Abstract
Lygodium microphyllum (Cav.) R. Br., a climbing fern native to the Pantropics of the Old World, is aggressively colonizing natural ecosystems in the Florida Peninsula. Here, we examined soil factors that might affect the fern’s invasiveness, specifically addressing the hypothesis that a release from natural belowground enemies contributes to its vigorous growth in Florida. We also investigated phenotypic differences of sporophytes raised from spores collected in Florida and the fern’s native range in Australia, hypothesizing that the Florida population would possess traits resulting in faster growth and superior competitive ability than the two Australian populations. We tested our hypotheses in parallel greenhouse experiments—one in Australia using soil from the fern’s native habitat, and another in Florida, USA, with soil from a recently colonized ecosystem. Fern growth rate and its principal determinants were expressed relative to the optimal growth with a common sand culture in each experiment and compared among treatments in which soil was altered through either sterilization or nutrient amendment, or both. Contrary to the expectation, the optimal growth rates in the sand culture were higher for Australian populations than the Florida population, while the comparatively poor growth of all populations in unaltered soil was stimulated by nutrient amendment and sterilization. The overall effect of sterilization, however, was muted under high-nutrient conditions, suggesting that the effect of soil sterilization may be due to greater nutrient availability in sterilized soils. The only exception was the local population from the site where the soil was collected for the experiment in Australia, which grew significantly faster in sterilized than in non-sterilized soil, and also more rapidly in response to soil insecticide application. Our results indicate that the invasiveness of L. microphyllum in Florida is not a simple phenotypic difference in inherent growth rate as predicted by the evolution of increased competitive ability hypothesis, but it may be mediated in part by release from soil-borne enemies that vary in their effectiveness even within the native geographical range of the fern.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
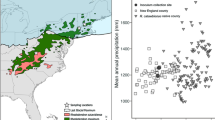
Among many non-native species in North America, Lygodium microphyllum (Cav.) R. Br., Old World climbing fern, is one of the most aggressive invasive species, threatening the greater Everglades ecosystem in South Florida (Pemberton and Ferriter 1998; Volin et al. 2004). It is native to wet tropical and subtropical regions of the Old World. Since it was first observed as a naturalized plant on the southeast coast of Florida in 1966 (Beckner 1968), it has rapidly expanded its geographical distribution (Fig. 1). A recently developed landscape model for L. microphyllum shows that, in the absence of aggressive control measures, this species’ infestations could exceed the current combined coverage of the top five most invasive species in Florida by 2014 (Volin et al. 2004).
Native, introduced, and predicted distribution of Lygodium microphyllum (adapted from Pemberton 1998; Goolsby 2004; Volin et al. 2004). Spore–source populations of plants used in the two studies include the introduced Florida population, the native Iron Range population (reputed source of those originally introduced to Florida), and the native Stradbroke Island population. The study conducted in Florida, USA, only used plants originating from the Florida source population, whereas the study conducted in southeast Queensland, Australia, included plants from all three source populations
Lygodium microphyllum possesses several life history characteristics that may enhance its competitive ability in Florida, including plastic reproductive strategies, prolific and continuous spore production, and the ability to grow rapidly across gradients in light (Lott et al. 2003; Volin et al. 2004) and hydrology (Gandiaga et al. 2009). Furthermore, L. microphyllum appears to optimize its morphological and physiological characteristics to maximize photosynthetic area and minimize carbon costs in tissue construction (M. Lott and J. Volin, unpublished data). Little is known, however, about the ecology of L. microphyllum within its native range, and hence the key question remains as to why, despite its life history characteristics, it is a comparatively benign constituent of native range communities.
There are many potential reasons why plants become invasive when introduced to a new environment, and one of the most frequently cited is release from natural enemies. The enemy release hypothesis (e.g., Elton 1958) states that within a species’ introduced range there are fewer natural herbivores or pathogens. Consequently, the species responds with greater growth, abundance, and habitat distribution, and eventually becomes invasive. Release from enemies is considered a contributing factor in the spread of many invasive species (Elton 1958; Keane and Crawley 2002; Mitchell and Power 2003; Callaway et al. 2004; DeWalt et al. 2004; Blumenthal 2006) and may play a role in the ability of L. microphyllum to readily colonize ecosystems outside its natural environment (Volin et al. 2004). Surveys of natural enemies within its native range have revealed moderate impacts by leaf herbivores with only a few potentially effective biocontrol candidates (Goolsby et al. 2003; Goolsby et al. 2006), but a parallel search has not been conducted belowground. Indeed, while studies examining invasive plant release from aboveground natural enemies are numerous (Liu and Stiling 2006), those concerning belowground natural enemies are much less common (e.g., Reinhart et al. 2003; Callaway et al. 2004).
