollen-grain viability was very sensitive to temperature fluctuation.
After 8 h, pistils of intact recipient flowers had significantly germinating pollen grains than flowers with tepals removed (t = 17.58, P < 0.01 in 2007; t = 17.77, P < 0.01 in 2008), and more pollen tubes reached midway down the style after 24 h (t = 9.12, P < 0.01 in 2007; t = 14.98, P < 0.01 in 2008). The mean number of germinating pollen grains (after 8 h) for control flowers averaged 10.48 ± 1.76 SD in 2007 and 12.46 ± 1.46 SD in 2008, and the number of pollen tubes reaching midway after 24 h in control flowers averaged 5.1 ± 1.22 SD in 2007 and 6.96 ± 1.41 SD in 2008. For flowers with tepals removed, the number of germinating pollen grains on stigmas for 8 h was averaged 5.38 ± 1.05 SD in 2007 and 7.96 ± 1.21 SD in 2008, and the number of pollen tubes reaching the midway of styles for 24 h was averaged 3.02 ± 1.06 SD in 2007 and 3.26 ± 1.03 SD in 2008. The results show that naturally sun-tracking flowers were more favorable for pollen germination than flowers with tepals removed.
Visits by insects to Anemone rivualris flowers were infrequent, and the primary visiting insects were bees or flies (Fig. 1f). Bagging experiments showed that this species is self-compatible, but low in seed set (Fig. 7). The number of seeds per flower ranged from 30 to 100 under natural conditions. The seed set of flowers with tepals removed was significantly lower than that of control flowers (F = 54.127, P < 0.01 in 2007; F = 60.82, P < 0.01 in 2008). For flowers without tepals, seed set from supplemental pollination was slightly lower than that of natural pollination, while seed set of intact flowers with both supplemental and natural pollination was obviously higher (Fig. 7). There were proportionally more seeds per flower for control flowers (84.23 ± 7.58 SD in 2007; 71.5 ± 7.79 SD in 2008) than for those with tepals removed (59.28 ± 20.41 SD in 2007; 36.47 ± 17.55 SD in 2008). Under supplemental pollination in 2008, there were fewer seeds per flower for the flowers with tepals removed (29.72 ± 17.56 SD) than for intact flowers (77.86 ± 12.87 SD). Meanwhile the control flowers produced larger seeds than those with tepals removed (F = 0.16, P < 0.01 in 2007; F = 0.394, P < 0.01 in 2008). The mean seed size of control flowers was 23.13 ± 4.47 (SD) mm2 in 2007 and 25.7 ± 3.33 (SD) mm2 in 2008, and that of flowers without tepals was 17.32 ± 4.35 (SD) mm2 in 2007 and 21.38 ± 3.12 (SD) mm2 in 2008. Consequently, the heliotropic movement with tepals of A. rivularis could aid seed production.
The responses of flowers to light and temperature
Solar tracking movement of peduncles was differently influenced by various treatments. First, peduncles angles under natural condition were about the same as those of 10/10°C and 30/30°C (DT:/NT:) at day time, whereas they were significantly different at night (1200: F = 1.26, P = 0.09; 2000: F = 6.7, P < 0.01). The peduncles would bend severely (angle was about 50°–70°) and rapidly under 10°C NT, whereas the angular deviation under 30°C NT was greater than 75°. When using cycles of a 6-h exposure to 10°C in light followed by 6-h exposure to 30°C in darkness, we found the flower stalks failed to fully straighten in light and did not bend significantly in darkness. Thus, lower temperature appears to hasten bending of the flowers peduncles, while higher temperature seems to reduce movement in A. rivularis. Secondly, flower peduncles remained vertically oriented in 24-h light, but never fully attained vertical orientation in 24-h darkness (angle was about 60°–80°). Flower stalks which had been kept in darkness could attain vertical orientation after 2 h of light exposure. When the flowers were transferred to darkness, after 4 h they bent to an angle <75°. Moreover, flower peduncles of A. rivularis became vertically oriented only when exposed to light containing the blue part of the spectrum; the heliotropic speed and extension in control and blue-light treatments were similar. In contrast, flowers beneath red-light-transmitting boxes were significantly less heliotropic, with an angle <75°. Therefore, light may be the chief factor to affect floral heliotropism in A. rivularis, and the flowers are sensitive to blue light rather than red light. Furthermore, the physiological mechanisms of heliotropism in A. rivularis require more elaborate investigation.
