Abstract
Vascular epiphytes are a conspicuous and highly diverse group in tropical wet forests; yet, we understand little about their mineral nutrition across sites. In this study, we examined the mineral nutrition of three dominant vascular epiphyte groups: ferns, orchids, and bromeliads, and their host trees from samples collected along a 2600 m elevational gradient in the tropical wet forests of Costa Rica. We predicted that the mineral nutrition of ferns, orchids, and bromeliads would differ because of their putative differences in nutrient acquisition mechanisms and nutrient sources—atmospherically dependent, foliar feeding bromeliads would have lower nitrogen (N) and phosphorous (P) concentrations and more depleted δ15N values than those in canopy soil-rooted ferns because canopy soil is higher in available N, and more enriched in δ15N than the atmospheric sources of precipitation and throughfall. We also predicted that epiphyte foliar chemistry would mirror that of host trees because of the likely contribution of host trees to the nutrient cycle of epiphytes via foliar leaching and litter contributions to canopy soil. In the same vein, we predicted that epiphyte and host tree foliar chemistry would vary with elevation reflecting ecosystem-level nutrients—soil N availability increases and P availability decreases with increasing elevation. Our results confirmed that canopy soil-rooted epiphytes had higher N concentrations than atmospheric epiphytes; however, our predictions were not confirmed with respect to P which did not vary among groups indicating fixed P availability within sites. In addition, foliar δ15N values did not match our prediction in that canopy soil-rooted as well as atmospheric epiphytes had variable signatures. Discriminant function analysis (DFA) on foliar measurements determined that ferns, orchids, and bromeliads are statistically distinct in mineral nutrition. We also found that P concentrations of ferns and orchids, but not bromeliads, were significantly correlated with those of host trees indicating a possible link in their mineral nutrition’s via canopy soil. Interestingly, we did not find any patterns of epiphyte foliar chemistry with elevation. These data indicate that the mineral nutrition of the studied epiphyte groups are distinct and highly variable within sites and the diverse uptake mechanisms of these epiphyte groups enhance resource partitioning which may be a mechanism for species richness maintenance in tropical forest canopies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants growing as epiphytes in the canopy habitat are spatially and physically removed from terrestrial resource pools. Such independence poses opportunities and constraints on plant nutrient acquisition, which are quite different from plants rooted directly in the forest floor soil matrix. The lack of forest floor contact combined with the diversity of nutrient and water sources in the canopy habitat (i.e., precipitation, throughfall, cloud water) has played a role in shaping the diversity of functional morphologies and nutrient and water acquisition mechanisms in epiphytes. Such diversity includes functional morphologies with elaborate nutrient-uptake mechanisms such as tank bromeliads that have foliar uptake and pendant orchids with velamen-coated aerial root uptake (Madison 1977; Martin 1994; Pittendrigh 1948). These variable uptake mechanisms may enhance the capacity of epiphytes to partition resources in the canopy and thus contribute to maintaining high species richness in the canopy habitat. Understanding the dynamic nature of the canopy habitat and the epiphyte response is critical to understanding how this ecosystem functions.
Within the canopy matrix, the most nutrient rich source is the canopy soil which has greater plant available N than forest floor soils but equivalent available P (Cardelús et al. 2009). However, canopy soil is a sparse resource with estimates of accumulation approximately 2 t ha−1 for montane and lowland wet forests (Nadkarni 1984; Cardelús et al., unpublished data). Other potential nutrient sources for epiphytes are from within the canopy such as host tree foliar leaching (Hietz et al. 2002) and throughfall, and extra-canopy or atmospheric sources of N through wet and dry deposition (Clark et al. 1998a, b; Liu et al. 2002), and N derived from fixation of atmospheric N2 (Bentley and Carpenter 1984; Bermudes and Benzing 1991; Cleveland et al. 1999; Forman 1975; Freiberg 1998; Stewart et al. 1995).
The nutrient pools of the canopy are reflected in epiphyte mineral nutrition. Among epiphyte groups, research has shown that bromeliads have lower N concentrations and more depleted δ15N values than other epiphyte taxa putatively because of their dependence on throughfall and atmospheric sources of N which are lower in N and more depleted in δ15N than canopy soil (Hietz et al. 1999). Position within the crown has also been shown to affect epiphyte nutrient concentrations and δ15N values, with epiphytes in the outer-crown having lower N concentrations and more depleted δ15N values than inner-crown epiphytes likely due to the lower concentrations of N and more depleted δ15N values of precipitation arriving to epiphytes in the outer-crown (Hietz et al. 2002). Source δ15N signatures of bulk precipitation, throughfall and canopy soil, however, have been shown to overlap in a lowland forest in Costa Rica making source signal interpretation within the canopy difficult (Wania et al. 2002).