In the absence of natural enemies, unprecedented selective regimes are hypothesized to result in the rapid evolution of reduced defensive traits and faster growth rates, culminating in an increased competitive ability (Evolution of Increased Competitive Ability, or EICA hypothesis: Blossey and Nötzold 1995; Rogers and Siemann 2004). Several common garden studies comparing genotypes of a given invasive species from its introduced versus native ranges have observed significant differences between them (Blossey and Nötzold 1995; Siemann and Rogers 2001; Wolfe 2002). For example, Siemann and Rogers (2001) found lower tannin contents and faster growth rates in introduced populations of Chinese tallow in Texas compared to native populations. Changes in biomass allocation patterns may also contribute to higher growth rates between populations. A rigorous test of this hypothesis, however, would require inclusion of multiple populations from broad geographical locations because the invading population may represent only a small subset of genetic variation within the native range (Muller-Scharer et al. 2004).
In this study, we address the hypotheses that the behavior of L. microphyllum in Florida ecosystems can be explained in part by a release from natural belowground enemies. We also tested the prediction by the EICA hypothesis that the Florida phenotype would possess traits that result in faster growth and superior competitive ability compared to the phenotypes sampled in Australia where the species is native, when they are grown under the same standardized conditions. In order to test these hypotheses, we conducted two parallel greenhouse experiments: one in Florida with soil from a habitat invaded by L. microphyllum, and the other in Australia with soil from the fern’s native habitat. In each case, we compared L. microphyllum growth and related attributes across treatments in a complete factorial design including soil sterilization to eliminate the belowground natural enemies, and nutrient amendment to examine the possible interaction with soil nutrient availability. In both studies, we used sand culture with optimal supply of nutrients as the reference to facilitate comparisons between continents. In our Florida study, we used plants grown from spores collected at 26°N latitude in Florida, whereas in Australia we used plants grown from spores collected in three populations: the Florida population, one from the Iron Range located at 12°S latitude in northeast Queensland, Australia, and one from Stradbroke Island, located at 27°S latitude in southeast Queensland (Fig. 1). The Iron Range is the reputed original source of the Florida population (Goolsby et al. 2006). Finally, in Australia, we also incorporated three additional treatments: soil application of fungicide, insecticide, and a combination of the fungicide and insecticide.
The intent of our experimental design was to disentangle two major effects of soil sterilization: elimination of beneficial and harmful soil biota (Trevors 1996), and alteration of nutrient form and/or availability (e.g., Schmidt et al. 1997). To our knowledge, these potentially confounded effects have not been examined simultaneously in the invasive plant literature. We predicted that sterilization and nutrient amendment of Australian soil would positively and additively affect fern growth and associated traits due to likely presence of deleterious soil organisms. In contrast, we predicted an absence of sterilization effect at high nutrient availability in Florida soil, in which nutrient limitation rather than biotic antagonists is likely to constrain the fern growth. These outcomes, in combination with positive fern responses to pesticide application in Australian soil, would support the role of release of belowground natural enemies in L. microphyllum invasion in Florida.
Materials and methods
Plant material, treatments, and growth measurements for the Florida study
For the Florida study, fertile leaves of L. microphyllum were collected from the Big Cypress Seminole Indian Reservation in Hendry County, Florida in June 2004. Spore germination and gametophyte growth methods were completed as detailed in Lott et al. (2003). In November 2004, the sporophytes were individually transferred to propagation flats containing commercial siliceous sand, which contained less than detectable amounts of nutrients. After transplanting, the young sporelings were watered with modified half-strength Hoagland’s nutrient solution and grown under transparent domes, which retained a higher than ambient humidity.