Discussion
Tepals drive heliotropic movement
Solar-tracking by flowers of diverse plant species has involved various forms of repeated movement from different floral parts, including petals, peduncles, carpels, and stamens (Simons 1992). For instance, the gynoecia of flowers in Dryas integrifolia M. Vahl (Rosaceae) adjust in orientation toward the sun (Krannitz 1996). Another example is Papaver radicatum Rottb. (Papaveraceae); this Ranunculales member lost sun-tracking behavior when the petals were removed (Corbett et al. 1992). Also within the Ranunculaceae, the flower heliotropic movement of R. adoneus was directed by peduncles, as even with the flowers entirely removed its peduncles could still accurately track the sun (Sherry and Galen 1998). The function of petals as organs of thermal regulation has also been investigated experimentally in many flower heliotropic species (Kevan 1975; Kjellberg et al. 1982; Corbett et al. 1992). In these studies, the petals were found to concentrate light upon the gynoecia, suggesting there may be little benefit in solar-tracking in the absence of petals. For example, when flowers of Dryas octopetala L. had their petals removed, they ceased heliotropic behavior and produced fewer and lighter seeds than natural flowers (Kjellberg et al. 1982).
Anemone is large genus of around 150 species mainly distributed in the alpine areas of the North Temperate Zone, but flower heliotropism in Anemone has not been previously studied in detail. Our data indicate that the intact flowers of A. rivularis track the sun accurately during the daytime, and tepals of this species are necessary for its sun-tracking. Because heliotropic behavior disappeared completely when tepals were removed; moreover, only the peduncles of control flowers and flowers with pistils and stamens removed were found to curve and to show differential cell elongation. As such, heliotropism in A. rivularis is different than that of R. adoneus in North America (Sherry and Galen 1998). The bowl-shaped tepals drive the flowers of A. rivularis heliotropic movement similarly to the movements of D. octopetala and P. radicatum (Kjellberg et al. 1982; Corbett et al. 1992).
Furthermore, our study demonstrated a close relationship between diurnal heliotropic movements and temperature of flower interior in A. rivularis. Flowers with tepals could provide a relatively narrow range of temperatures, in comparison with flowers lacking tepals. That means tepals might protect flower from overheating damage during the daytime, and the closing movement of tepals would protect flower from chilling damage at night. It suggests that tepals of A. rivularis play an important role on not only triggering solar-tracking movement but also regulating the thermal condition in flowers. Finally, the shelter offered by tepals in A. rivularis provides thermal and reproductive benefits in the alpine Himalaya environment.
Heliotropic mechanism in Anemone rivularis
Our results showed that the heliotropic movements by A. rivularis ceased upon removal of the tepals, which suggests that they function in light perception. In addition, A. rivularis heliotropism is a blue-light-dependent phenomenon and is insensitive to red light, in accordance with heliotropic behavior in R. adoneus flowers (Stanton and Galen 1993). Their solar-tracking in blue-transmitting boxes was indistinguishable from that by control flowers outside. Thus, we assume that the photosignal is received by tepals, which drive the peduncles to bend due to differential cell elongation along the peduncle.