Across studies of foliar nutrient concentrations of epiphytes and forest floor-rooted plants within the same forests, the former consistently have lower nutrient concentrations indicating the low nutrient sources found in the canopy (Hietz et al. 2002; Hofstede et al. 1993; Watkins et al. 2007) and more depleted δ15N values confirming different N sources (Hietz et al. 1999, 2002). Given canopy nutrient poverty, it is not surprising that epiphytes present a diversity of nutrient acquisition mechanisms: from uptake of nutrients across absorptive foliar surfaces in epiphytic bromeliads (Martin 1994; Pittendrigh 1948) and velamen-coated roots exposed to the atmosphere in epiphytic orchids (Madison 1977). While nutrient poverty could be the underlying pressure that has shaped the radiation of epiphytic plants, the diversity of nutrient sources has also likely played an important role in the evolution of this group.
The role of the host tree in canopy nutrient cycling remains unclear. On the one hand, recent work has shown a disconnect between epiphytes and host tree N sources (Hietz et al. 2002; Stewart et al. 1995), in part due to the supposition that canopy soil is largely made up of decomposed epiphyte matter and not host tree leaves. This is evidenced by the depleted δ15N signatures of both canopy soil and epiphyte foliar tissue relative to forest floor soil and the host tree foliar tissue (Hietz et al. 2002; Stewart et al. 1995). Furthermore, an in situ study of leaf litter inputs to canopy soil in Monteverde, Costa Rica, showed that host tree leaves were blown out of the crown before decomposition occurred (Nadkarni and Matelson 1991). On the other hand, however, other recent work indicates that the host tree does in fact influence canopy soil nutrient cycling (Cardelús et al. 2009) as well as the fundamental ecosystem process of litter decomposition (Cardelús, in press). In addition, in an experimental fertilization study on the forest floor in Hawaii, researchers found a significant response via growth and richness of epiphytes in P fertilized plots likely from host tree foliar leaching, but no response was seen with N fertilized plots (Benner et al. 2007; Benner and Vitousek 2007). Thus, the influence of the host tree may be more important for P than N dynamics.
While diversity of epiphytes was examined in an earlier article (Cardelús et al. 2006), here, we examine how the mineral nutrition of dominant epiphyte groups vary from each other, from their host tree and among elevations. In this study, we measured the leaf construction and mineral nutrition of ferns, orchids, bromeliads, and their forest floor-rooted host trees, at six elevations along a continuous wet forest (>3000 mm precipitation) elevational gradient. Elevational gradients offer opportunities to examine how mineral nutrition varies with changes in macroclimate (5.2–6.5°C decrease in mean annual temperature per 1000 m elevation), potential nutrient sources (cloud forest begins at 900 m) and known available nutrients (N availability increases and P availability decreases with increasing elevation) (Marrs et al. 1988; Titiz 2005).
We measured specific leaf weight (SLW, g m−2) because it is a leaf trait that co-varies with multiple life history traits including leaf lifespan, photosynthetic capacity, and leaf N and P concentrations (Reich et al. 1991, 1997; Wright et al. 2004, 2005b). Because of the differences among ferns, orchids, bromeliads, and their host trees in nutrient acquisition mechanisms, we hypothesized that nutrient source and water availability would be reflected in their leaf functional morphology (as expressed by SLW) and mineral nutrition: epiphytes with access to canopy soil will have lower SLW, greater concentrations of N and P and exhibit less water stress (more depleted δ13C signatures) than epiphytes with no canopy soil use. Host trees, in turn, would exhibit lower SLW, and greater N and P concentrations than all the epiphyte groups and little to no water stress reflecting their greater nutrient and water source pools. We also hypothesized that epiphyte foliar chemistry would mirror that of host trees because of the likely contribution of host trees to the nutrient cycle of epiphytes via foliar leaching and litter. Further, we hypothesized that nutrient status of epiphytes and host trees would reflect known forest floor soil nutrient status.
Materials and methods
Study site
We worked along the Volcán Barva – La Selva transect (the Barva transect), a 3000 m elevational gradient located in northeastern Costa Rica. It is composed of four tropical wet forest types: upper montane, montane, premontane, and lowland forest, and is the last contiguous elevational gradient of central America (Hartshorn and Peralta 1988; Norman 1985). We chose to work along this gradient because it provides a large range of variation in terrestrial nutrient availability (Edwards 1982; Grieve et al. 1990; Marrs et al. 1988; Titiz 2005), as well as a large range of epiphyte functional types and high species richness (Cardelús et al. 2006).
Study design
The plants sampled for this study were part of a larger survey of the diversity and distribution of vascular epiphytes in the inner-crown of canopy trees at six elevations along the Barva transect in Costa Rica (30, 500, 1000, 1600, 2000, 2600 m). In 2001, 5–10 trees were chosen randomly from a subselection of 20 intact, healthy canopy trees at each study site (tree identity in Cardelús et al. 2006), except at the 30 m site, which was sampled separately in 2004. Vascular epiphytes were sampled in situ from two transects established within the inner-crown of each study tree. Canopies were accessed using single-rope climbing techniques (Perry 1978). Ten canopy trees were sampled at the 30, 1000, 1600, and 2000 m sites; nine trees were sampled at 500 m, and five trees were sampled at the 2600 m site, where epiphyte species richness stopped increasing after the second tree was sampled. Transects were 4.0 × 0.5 m extending from the trunk. Each transect was divided into quadrats of 0.5 m × 0.5 m, and all epiphytes within each quadrat were collected. Sun leaves of the host trees were sampled using a crossbow. All specimens were pressed and processed at the base camp using 70% ethanol for initial preservation (Methods in Supplement). Duplicates were deposited at the Museo Nacional and Instituto Nacional de Biodiversidad.