In March 2005, 38 plants were transplanted into 3.8 l pots and moved to a 50% shade enclosure. Thirty-two plants (eight per treatment) were evenly allocated, after sorting by size class, to two soil treatments and two nutrient treatments: untreated soil, sterilized soil, nutrient-amended soil, and nutrient-amended/sterilized soil. The sandy spodosol was collected in the field from the rooting zone of a dense L. microphyllum population in the Big Cypress Seminole Indian Reservation. In addition, six plants were grown in sand with amended nutrients. For the nutrient treatment, each pot received 25 g of a slow-release fertilizer (Dynamite Plant Food™ 18:6:8 NPK plus micronutrients) at the beginning of the study. For the soil sterilization treatment, the soil importation and soil sterilization protocol of the United State Department of Agriculture for steam heat was used. Pots were watered to maintain the soil moisture at field capacity throughout the study.
At the beginning of the study, eight plants were randomly selected, destructively harvested and separated into roots, rhizomes, rachises, and pinnae. Leaf area was measured using a LI-3100 leaf area meter (LiCor Biosciences, Lincoln, NE, USA). The total rachis length (sum of the length of all rachises) of each plant was measured and an allometric relationship between total plant mass and total rachis length was determined (r 2 = 0.84, P < 0.001). Soil was thoroughly washed from roots and all plant components were dried to a constant mass at 70°C before weighing. The total rachis length was also measured at the beginning of the study for all experimental plants, from which the initial mass of each plant was estimated with the allometric relationship.
At the end of the study (62 days after treatments began), all the treatment plants were destructively harvested and their components separated, dried, and weighed, thus allowing for calculation of allocational determinants of growth including root mass ratio (RMR), rhizome mass ratio (RhiMR), rachis mass ratio (RacMR), and pinnae mass ratio (LMR). Before drying, the leaf area of all pinnae, or a subsample thereof, was measured to allow for determination of specific leaf area (SLA), a morphological determinant of growth. Relative growth rate (RGR, mg g−1 day−1) was calculated for individual plants during the 50-day interval, where RGR = [ln(final dry mass) − ln(initial dry mass)]/days (Evans 1972).
Plant material, treatments, and growth measurements for the Australia study
The parallel experiment in Queensland, Australia, used soil collected from the rooting zone of a dense L. microphyllum population located in southeastern Queensland (27°S). The onset of the Australia experiment was offset by 6 months from Florida; because of the opposing latitudes of the Florida (26°N) and Australian (27°S) study locations, they took place under similar seasonal condition over the same experimental duration. In January 2005, L. microphyllum spores were collected from North Stradbroke Island (27°S), located just off the coast of Brisbane. Spores from two additional populations were also used, including the Florida population and another from the Iron Range (12°S) on the Cape York Peninsula, Australia. The Iron Range is the reputed L. microphyllum source location of those originally introduced to Florida (Goolsby et al. 2006). Iron Range spores were provided by the Australian Commonwealth Scientific and Research Organisation (CSIRO) Biocontrol Laboratory in Brisbane, Australia.
As in the Florida study, sporelings were individually transplanted into propagation flats containing commercial siliceous sand. At the initiation of the study, the plants were transplanted to 3.8 l pots and transferred to a 50% shade enclosure. Sixty plants per spore–source population (180 plants in total) were evenly distributed, after sorting by size class, across the following treatments: untreated soil, sterilized soil, nutrient-amended soil, nutrient-amended and sterilized soil, and sand. Therefore, 12 plants were assigned to each soil/nutrient/spore–source combination. At the beginning of the study, 15 plants per spore–source population were harvested to determine allometric relationships for estimating initial mass of the experimental plants.
In addition to the above treatment combinations of sterilization × nutrient amendment, a second experiment was conducted in Australia to test the effects of pesticide applications to eliminate soil-born insects and microbes. At the study’s outset, 12 additional plants per treatment for each spore–source population were randomly assigned to one of the following treatments: soil application of fungicide, insecticide, and insecticide/fungicide. Therefore, 36 additional plants per spore source were grown at the same time and in the same shade house. The pesticide treatment plants were grown in otherwise unaltered soil. All plants were grown for an average of 64 days, and then destructively harvested in the same way as in Florida.