Meanwhile, the tepals of A. rivularis could shield flowers to maintain the gynoecia at a physiologically functional temperature. This was seen when control flowers maintained lower temperature around gynoecia than the ambient air temperature at the noon, while flowers with tepals removed did not (Fig. 5). The thermal control found on flowers appears to be due to light reflection of solar radiation and transpiration. The tepals of A. rivularis seem to be reflectors acting as small parabolic mirrors, as previous reports for parabolic flowers (Hocking and Sharplin 1965; Kevan 1975; Knutson 1981; Corbett et al. 1992). This would have the function of thermal regulation to protect the sexual organs from excess solar radiation on flowers. Galen (2006) documented that solar-tracking flowers of snow buttercups are warmer, take up more water, but lose water more rapidly through transpiration than non-tracking flowers. Therefore, transpiration may also represent one of the potent mechanisms for heat stress avoidance in A. rivularis flowers. As ambient temperatures rise to undesirable levels, there seems to be a balance between prevention of overheating and reduction of water loss by the tepals. Further experiments with additional treatment are needed to fully elucidate this mechanism.
Knowledge of adaptation of flower heliotropic behavior to changes in environmental conditions is still incomplete. Flower peduncles of A. rivualris pretreated in darkness straightened rapidly in response to light, but did not fully straighten if kept in darkness. Moreover, exposure to lower temperature change resulted in slower and ultimately incomplete peduncle straightening, while higher temperatures had the opposite effect. Consequently, we assume that heliotropic straightening and subsequent curvature of peduncles are independently controlled processes. Furthermore, flower heliotropism in this species is controlled predominantly by immediate stimuli, rather than by circadian rhythms. More precisely, light provides the immediate stimulus essential for solar-tracking behavior.
Heliotropism in different environments
Floral traits are subject to the evolution of various strategies aimed at minimizing damage (Stirton 1983; Dafni 1996). In different environments, the heliotropism displays a variety of mechanisms. For example, in alpine and arctic environments, heliotropism likely has a positive effect on flower and seed development in some species (such as Dryas integrifolia, Dryas octopetala, and Papaver radicatum). These species’ flowers are paraboloid antenna in shape, which increases floral temperature or/and attracts more insect visits (Kevan 1972; Kjellberg et al. 1982; Corbett et al. 1992; Krannitz 1996; Wada 1998); likewise, a Ranunculaceae species (e.g., Adonis ramose) in northern Japan, flower heliotropism could increase reproductive success by increasing pollination, fertilization success, and/or seed development (Kudo 1995). In some North American alpine species (e.g., R. adoneus), flower heliotropism simultaneously raises organ temperature and transpiration to improve floral microenvironment and increase reproductive success (Stanton and Galen 1989; Galen and Stanton 2003; Galen 2006). Conversely, plants with heliotropic flowers in the tropical or subtropical areas tend to adopt another strategy, developing adaptive mechanisms to avoid extreme temperatures and solar radiation (such as Helianthus annuus L., Ipomoea aquatica Forsskal, I. pes-caprae (L.) Sweet, and Merremia borneensis Merr.) (Lang and Begg 1979; Patino and Grace 2002; Patino et al. 2002).
Remarkably, in alpine Himalaya region, the natural tendency of flowers in A. rivularis to track the sun resulted in a relatively lower temperature of the flower interior at the noon, when this species is subjected to an air temperature near the maximum for the day time. The tepals of A. rivularis seem to adjust floral temperature to optimal levels, because a relative stable floral microenvironment is necessary for the development of sexual organs. It is reasonable to assume that diurnal heliotropism represents a mechanism for avoidance of heat stress, as well as for minimizing convective heat loss. Thus, the flowers of A. rivularis are physiologically and structurally equipped to balance the floral microenvironment, with the purpose of improving reproductive success.