No species from our three epiphyte taxa (Cardelús et al. 2006) nor tree taxa (Lieberman et al. 1996) spanned the entire elevational gradient. We selected a subsample of 195 individual epiphytes, 97 species from 15 genera, as well as 43 host trees for element and isotope analyses; we did not include any genera with fewer than three individuals (Table 1). The number of individuals per genera analyzed was representative of their abundance along the elevational gradient. The most abundant and species rich ferns in our samples were members of the genera Elaphoglossum, which spanned five elevations, and Hymenophyllum, which spanned all the six elevations. The most abundant and species rich orchid genera were Pleurothalis, Stelis, Maxillaria, and Scaphyglottis with no genus spanning the entire elevational range. Bromeliad species were all members of the subfamily Tillandsioideae, from three genera: Guzmania, Tillandsia, and Werauhia. Werauhia was the most abundant genus and spanned five elevations. Although there may be variations in mineral nutrition among species and genera, all the epiphytes studied were collected from the same microsite within the canopy, the inner-crown, which does not vary in microclimate (Cardelús and Chazdon 2005) or canopy soil nutrient status (Cardelús et al. 2009) among lower branches within the same tree.
We chose ferns, orchids, and bromeliads as our focal taxa for two reasons. First, they account for 52% of the vascular epiphyte species richness along the Barva Transect (Cardelús et al. 2006) which is similar to their contribution to neotropical vascular epiphyte floras in general (Kromer et al. 2005; Küper et al. 2004; Nieder et al. 1998, 2001). Second, they have differing nutrient-uptake mechanisms: bromeliads have absorptive trichomes on their foliar surfaces and access nutrients from their foliar surfaces from the atmospheric sources and/or their tanks which collect precipitation and throughfall; orchids, have velamen-coated roots that are found embedded in canopy soil, suspended above or below branches, or grasping the branch, and can thus take up nutrients from the canopy soil directly, throughfall, and atmospheric sources; and ferns have roots embedded in canopy soil and take up nutrients from the soil matrix.
Nutrient analyses
Prior to grinding, SLW (g m−2) was measured on dried samples either by taking the average mass of 5 hole punches with a known area in the laminar region or, for dissected leaves, taking the length, width, and mass of multiple sections of the lamina. All the samples were then ground using a Wiley Mill (Thomas Scientific, Swedesboro, NJ) and passed through a #40 screen. Total C and N, and natural abundance δ15N and δ13C were determined on a Costech Analytical Elemental Analyzer (Valencia, California) coupled to a Delta Plus Isotope Ratio mass spectrometer (Brenen, Germany) in the Department of Soil and Water Science at the University of Florida. Total P was measured using an ash digestion (Jones and Case 1996) followed by colorimetric determination of ortho-phosphate on an Astoria Pacific colorimetric autoanalyzer (Clackamas, Oregon). We tested the potential effects of ethanol drying on foliar nutrient concentrations and isotopes and found that ethanol treatment did not significantly alter response variables (Supplement Table 1).
Statistical analyses
In order to address the hypothesis that mineral nutrition was different among taxa, we examined epiphyte nutrient data within focal taxa, at the family level for orchids and bromeliads, and at the group level for host trees and ferns, among elevations, and between focal taxa within elevations. The 2600 m site was excluded from among group statistical comparisons because of the lack of bromeliads sampled there. Analysis of variance and post-hoc Tukey tests were used to determine differences among elevations or groups, and a linear regression was performed to examine the relationships of variables with elevation. We used discriminant function analysis (DFA) to determine overall mineral nutrition differences among epiphytic taxa with combined data: SLW, %N, %P, δ15N, δ13C, and elevation.
Results
Within taxa among elevations
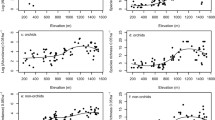
Seven foliar nutrient and physical properties were compared statistically within groups: SLW, %N, %P, percent carbon (%C), δ13C (‰), and δ15N (‰). Within epiphyte groups, SLW did not vary significantly among elevations but did vary among epiphyte groups within elevations (Table 2). Orchids, on average had the highest SLW among epiphyte groups (109.6 ± 4.2 g m−2), followed by bromeliads (80.5 ± 3.7 g m−2), and ferns (67.06 ± 4.60 g m−2) with the lowest. Similarly, host tree SLW did not vary significantly among elevations nor did it vary consistently from the epiphyte groups at any elevation (Fig. 1). Host tree average SLW (85.88 ± 4.40 g m−2) hovered below that of orchids and above that of ferns and bromeliads.
Nitrogen
Foliar N concentrations varied significantly among elevations within all three epiphyte groups, but not among elevations for host trees (Table 2). Each of the three epiphyte taxa had %N that varied in two sites, but these sites were not consistent among groups. Fern %N was significantly higher at the 1600 m site than the adjacent 1000 m site; orchid %N was significantly higher at the 2600 m site compared to the 500 m site and bromeliad %N was significantly higher at the 1600 m site than the 30 and 500 m sites (Fig. 2a). Ferns, overall, had the highest %N concentrations of the three groups. Host trees overall had twice the %N compared to the epiphytes (~1.10 ± 0.03 and ~2.37 ± 0.08, mean ± 1 SE epiphyte and host tree %N, respectively). No significant correlations between elevation and %N were detected for any group.