Several preliminary trials were conducted to determine the impacts of various types and concentrations of fungicides and insecticides. We assessed these trials visually, choosing to use a combination of two fungicides (Banrot® and LESCO® 4 Flowable Mancozeb) for the fungicide treatment and one systemic insecticide (Confidor®). Two weeks after the initiation of the study, a single soil drench was applied in designated treatment pots. Two hundred milliliter per pot was applied at a concentration of 0.8 g l−1, 1.5 g l−1, and 0.7 ml l−1 of Banrot®, LESCO® and Confidor®, respectively. Active ingredients for Banrot® are Etridiozole (15%) and Thiophanate-methyl (25%), and these chemicals provide systemic and contact activity against Cylindrocladium, Fusarium, Phythium, Phytophthora, Rhizoctonia, and Thielaviopsis. The active ingredient for LESCO® is Mancozeb (37%), which is a broad spectrum fungicide, and Confidor® is a systemic insecticide containing the active ingredient Imidacloprid, 1-[(6-chloro-3-pyridinyl)methyl]-N-nitro-2-imidazo-lidinimine (75%).
Measurement of leaf photosynthesis
Photosynthesis was measured at the final harvest of each study, using a LI-6400 portable photosynthesis system (Li-Cor Biosciences), on one or two fully expanded leaves from four to seven randomly chosen sporelings per population per treatment. Because of time constraints, photosynthesis was not measured on plants in the pesticide treatments. Measurements were conducted from 0830 to 1130 hours on clear days, and photon flux density (PFD) in the cuvette was maintained within ±10% of in situ PFD at the time of measurements using a red/blue LED light source (cuvette PFD range was 799–1001 μmol m−2 s−1, which was sufficient to saturate photosynthesis). Partial pressure of CO2 in the cuvette reference chamber was maintained at 40 Pa using the LI-6400 CO2 injector system.
Soil and leaf nutrient analyses
Nutrient concentrations of sterilized and non-sterilized soils were analyzed at the Analytical Research Laboratory at the University of Florida Institute of Food and Agriculture for the Florida experiment, and at the Graham Kerven Analytical Services at the School of Land and Food Sciences of the University of Queensland, for the Australia experiment, using identical extraction and analytical methods (Rayment and Higginson 1992). Leaf nitrogen concentration was determined for five to eight plants per treatment combination taken at the final harvest in each study with a C/N analyzer (Costech Analytical Model ECS4010).
Statistical analyses
In order to compare population-level responses to soil treatments across the Florida and Australia studies, it was necessary to standardize for differences in non-treatment conditions using the nutrient-amended siliceous sand as a common reference (hereafter referred to as “sand culture”). For each experiment, response ratios were calculated using variable means observed for that same population in sand culture as the denominator (e.g., response ratio for RGR = RGRtreatment/RGRsand). For the pesticide experiment in Australia, the unamended, non-sterilized soil was used as the control soil in the calculation of response ratios (e.g., response ratio for RGR = RGRtreatment/RGRcontrol).
Analysis of variance was used to assess the statistical significance of differences in plant traits among treatments, combinations of soil, and spore source, and their interactions, based on a completely randomized factorial design. Combinations of soil and spore source were as follows: Florida, Iron Range, and Stradbroke Island populations grown in Australian soil, and Florida population grown in Florida soil. Regression analysis was used to test for the influence of plant mass on RGR and its determinants (Poorter and Lambers 1986; Kruger et al. 1998; Volin et al. 2002). From this analysis, we determined that all parameters, except SLA and photosynthesis, were significantly influenced by plant mass. Therefore, RGR and its allocational determinants were normalized for plant mass (using analysis of covariance), whereas SLA and photosynthesis were not. In order to identify the intrinsic causes of variation in RGR across treatments, relationships between RGR and its determinants were analyzed using linear regression. Owing to the manner in which growth interval averages for LMR and SLA were calculated (using population averages for initial harvest values), these analyses were conducted with population/soil × treatment means as experimental units, using the general linear models procedure (PROC GLM) in SAS (SAS Institute Inc., Cary, NC, USA). Regressions and treatment effects were deemed significant when P < 0.05.
Results
Growth responses to soil sterilization and nutrient amendment
Under the conditions of high resource availability in sand culture, marginally significant variation in RGR was observed among the four spore–source populations (P = 0.09; Fig. 2). Australia populations, and Iron Range sporelings in particular, tended to grow more rapidly than the Florida population grown on either continent.