Heliotropism enhancing reproductive fitness
Flower heliotropism has been shown to enhance floral growth and reproductive success in many taxa (Kevan 1975; Smith 1975; Kjellberg et al. 1982; Young 1984; Molgaard 1989; Stanton and Galen 1989; Kudo 1995; Wada 1998; Luzar and Gottsberger 2001; Galen and Stanton 2003). In general, activity of pollinating insects is temperature dependent and the visiting frequency is higher in heliotropic flowers than in non-heliotropic ones (Kevan 1975; Stanton and Galen 1989; Kudo 1995; Krannitz 1996; Luzar and Gottsberger 2001; Orueta 2002). However, in our study, in comparison with seed production between supplemental and natural pollination of flowers with tepals removed (Fig. 7), suggesting that the maternal decreases might not be the result of pollinator limitation. Meanwhile, the seed set and pollen germination of flowers with tepals removed were evidently lower, compared with these of the intact flowers. Thus, the tepal-driving heliotropic movement appears to be adaptive for enhancing reproductive success.
Modification of floral temperature within a relatively narrow range is essential for reproductive functions including pollen development in the anther, pollen tube growth, viability of fertilized ovules, and ultimately seed set (Seymour 2001; Erickson and Markhart 2002; Tsukaya et al. 2002; Young et al. 2004; Galen 2006). The present study demonstrated that flower heliotropism in A. rivularis enhanced pollen viability and pollen quality via a paternal environmental effect, and the tepals closed overnight to provide a suitable microenvironment for pollen fertility. Meanwhile, the heliotropic behavior also showed a positive maternal function (via increased pollen germination and pollen-tube growth), increasing seed production rather than providing a reward for insect visitation. It is concluded that the flower heliotropism of A. rivularis is adaptive to enhance its reproductive success through contributions of paternal and maternal fitness in the alpine environment of the Himalayas.
References
Britz SJ (1979) Chloroplast and nuclear migration. In: Haupt W, Feinleib ME (eds) Physiology of plant movements. Springer, Berlin
Corbett AL, Krannitz PG, Aarssen LW (1992) The influence of petals on reproductive success in the arctic poppy (Papaver radicatum). Can J Bot 70:200–204
Dafni A (1996) Autumnal and winter pollination adaptations under Mediterranean conditions. Bocconea 5:171–181
Erickson AN, Markhart AH (2002) Flower developmental stage and organ sensitivity of bell pepper (Capsicum annum L.) to elevated temperature. Plant Cell Environ 25:123–130
Galen C (2006) Solar furnaces or swamp coolers: costs and benefits of water use by solar-tracking flowers of the alpine snow buttercup, Ranunculus adoneus. Oecologia 148:195–201
Galen C, Stanton ML (2003) Sunny-side up: flower heliotropism as a source of parental environmental effects on pollen quality and performance in the snow buttercup, Ranunculus adoneus (Ranunculaceae). Am J Bot 90:724–729
Hart JW (1990) Plant tropisms and other growth movements. Unwin Hyman, London
Hocking B, Sharplin CD (1965) Flower basking by arctic insects. Nature 206:215
Kevan PG (1972) Heliotropism in some arctic flowers. Can Field Nat 86:41–44
Kevan PG (1975) Sun-tracking solar furnaces in high arctic flowers: significance for pollination and insects. Science 189:723–726
Kjellberg B, Karlsson S, Kerstensson I (1982) Effects of heliotropic movements of flowers of Dryas octopetala L. on gynoecium temperature and seed development. Oecologia 54:10–13
Knutson RM (1981) Flowers that make heat while the sun shines. Nat Hist 90:75–80
Koller K (1980) Solar-tracking (phototropism) in leaves of Lavatera cretica and Malva parviflora. Year book, Carnegie Institution of Washington, Washington, pp 72–75
Koller D (1986) The control of leaf orientation by light. Photochem Photobiol 44:819–826
Körner C (2003) Alpine plant life: functional plant ecology of high mountain ecosystems, 2nd edn. Springer, New York