Mean (±1 SE) a foliar nitrogen (%N), b phosphorous (%P), and c N:P ratios (row three) for each study group: ferns, orchids, bromeliads, and host trees at each of the six study elevations along the Barva Transect, Costa Rica. Different lower case letters indicate significant differences within taxa among elevations (P < 0.05)
Phosphorus
Phosphorus concentrations varied among elevations within epiphyte taxa and host trees (Table 2), but varied little among epiphyte taxa within elevations, and there were consistent patterns in %P among ferns, orchids, and host trees among elevations (Fig. 2b). Mean fern and orchid %P varied up to twofold among elevations. Within both ferns and orchids, the 1000 and 2000 m sites had the lowest mean %P, whereas the 30 and 2600 m sites were the highest with the 1600 m site having intermediate values. Bromeliads were anomalous in that only one site varied: the 1600 m site had significant higher %P while the other sites ranked equivalently (Table 2). Within elevations, %P did not vary among epiphyte taxa except at the 30 m site where bromeliads had the lowest concentrations. Host trees, such as ferns and orchids, had the highest P concentrations at the 30, 1600 and 2600 m sites, and on average, had greater %P than all the epiphyte groups (~0.118 ± 0.006 and ~0.068 ± 0.038, mean ± 1 SE host tree and epiphyte %P, respectively). No correlations between %P and elevation were found for any study group; however, fern and orchid P concentrations correlated significantly and positively with host tree P concentrations (Fig. 3).
Nitrogen:phosphorus ratios were significantly different among elevations for all epiphyte taxa, but not among host trees (Fig. 2c, Table 2). Fern and orchid foliar N:P ratios were the lowest at the 30 m site and highest for ferns at the 2000 m site, and for orchids at the 1000 m site. Bromeliad N:P ratios were the highest at the 1000 m site and equivalent at the others. Across groups and elevations, only the 30 and 2000 m sites varied among taxa. At the 30 m site, host trees had significantly greater N:P ratios than ferns and orchids, but not the bromeliads, while at the 2000 m site, ferns had a greater N:P ratio than bromeliads and orchids, but not the host trees. No significant correlations were detected between N:P ratios and elevation for any group.
Isotopes
Natural abundance δ13C values were significantly different among the elevations for ferns and orchids, but not among bromeliads nor host trees (Fig. 4a). In ferns, the 1600 m site was significantly more depleted than the 1000 and 2000 m sites, and the 2000 m site was in turn more enriched than the 30 and 500 m sites (F 69 = 5.28, P = < 0.001). Similarly in orchids, the 1600 m site was significantly more depleted than the 300 and 2600 m sites (F 63 = 4.19, P = 0.003). Only two individuals had δ13C values in the range of crassulacean acid metabolism (δ13C = −16.11 and −15.05‰), both the same species of bromeliads at the 30 m site, Tillandsia festucoides, and both species were removed for statistical comparisons of δ13C among elevations and groups. There was significant variation within elevations among study groups in δ13C, with bromeliads consistently more enriched than ferns, but not always statistically so (Fig. 4a). Among the epiphyte groups, there was a pronounced depletion of δ13C at the 1600 m site. Interestingly, host tree δ13C signatures were significantly more enriched than ferns at most sites and did not exhibit a depletion at the 1600 m site (Fig. 4a).
Natural abundance δ15N signatures showed the greatest variation of all the nutrients measured both within taxa among elevations and among taxa within elevations, with the exception of bromeliads which showed no significant variation among elevations (Fig. 4b). Host trees were the only group that had a consistent pattern in relation to the other taxa in that it was more enriched in δ15N than all the epiphyte groups (Fig. 4b) and also the only group that had a linear, negative, and significant correlation with elevation (R 242 = 0.66, P = < 0.001).
Using DFA with SLW, %N, %P, δ15N, δ13C, and elevation, we found that the mineral nutrition of the epiphyte taxa were statistically distinct as indicated by non-overlapping 95% confidence intervals (Fig. 5; MANOVA: Pillai’s trace = 0.67, F = 13.38, P = < 0.001). The first discriminant function explained 57% of the data while the second discriminant function explained 39.9% with %P followed by %N having the highest axis loadings.
Discussions
Epiphytic plants inhabiting the forest canopy often exhibit a suite of morphological characters that are unique from plants directly rooted in terrestrial substrates. Such functional suites include characters from modified leaves to velamen-coated aerial roots that function directly in uptake of water and nutrients from the atmosphere. We hypothesized that the differences in adaptations to nutrient uptake observed in the major epiphyte taxa would be reflected in their leaf construction and mineral nutrition: canopy soil-rooted epiphytes would have lower SLW and greater N and P concentrations than non-canopy soil-rooted taxa as well as lower water stress. We also hypothesized that epiphyte mineral nutrition would track that of host trees because of their inherent link through habitat, and that the mineral nutrition of epiphytes and host trees would also track that of the ecosystem.