Relative growth rate (RGR) of the three spore–source populations growing in nutrient-amended sand culture, where FF is the Florida spore–source population growing in Florida, and AF, IR, and SI are the Florida, Iron Range, and Stradbroke Island source populations, respectively, growing in Australia. Data are means (±SE), based on n = 6–12 plants per treatment/source
Across all four population/soil combinations, RGR response ratios were lowest in the untreated soil (no sterilization and no nutrient amendment), averaging 0.50–0.79 (Fig. 3a). In soils without nutrient amendment, sterilization increased RGR response ratios (P = 0.01 across population/soil combinations), which averaged 0.73–0.92. In contrast, sterilization typically did not stimulate RGR in nutrient-amended soils (Fig. 3a). A notable exception to this was the response of Stradbroke Island ferns, which grew faster (P < 0.01) in sterilized than non-sterilized soils regardless of nutrient amendment. Across sterilization treatments, nutrient amendment generally increased growth rates (P = 0.01; Fig. 3a). Consequently, growth in high-nutrient soils essentially matched or exceeded that in sand culture.
Response ratios of a relative growth rate (RGR), b leaf mass ratio (LMR), c photosynthesis, and d leaf nitrogen (N) for the combination of spore–source and soil treatments where FF is the Florida spore–source individuals grown in Florida soil, and AF, IR, and SI are the Florida, Iron Range, and Stradbroke Island spore–sources, respectively, grown in Australia soil. Plants in both studies were grown in soils which were either untreated (□), sterilized ( ), nutrient-amended (
), nutrient-amended ( ), or nutrient-amended and sterilized (
), or nutrient-amended and sterilized ( ). Data are means ± SE
). Data are means ± SE
Responses of growth determinants to soil sterilization and nutrient amendment
In general, treatment responses of leaf mass ratio (LMR) resembled those of RGR (Fig. 3b). LMR response ratios were lowest in the untreated soils and, for the Florida population grown in Florida, increased as a result of sterilization (P = 0.01). LMR also increased markedly in high-nutrient soils (P < 0.0001), where response ratios did not differ significantly from unity. As with RGR, LMR did not respond to sterilization in the high-nutrient soils (P = 0.02, sterilization by nutrient interaction). Unlike RGR, there was no significant interaction between population/soil and nutrient amendment with respect to LMR response ratio. Overall, treatment responses of relative biomass distribution belowground, which included both roots and rhizomes, were mirror opposites to those of LMR, whereas neither rachis biomass ratio nor SLA responded significantly to treatments (data not shown). Relative to that in unaltered soil, photosynthesis was enhanced by both sterilization (P = 0.06) and nutrient amendment (P = 0.02), although, save for the Florida population in Florida soil, treatment response ratios did not differ significantly within population/soil combinations (Fig. 3c).
Soil and leaf nutrient analyses
Qualitatively, the effects of sterilization on soil nutrient status were consistent in both Florida and Australia; levels of ammonium (NH4), extractable phosphorus, and potassium increased while those of nitrate (NO3) decreased slightly (Table 1). Across treatments, response ratios of leaf nitrogen concentration (Nleaf) showed a similar pattern as those for RGR, where Nleaf in untreated soil was significantly lower than that in sterilized soil (Fig. 3d). Furthermore, the response to sterilization disappeared in nutrient-amended soils. Interestingly, there was a significant population × sterilization interaction on Nleaf (P = 0.05). Relative to other spore–source populations, the Stradbroke Island population had a very low Nleaf response ratio in untreated soil (Fig. 3d), but sterilization markedly increased the Nleaf response ratio to a level similar to other population/soil combinations.
Growth and associated responses to pesticides
RGR response ratios in the fungicide treatment did not differ significantly from unity for any population (Fig. 4). The same was observed for RGR responses to insecticide and combined pesticide applications, except for those of the Stradbroke Island ferns, which were significantly higher than unity in both treatments. Response ratios for LMR, RacMR, belowground mass ratio, and SLA in the pesticide treatments did not differ significantly from unity (data not shown).
Relationships between growth and its determinants
Among the measured growth determinants, LMR, calculated as an average of first and second harvest values, explained the most variation in RGR (r 2 = 0.75, P < 0.0001) across population/soils and treatments (Fig. 5). RGR was also positively but weakly related to photosynthesis (r 2 = 0.36, P = 0.005), whereas SLA was not a significant explanatory variable (r 2 = 0.03, P = 0.44). The addition of photosynthesis and/or SLA in a multiple regression with LMR did not significantly increase the amount of RGR variation explained (data not shown). The relationship between RGR and LMR did not differ among treatments except in the case of the Stradbroke Island ferns (Fig. 5). In that population/soil, the slope of the relationship was 84% higher (P = 0.01) for sterilization and insecticide treatments (pooled) versus treatments without sterilization or insecticide (i.e., unaltered, non-sterilized but nutrient-amended, and non-sterilized with fungicide).