Krannitz PG (1996) Reproductive ecology of Dryas integrifolia in the high Arctic semi-desert. Can J Bot 74:1451–1460
Kudo G (1995) Ecological significance of flower heliotropism in the spring ephemeral Adonis ramosa (Ranunculaceae). Oikos 72:14–20
Lang ARG, Begg JE (1979) Movements of Helianthus annuus leaves and heads. J Appl Ecol 16:299–305
Luzar N, Gottsberger G (2001) Flower heliotropism and floral heating of five alpine plant species and the effect on flower visiting in Ranunculus montanus in the Austrian alps. Arct Antarct Alp Res 33:93–99
Molgaard P (1989) Temperature relations of yellow and white flowered Papaver radicatum in north Greenland. Arct Antarct Alp Res 21:83–90
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Orueta D (2002) Thermal relationships between Calendula arvensis inflorescences and Usia aurata bombyliid flies. Ecology 83:3073–3085
Patino S, Grace J (2002) The cooling of convolvulaceous flowers in a tropical environment. Plant Cell Environ 25:41–51
Patino S, Jeffree C, Grace J (2002) The ecological role of orientation in tropical convolvulaceous flowers. Oecologia 130:373–379
Seymour RS (2001) Biophysics and physiology of temperature regulation in thermogenic flowers. Biosci Rep 21:223–236
Sherry RA, Galen C (1998) The mechanism of floral heliotropism in the snow buttercup, Ranunculus adoneus. Plant Cell Environ 21:983–993
Simons P (1992) The action plant: movement and nervous behavior in plants. Blackwell, Cambridge, MA
Smith AP (1975) Insect pollination and helioptropism in Oritrophium limnophilum (Compositae) of the Andean Paramo. Biotropica 7:284–286
Stanton ML, Galen C (1989) Consequences of flower heliotropism for reproduction in an alpine buttercup (Ranunculus adoneus). Oecologia 78:477–485
Stanton ML, Galen C (1993) Blue light controls solar-tracking by flowers of an alpine plant. Plant Cell Environ 16:983–989
Stirton CH (1983) Nocturnal petal movements in the Asteraceae. Bothalia 14:1003–1006
Totland O (1996) Flower heliotropism in an Alpine population of Ranunculus acris (Ranunculaceae): effects on flower temperature, insect visitation, and seed production. Am J Bot 83:452–458
Tsukaya H, Fujikawa K, Wu SG (2002) Thermal insulation and accumulation of heat in the downy inflorescences of Saussurea medusa (Asteraceae) at high elevation in Yunnan, China. J Plant Res 115:263–268
Vogelmann TC (1984) Site of light perception and motor cells in a sun-tracking lupine (Lupinus succulentus). Physiol Plant 62:335–340
Wada N (1998) Sun-tracking flower movement and seed production of mountain avens, Dryas octopetala L. in the high arctic, Ny-Alesund, Svalbard. Proc NIPR Symp Polar Biol 11:128–136
Wu ZY, Wu SG (1998) A proposal for a new floristic kingdom (realm). In: Zhang AL, Wu SG (eds) Floristic characteristics and diversity of East Asian plants. China Higher Education Press/Springer, Beijing/New York, pp 3–42
Young TP (1984) Solar irradiation increases floral development rates in Afro-alpine Lobelia telekii. Biotropica 16:243–245
Young LW, Wilen RW, Bonham-Smith PC (2004) High temperature stress of Brassica napus during flowering reduces micro-and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J Exp Bot 55:485–495
Acknowledgments
We are grateful to Wei Zhou and Chun-Fang Li for their kind assistance with experiments and statistics. We also thank Dr. Zachary M. Larson-Rabin from the University of Wisconsin-Madison, USA, for revising an earlier version of this manuscript. This study was supported by National Basic Research Program of China (973 Program, No. 2007CB411603).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S., Ai, HL., Yu, WB. et al. Flower heliotropism of Anemone rivularis (Ranunculaceae) in the Himalayas: effects on floral temperature and reproductive fitness. Plant Ecol 209, 301–312 (2010). https://doi.org/10.1007/s11258-010-9739-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-010-9739-4