The differences in the functional morphology of epiphytic taxa are evident by examining their differences in SLW. Orchids, on average, had higher SLW than ferns and bromeliads while ferns had the lowest (Fig. 1). These results suggest that orchids have thicker, longer-lived leaves with lower photosynthetic capacity per unit area than ferns at most sites along the gradient (Reich et al. 1991; Reich et al. 1997; Wright et al. 2005a). The lack of significant variation in SLW among elevations indicates that leaf construction within representative groups is conserved. Contrary to our prediction, forest floor-rooted host tree leaves had SLW comparable to that of epiphytes indicating fewer leaf functional differences.
We found that among epiphyte groups, ferns with exclusive dependence on canopy soil had the highest N concentrations overall, non-soil-rooted bromeliads had the lowest, and orchids were intermediate (Fig. 2). Although nutrient concentrations cannot always be directly attributed to nutrient source pool or size, the fact that the ferns had the highest N content suggests that plants rooted in canopy soil have access to more N than those that are dependent on more stochastic extra-canopy N sources—a pattern found in other forests (Hietz et al. 2002; Wania et al. 2002). Not surprisingly, forest floor-rooted host trees had the highest N concentrations when compared to all the epiphytes across elevations (Fig. 1a), likely due to the larger source pool of forest floor soils. Another interpretation of the variation in N concentrations among taxa is that they have different N requirements, for example, the low N concentrations of bromeliads could be due to lower N requirements because of higher N use efficiency.
In contrast to N concentrations, P concentrations among elevations varied significantly, but not among epiphyte taxa (Fig. 2). These results suggest that N concentrations in the canopy are dynamic and vary among sources within sites, whereas the consistent P concentrations among taxa within elevations indicates fixed P availability. Further, the correlations between fern and orchid P concentrations and those of host trees (Fig. 3) also indicate that P is tightly cycled in the system. Some evidence suggests that canopy soil is composed of both epiphyte and host tree litter inputs (Cardelús et al. 2009; Hietz et al. 2002) which would explain the similar trends between host trees and ferns and orchids and may suggest a link between their P cycles.
The invariability of P concentrations among taxa within elevations combined with the correlations of P concentrations lends further evidence to mounting data indicating that the canopy habitat is P and not N-limited (Benner et al. 2007; Benner and Vitousek 2007; Cardelús, in press; Cardelús et al. 2009; Winkler and Zotz 2008; Zotz 2004; Zotz and Richter 2006). In a recent empirical study of forest floor N and P fertilization, epiphyte diversity and biomass increased significantly with P but not N fertilization (Benner et al. 2007), while other studies on individual species have shown that P is more readily retranslocated than N during leaf senescence (Zotz 2004). Further evidence of canopy habitat P limitation is that litter decay is highly correlated with lignin:P ratios, and not with lignin:N ratios which is usually the case (Cardelús, in press).
The 1600 m site along our transect was notable in that the P concentrations were high for all groups (Fig. 2). Our sample transect occurred along a dormant volcano and geothermal streams with high total P output have been found at sites of lower elevations (Pringle et al. 1993). However, soluble reactive phosphorous was high at a stream near the 2000 m site (Pringle et al. 1993), suggesting that geothermal inputs may be present at higher elevations along this gradient. There are no additional anthropogenic sources of P close to the site.
While δ15N signatures have successfully been used to interpret source nutrients (Dawson et al. 2002; Robinson et al. 2000), we found our data difficult to interpret because of the high variability and lack of consistent patterns within or among epiphyte taxa (Fig. 4b). This high variability is likely a combination of source nutrient signature, a possible series of integrated fractionation events (Dawson et al. 2002; Robinson et al. 2000), and variability among species within each taxon. The most notable result for δ15N values was the depletion trend with increasing elevation in host trees. Although this depletion in δ15N values with elevation could be caused by changes in N sources with increasing elevation, this trend may also reflect fewer N losses in the more N-limited higher elevations (Amundson et al. 2003; Martinelli et al. 1999). This pattern is supported by the observation that available soil N decreases with increasing elevation on the Barva Transect (Marrs et al. 1988). Although the correlation between host tree δ15N values and elevation is strong, it may be undetected in soil-rooted epiphytes because they are accessing soil nutrients that have both epiphyte and host tree inputs.