Relationship between relative growth rate (RGR) and the growth interval average (i.e., average of values from initial and final harvest) for leaf mass ratio (LMR) across all population/soil combinations and treatments. Data are population/soil × treatment means. (Overall regression equation: RGR = 213*LMR − 76, R 2 = 0.75, P < 0.0001, n = 29.) Symbols denote treatment means for Stradbroke Island ferns in sterilized or insecticide-drenched soil (△), treatment means for Stradbroke Island ferns without sterilization or insecticide (i.e., unaltered, non-sterilized but nutrient-amended, and non-sterilized with fungicide, ○), and all other population/soil × treatment means (●). Regressions based on each of the two Stradbroke Island data groups differ in slope (365 vs. 198, P = 0.01)
Discussion
Our results provide a partial support for the hypothesis that release from natural soil-borne enemies may contribute to L. microphyllum’s invasiveness in Florida. In particular, the relatively large benefit of soil sterilization to the growth of the sporophytes from the spores from Stradbroke Island, across nutrient levels (Fig. 3), pointed to a possible elimination of growth-inhibiting soil biota. This inference was reinforced by positive growth responses of that same population to insecticide application. Interestingly, the three Australian populations appeared to differ in their susceptibility to potentially deleterious elements in the particular soil used in the study: the Australian soil, which was collected from a site only 40 km from the Stradbroke Island population, may contain insect pests that can more effectively attack local genotypes than those from distant regions, such as the Iron Range (2,400 km away).
The plausibility of geographical variation in host-pest matching within the species native range has similarly been found for the interaction of L. microphyllum with aboveground natural enemies; Goolsby et al. (2003) found an eriophyid mite, Floracarus perrepae, that was the most widely distributed of the herbivores and qualitatively appeared to have a significant negative impact on L. microphyllum in its native range. Based on DNA sequences, Goolsby et al. (2006) found that the L. microphyllum population in Florida is most closely related to native populations sampled in northern Queensland, Australia (the Iron Range population in our study) and Papua New Guinea. Furthermore, they found that the mite collected from the Iron Range caused the most detrimental impact on northern Australian/Florida fern populations. Similarly, the mite from southern Queensland (Stradbroke Island population in our study) caused more damage to the fern originating from southern Queensland locations. In this study, we do not suggest a role for the mite, which has no belowground stage in its life cycle. Instead, belowground natural enemies may also be more effective in attacking local host populations than distant ones.
These considerations point to the importance of testing the “release from natural enemies” hypothesis not only in the introduced ranges (Maron and Vilá 2001; Keane and Crawley 2002), but also within the native range (DeWalt et al. 2004; Hierro et al. 2006). Yet, to date, these studies have generated mixed results. For example, Reinhart et al. (2003) and Callaway et al. (2004) found that invasion of their study species was facilitated by a release from native-range pathogens in newly colonized soils. On the other hand, Beckstead and Parker (2003) found that soil pathogens were equally virulent in both the introduced and native range of the dune grass, Ammophila arenaria.
Aside from that of Stradbroke Island ferns, growth did not respond significantly to sterilization in high-nutrient soil, implying that the growth-enhancing effect of sterilization in most cases could be explained by increased nutrient availability (Schmidt et al. 1997). In the absence of sterilization or nutrient amendment, comparatively low-nutrient availability (Table 1) clearly constrained fern growth in the Australian soil. Growth responses to nutrient amendment were mediated by increases in LMR and, to a lesser extent, photosynthesis, both of which are typically sensitive to variation in plant and soil nutrient status (Poorter et al. 1995; Aerts and Chapin 2000). For reasons unknown, however, responses of the two growth determinants to added nutrients did not culminate in commensurate growth stimulation in the Florida soil.
Overall, LMR was the most influential determinant of RGR variation in this study, which is uncommon but reasonable given the general lack of pronounced and/or significant treatment differences in SLA and photosynthesis (Kruger and Volin 2006). Based, then, on the assumption that the proportion of plant mass in leaves governed most of the observed variation in fern carbon gain, we contend that treatment differences in the RGR versus LMR relationship are consistent with our inference regarding detrimental influences of soil biota on Stradbroke Island ferns. Namely, through putative elimination of, or reduction in, populations of detrimental soil insects, sterilization, or insecticide application resulted in a higher RGR at a given LMR. This implied that the hypothetical insects inhibited growth by consuming or otherwise mediating a loss of fixed carbon from plant root systems. An alternative explanation, that the contrast in RGR versus LMR trends resulted from associated treatment differences in photosynthesis or SLA, was not supported by our findings (e.g., Fig. 3c).