Plants under water stress often exhibit high water use efficiency (WUE) which is seen by the enrichment of δ13C signatures. Among epiphyte taxa, we found that bromeliads had the most enriched δ13C values, on average, indicating that they have greater WUE than ferns and orchids. The more enriched signatures at the base of the mountain and the peak are not surprising given the high temperatures and lack of cloud cover in the former and the high winds in the latter, which can decrease water supply (Hartshorn and Peralta 1988). The 1600 m site is where the WUE of all the epiphyte groups is low, i.e., most depleted δ13C values (Fig. 3a). Although this is not the site with the highest inputs of precipitation, it is the middle of the cloud forest band which may contribute significant amounts of water to the site. Because of the limited water storage capacity of the canopy habitat due to the low abundance of canopy soil (Nadkarni 1984; Cardelús et al., unpublished data), epiphytes have been shown to have δ13C values that are often more enriched than terrestrially rooted plants (Stewart et al. 1995; Watkins et al. 2007). However, host trees did not have depleted δ13C values compared to epiphytes (Fig. 3a). Rather, host tree δ13C values were intermediate among epiphytes at lower elevations and the highest at upper elevation indicating lower water use efficiency than epiphytes at upper elevations (Fig. 4a). This finding, at first, seemed counterintuitive given that host trees have the water resources of the forest floor soils. However, one possible explanation for this is the cloud cover. Cloud cover begins at mid elevation, and the cloud layer often sits above the forest floor and could be delivering inputs of water to the canopy and not directly to the forest floor resulting in greater water stress on the forest floor than in the canopy.
The variation among epiphytes groups is exemplified in the DFA which showed that ferns, orchids, and bromeliads have statistically distinct mineral nutritions. These differences are likely due, in part, to their differences in nutrient-uptake mechanism as well as nutrient source. Elaboration of leaves and stems for nutrient capture aboveground increases the diversity of nutrient sources that the epiphytic plant community can use and may ultimately facilitate and maintain the high morphological and taxonomic diversity of canopy communities (Lesica and Antibus 1990; Nadkarni et al. 2004; Putz and Holbrook 1989) .
The significantly greater N concentrations and significantly depleted δ15N values of ferns, orchids, and bromeliads than host trees supports the hypothesis that species of canopy habitats rely more on atmospheric or within-canopy derived N sources, while forest floor-rooted trees derive N from a more N-rich and highly fractionated N soil pool. These results are consistent with findings that canopy plants have lower N concentrations and have more depleted δ15N values than forest floor-rooted plants (Hietz et al. 1999; Hietz et al. 2002; Stewart et al. 1995; Watkins et al. 2007). Two possible mechanisms for the more depleted δ15N values of epiphytes compared to host trees are that (1) the majority of organic inputs into the canopy are depleted in δ15N—epiphytes depleted in δ15N derived from the atmosphere die and decompose in place (Hietz et al. 2002)—and (2) external inputs into the canopy are depleted in δ15N. The former is supported by data from a leaf retention study done in Monteverde, which suggests that host tree litter decomposition is minimal because the leaves are blown out of the canopy within two weeks of leaf fall (Nadkarni and Matelson 1992). However, other studies have shown canopy soil to be isotopically enriched relative to epiphytes, mirroring the isotopic signature of host tree leaves (Cardelús et al. 2009; Hietz et al. 2002) suggesting that decomposed host tree foliage contributes significantly to canopy soil.
A nutritional linkage between host trees and epiphytes exists for P (Fig. 3) with less evidence from this study linking N cycles. It seems clear that the canopy resource status is largely disconnected from terrestrial resource status which is especially true of those taxa not rooted in canopy soil. Our data suggest that the host tree has a differential role in the N nutrition of epiphyte groups. Epiphytes rooted in canopy soil have access to nutrients from decomposed host tree litter (Cardelús et al. 2009) as well as a larger nutrient pool derived from decaying epiphyte litter than non-canopy soil-rooted epiphytes, as indicated by the significantly higher N concentrations of canopy soil-rooted ferns at most sites (Fig. 2; Table 2). Given the lack of variation among groups at each site, our data also suggest that P is more limiting in the canopy habitat compared to the forest floor. The mineral nutrition of epiphytes compared to host trees also indicates that the canopy habitat is either nutrient poor or that nutrients are less available compared to the forest floor, or both.
We found some surprisingly broad (consistent) patterns of mineral nutrition across diverse groups of epiphytes. For example, ferns and orchid P concentrations were closely correlated with the concentrations of host trees. In addition, host tree δ15N values correlated negatively with elevation and epiphyte foliar nutrient concentrations were lower than host trees. However, most surprising were the lack of patterns both among epiphyte groups and between epiphyte groups and elevation. This finding is consistent with one known study on epiphytic and terrestrial ferns along a disjunct elevational gradient, which were found to have increasing foliar N concentrations with increasing elevation, contrary to forest floor soils N concentrations (Wegner et al. 2003). These results, however, are in contrast to a number of articles on terrestrial-based taxa showing that along temperature/latitudinal gradients there is a trend of decreasing foliar N and P concentrations with increasing mean annual temperature (McGroddy et al. 2004; Reich and Oleksyn 2004). The inconsistency of our data with those of large and diverse datasets on predominantly forest floor-rooted taxa suggests that the nutrient dynamics of epiphyte communities are radically different from those rooted in the forest floor soil and thus, must be examined as a separate group.
References
Amundson R, Austin AT, Schuur EAG, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Glob Biogeochem Cycles 17:1031
Benner JW, Vitousek PM (2007) Development of a diverse epiphyte community in response to phosphorus fertilization. Ecol Lett 10:628–636
Benner JW, Conroy S, Lunch CK, Toyoda N, Vitousek PM (2007) Phosphorus fertilization increases the abundance and nitrogenase activity of the cyanolichen Pseudocyphellaria crocata in Hawaiian Montane Forests. Biotropica 39:400–405
Bentley BL, Carpenter EJ (1984) Direct transfer of newly-fixed nitrogen from free-living epiphyllous microorganisms to their host plant. Oecologia 63:52–56
Bermudes D, Benzing DH (1991) Nitrogen fixation in association with Ecuadorean bromeliads. J Trop Ecol 7:531–536
Cardelús CL (in press) Litter decomposition in the canopy and forest floor of three tree species in a tropical rainforest, Costa Rica. Biotropica
Cardelús CL, Chazdon RL (2005) Inner-crown microenvironments of two emergent tree species in a lowland wet forest. Biotropica 37:238–244
Cardelús CL, Colwell RK, Watkins James E Jr (2006) Vascular epiphyte distribution patterns: explaining the mid-elevation richness peak. J Ecol 94:144–156
Cardelús CL, Mack MC, Woods CL, DeMarco J, Treseder KK (2009) Nutrient cycling in canopy and terrestrial soils at a lowland rainforest site, Costa Rica. Plant Soil 318:47–61
Clark KL, Nadkarni NM, Schaefer D, Gholz HL (1998a) Atmospheric deposition and net retention of ions by the canopy in a tropical montane forest, Monteverde, Costa Rica. J Trop Ecol 14:27–45
Clark KL, Nadkarni NM, Schaefer D, Gholz HL (1998b) Cloud water and precipitation chemistry in a tropical montane forests, Monteverde, Costa Rica. Atmos Environ 32:1595–1603
Cleveland CC, Townsend AR, Schimel DS, Fisher H, Howarth RW, Hedin LO, Perakis SS, Latty EF, Von Fischer JC, Elseroad A, Wasson MF (1999) Global patterns of terrestrial biological nitrogen (N-2) fixation in natural ecosystems. Glob Biogeochem Cycles 13:623–645
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559
Edwards PJ (1982) Studies of mineral cycling in a montane rain forest in New Guinea. V. Rates of cyling in throughfall and litterfall. J Ecol 70:807–827
Forman RT (1975) Canopy lichens with blue-green algae: a nitrogen source in a Colombian rainforest. Ecology 56:1176–1184
Freiberg E (1998) Microclimatic parameters influencing nitrogen fixation in the phyllosphere in a Costa Rican premontane rain forest. Oecolgia 17:9–18
Grieve IC, Proctor J, Cousins SA (1990) Soil variation with altitude on Volcan Barva, Costa Rica. CATENA 17:525–534
Hartshorn G, Peralta R (1988) Preliminary description of primary forests along the La Selva-Volcán Barva altitudinal transect, Costa Rica. In: Almeda F, Pringle CM (eds) Tropical rainforests: diversity and conservation. California Academy of Sciences and Pacific Division, American Association for the Advancement of Science, San Francisco, pp 281–306
Hietz P, Wanek W, Popp M (1999) Stable isotopic composition of carbon and nitrogen and nitrogen content in vascular epiphytes along an altitudinal transect. Plant Cell Environ 22:1435–1443
Hietz P, Wanek W, Wania R, Nadkarni NM (2002) Nitrogen-15 natural abundance in a montane cloud forest canopy as an indicator of nitrogen cycling and epiphyte nutrition. Oecologia 131:350–355
Hofstede RGM, Wolf JHD, Benzing DH (1993) Epiphytic biomass and nutrient status of a Colombian upper montane rain forest. Selbyana 14:37–45
Jones JB, Case BW (1996) Soil testing and plant analysis no. 3. In: Sparks DL (ed) Methods of soil analysis Part 3: chemical methods. Soil Science Society of America, Madison, pp 389–415
Kromer T, Kessler M, Gradstein RS, Acebey A (2005) Diversity patterns of vascular epiphytes along an elevational gradient in the Andes. J Biogeogr 32:1799–1809
Küper W, Kreft H, Nieder J, Koster N, Barthlott W (2004) Large-scale diversity patterns of vascular epiphytes in Neotropical montane rain forests. J Biogeogr 31:1477–1487
Lesica P, Antibus RK (1990) The occurrence of mycorrhizae in vascular epiphytes of two Costa Rican rain forests. Biotropica 22:250–258
Lieberman D, Lieberman M, Peralta R, Hartshorn GS (1996) Tropical forest structure and composition on a large-scale altitudinal gradient in Costa Rica. J Ecol 84:137–152
Liu WY, Fox JED, Xu ZF (2002) Nutrient fluxes in bulk precipitation, throughfall and stemflow in montane subtropical moist forest on Ailao Mountains in Yunnan, south-west China. J Trop Ecol 18:527–548
Madison M (1977) Vascular epiphytes: their systematic occurrence and salient features. Selbyana 2:1–13
Marrs RH, Proctor J, Heaney A, Mountford MD (1988) Changes in soil nitrogen-mineralization and nitrification along an altitudinal transect in tropical rain forest in Costa Rica. J Ecol 76:466–482
Martin CE (1994) Physiological ecology of the Bromeliaceae. Bot Rev 60:1–82