Our results did not indicate an increased competitive ability as predicted by the EICA Hypothesis (Blossey and NÖtzold 1995), which may explain patterns observed in some invasive species (Siemann and Rogers 2001; Blair and Wolfe 2004; Meyer et al. 2005), but not in others (Willis et al. 1999; Willis et al. 2000; Buschmann et al. 2005; Franks et al. 2008). A key tenet of EICA is that release from natural enemies leads to selection for genotypes with low defense and inherently fast growth rates (Siemann and Rogers 2001). In our study, Florida populations grew, if anything, more slowly than Australian populations in nutrient-amended sand culture when they were grown side-by-side in Australia (AF versus IR or SI in Fig. 2).
A release from natural enemies belowground would aid in explaining L. microphyllum’s invasiveness in its introduced range, but further study is needed to more clearly elucidate the potential agents involved. Also unclear is the importance of potential variations within the fern’s native and introduced ranges with respect to myriad biotic and abiotic environmental factors that cannot be fully incorporated in nursery studies with potted plants. The next logical step is to examine L. microphyllum growth and ecophysiology in field common gardens, in native versus introduced habitats, ideally at multiple geographical localities.
References
Aerts R, Chapin FS III (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Beckner J (1968) Lygodium microphyllum, another fern escaped in Florida. Am Fern J 58:93
Beckstead J, Parker IM (2003) Invasiveness of Ammophila arenaria: release from soil-borne pathogens? Ecology 84:2823–2831
Blair AC, Wolfe LM (2004) The evolution of an invasive plant: an experimental study with Silene latifolia. Ecology 85:3035–3042
Blossey B, Nötzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants—a hypothesis. J Ecol 83:887–889
Blumenthal DM (2006) Interactions between resource availability and enemy release in plant invasion. Ecol Lett 9:887–895
Buschmann H, Edwards PJ, Dietz H (2005) Variation in growth pattern and response to slug gamage among native and invasive provenances of four perennial Brassicaceae species. J Ecol 93:322–334
Callaway RM, Thelen GC, Rodriguez A, Holben W (2004) Soil biota and exotic plant invasion. Nature 427:731–733
DeWalt SJ, Denslow JS, Hamrick JL (2004) Biomass allocation, growth, and photosynthesis of genotypes from native and introduced ranges of the tropical shrub Clidemia hirta. Oecologia 138:521–531
Elton CS (1958) The ecology of invasions of animals and plants. Meuthen, London
Evans CG (1972) The quantitative analysis of plant growth. University of California Press, Berkeley
Franks SJ, Pratt PD, Dray FA, Simms EL (2008) No evolution of increased competitive ability or decreased allocation to defense in Melaleuca quinquenerevia since release from natural enemies. Biol Invasions 10:455–466
Gandiaga S, Volin JC, Kruger EL, Kitajima K (2009) Effects of hydrology on the growth and physiology of an invasive exotic, Lygodium microphyllum (Old World climbing fern). Weed Res 49:283–290
Goolsby JA (2004) Potential distribution of the invasive Old World climbing fern, Lygodium microphyllum in North and South America. Nat Area J 24:351–353
Goolsby JA, Wright AD, Pemberton RW (2003) Exploratory surveys in Australia and Asia for natural enemies of Old World Climbing fern, Lygodium microphyllum: Lygodiaceae. Biol Control 28:33–46
Goolsby JA, DeBarro PJ, Makinson JR, Pemberton RW, Hartley DM, Frohlich DR (2006) Matching the origin of an invasive weed for selecation of a herbivore haplotype for a biological control programme. Mol Ecol 15:287–297
Hierro JL, Villarreal D, Eren O, Graham JM, Callaway RM (2006) Disturbance facilitates invasion: the effects are stronger abroad than at home. Am Nat 168:144–156
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kruger EL, Volin JC (2006) Reexamining the empirical relation between plant growth and leaf photosynthesis. Funct Plant Biol 33:421–429
Kruger EL, Volin JC, Lindroth RL (1998) Influences of atmospheric CO2 enrichment on the responses of sugar maple and trembling aspen to defoliation. New Phytol 140:85–94
Liu H, Stiling P (2006) Testing the enemy release hypothesis: a review and meta-analysis. Biol Invasions 8:1535–1545