Martinelli LA, Piccolo MC, Townsend AR, Vitousek PM, Cuevas E, McDowell W, Robertson GP, Santos OC, Treseder K (1999) Nitrogen stable isotopic composition of leaves and soil: tropical versus temperate forests. Biogeochemistry 46:45–65
McGroddy ME, Daufresne T, Hedin LO (2004) Scaling of C: N: P stoichiometry in forest ecosystems worldwide. Ecology 85:2390–2401
Nadkarni N (1984) Epiphyte biomass and nutrient capital of a neotropical elfin forest. Biotropica 16:249–256
Nadkarni NM, Matelson TJ (1991) Litter dynamics within the canopy of a neotropical cloud forest, Monteverde, Costa Rica. Ecology 72:849–860
Nadkarni NM, Matelson TJ (1992) Biomass and nutrient dynamics of epiphytic litterfall in a neotropical montane forest, Costa-Rica. Biotropica 24:24–30
Nadkarni NM, Schaefer D, Matelson TJ, Solano R (2004) Biomass and nutrient pools of canopy and terrestrial components in a primary and a secondary montane cloud forest, Costa Rica. For Ecol Manag 198:223–236
Nieder J, Engwald S, Barthlott W (1998) Patterns of epiphyte diversity in neotropical rain forests. Selbyana 19:290
Nieder J, Prosperí J, Michaloud G (2001) Epiphytes and their contribution to canopy diversity. Plant Ecol 153:51–63
Norman C (1985) Virgin rain forest reprieved. Science 227:273
Perry DR (1978) A method of access into the crowns of emergent and canopy trees. Biotropica 10:155–157
Pittendrigh CS (1948) The bromeliad-Anopheles-malaria complex in Trinidad. I-The bromeliad flora. Evol Int J Org Evol 2:58–89
Pringle CM, Rowe GL, Triska FJ, Fernandez JF, West J (1993) Landscape linkages between geothermal activity and solute composition and ecological response in surface waters draining the Atlantic slope of Costa-Rica. Limnol Oceanogr 38:753–774
Putz FE, Holbrook NM (1989) Strangler fig rooting habits and nutrient relations in the llanos of Venezuela. Am J Bot 76:781–788
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. PNAS 101:11001–11006
Reich PB, Uhl C, Walters MB, Ellsworth DS (1991) Leaf lifespan as a determinant of leaf structure and function among 23 Amazonian tree species. Oecologia 8:16–24
Reich PB, Walters MB, Ellsworth D (1997) From tropics to Tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Robinson D, Handley LL, Scrimgeour CM, Gordon DC (2000) Using stable isotope natural abundances (δ15N and δ13C) to integrate the stress responses of wild barley (Hordeum spontaneum C. Kock.) genotypes. J Exp Bot 51:41–50
Stewart GR, Schmidt S, Handley LL, Turnbull MH, Erskine PD, Joly CA (1995) 15N Natural abundance of vascular rainforest epiphytes: implications for nitrogen source and acquisition. Plant Cell Environ 18:85–90
Titiz B (2005) Signatures in the soil: Soil charcoal and phosphorus distribution patterns along an elevational gradient in a Costa Rican tropical rainforest. University of Denver, United States – Colorado
Wania R, Hietz P, Wanek W (2002) Natural 15N abundance of epiphytes depends on the position within the forest canopy: source signals and isotope fractionation. Plant Cell Environ 25:581–589
Watkins JE Jr, Rundel PW, Cardelús CL (2007) The influence of life form on carbon and nitrogen relationships in tropical rainforest ferns. Oecologia 153:225–232
Wegner C, Wunderlich M, Kessler M, Schawe M (2003) Foliar C:N ratio of ferns along an Andean elevational gradient. Biotropica 35:486–490
Winkler U, Zotz G (2008) Highly efficient uptake of phosphorus in epiphytic bromeliads. Ann Bot 103:477–484
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N, Poorter H, Villar R, Warton DI, Westoby M (2005a) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Groom PK, Hikosaka K, Lee W, Lusk CH, Niinemets U, Oleksyn J, Osada N, Poorter H, Warton DI, Westoby M (2005b) Modulation of leaf economic traits and trait relationships by climate. Glob Ecol Biogeogr 14:411–421
Zotz G (2004) The resorption of phosphorus is greater than that of nitrogen in senescing leaves of vascular epiphytes from lowland Panama. J Trop Ecol 20:693–696
Zotz G, Richter A (2006) Changes in carbohydrate and nutrient contents throughout a reproductive cycle indicate that phosphorus is a limiting nutrient in the epiphytic bromeliad, Werauhia sanguinolenta. Ann Bot 97:745–754
Acknowledgments
We thank the Ecology Lab Group at the University of Florida for helpful discussions throughout this study and J. DeMarco, A. Mishra, and G. Crummer for essential help with lab work and M. Blanco for plant identification. This article is based on research supported by the National Science Foundation under Grant No. 0309827 to CC, and a Mellon Foundation Grant to MCM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cardelús, C.L., Mack, M.C. The nutrient status of epiphytes and their host trees along an elevational gradient in Costa Rica. Plant Ecol 207, 25–37 (2010). https://doi.org/10.1007/s11258-009-9651-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-009-9651-y