Lott MS, Volin JC, Pemberton RW, Austin DF (2003) The reproductive biology of Lygodium microphyllum and L. japonicum (Schizaeaceae) and its implications for invasive potential. Am J Bot 90:1144–1152
Maron JL, Vilá M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
Meyer G, Clare R, Weber E (2005) An experimental test of the evolution of increased competitive ability hypothesis in goldenrod, Solidago gigantea. Oecologia 144:299–307
Mitchell CE, Power AG (2003) Release of invasive plants from fungal and viral pathogens. Nature 421:625–627
Muller-Scharer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19:417–422
Pemberton RW (1998) The potential of biological control to manage Old World climbing fern (Lygodium microphyllum), an invasive weed in Florida. Am Fern J 88:176–182
Pemberton RW, Ferriter A (1998) Old World climbing fern (Lygodium microphyllum), a dangerous invasive weed in Florida. Am Fern J 88:165–175
Poorter H, Lambers H (1986) Growth and competitive ability of a highly plastic and a marginally plastic genotype of Plantago major in a fluctuating environment. Physiol Plant 67:217–222
Poorter H, van Devijver CADM, Boot RGA, Lambers H (1995) Growth and carbon economy of a fast-growing and a slow-growing grass species as dependent on nitrate supply. Plant Soil 171:217–227
Rayment GE, Higginson FR (1992) The Australian handbook of soil and water chemical methods. Inkata Press, Melbourne
Reinhart KO, Packer A, van der Putten WH, Clay K (2003) Plant-soil biota interactions and spatial distribution of black cherry in its native and invasive ranges. Ecol Lett 6:1046–1050
Rogers WE, Siemann E (2004) Invasive ecotypes tolerate herbivory more effectively than native ecotypes of the Chinese tallow tree Sapium sebiferum. J Appl Ecol 41:561–570
Schmidt IK, Michelsen A, Jonasson S (1997) Effects of labile soil carbon on nutrient partitioning between an arctic graminoid and microbes. Oecologia 112:557–565
Siemann E, Rogers WE (2001) Genetic differences in growth of an invasive tree species. Ecol Lett 4:514–518
Trevors JT (1996) Sterilization and inhibition of microbial activity in soil. J Microbiol Methods 26:53–59
Volin JC, Kruger EL, Lindroth RL (2002) Responses of deciduous broadleaf trees to defoliation in a CO2 enriched atmosphere. Tree Physiol 22:435–448
Volin JC, Lott MS, Muss JD, Owen D (2004) Predicting rapid invasion of the Florida Everglades by Old World Climbing Fern (Lygodium microphyllum). Divers Distrib 10:439–446
Willis AJ, Thomas MB, Lawton JH (1999) Is the increased vigour of invasive weeds explained by a trade-off between growth and herbivore resistance? Oecologia 120:632–640
Willis AJ, Memmott J, Forrester RI (2000) Is there evidence for the post-invasion evolution of increased size among invasive plant species? Ecol Lett 3:275–283
Wolfe LM (2002) Why alien invaders succeed: support for the escape-from-enemy hypothesis. Am Nat 160:705–711
Acknowledgments
The research was funded by competitive grants from the Florida Department of Environmental Protection and the South Florida Water Management District. A special note of acknowledgment goes to Dr. Susanne Schmidt, her post docs and students as well as her colleagues, Drs. Liz Aitken and Gimme Walter at the University of Queensland, who provided space and stimulating discussions during the Australian portion of the study. Special thanks go to Matthew Purcell, Tony Wright, and colleagues at the Commonwealth Scientific and Industrial Research Organization’s Australian Biological Control Laboratory: without their substantial assistance in the field, access to their growth facilities, and introduction to a proper Australian pie, the Australia study could not have been accomplished. Finally, we would like to thank many students, especially Andrew Ridley, on both sides of the World who helped with the harvests. The experiments comply with the current laws of both countries where they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Volin, J.C., Kruger, E.L., Volin, V.C. et al. Does release from natural belowground enemies help explain the invasiveness of Lygodium microphyllum? A cross-continental comparison. Plant Ecol 208, 223–234 (2010). https://doi.org/10.1007/s11258-009-9700-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-009-9700-6