Abstract
The survival of alpine species in changing climates depends on dispersal or adaptation. However, it is unclear whether trait variability along elevation/climatic gradients is adaptive or represents stress towards lower/warmer elevations, particularly for the endangered endemics for which protected status and plant longevity preclude experimental study. We chose one such species, known for its phenotypic variability (Primula glaucescens, endemic to the southern Alps), and quantified key functional traits in situ throughout its range, correlating these with elevation as a proxy for climate. Larger leaves were evident towards lower elevations, but tissue nitrogen dilution and limited regenerative fitness were symptomatic of stress. Specific leaf area, a correlate of relative growth rate, was consistently low: the entire species exhibits conservative leaf economy and inherently slow growth. This seemingly variable species exhibits superficial variability around a fundamentally conservative, cold-adapted survival strategy, and thus phenotypic variability is unlikely to facilitate the persistence of alpine endemics during rapid climate warming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species’ range boundaries are closely associated with climate (Huntley et al. 1995; Jump and Woodward 2003; Franco et al. 2006), and anthropogenic climate warming is currently forcing the global mean range of species upwards in elevation at a rate of 6.1 m y−1 and towards the poles at a rate of 6.1 km y−1 (Parmesan and Yohe 2003; Root et al. 2003). Migration is exceptionally rapid for some organisms, particularly arthropods (Hickling et al. 2006), whereas alpine plants may exhibit vertical migration rates as low as 0.1 m y−1 (Miller-Rushing and Primack 2004), with an average of 2.4 m y−1 in the southern European Alps (Parolo and Rossi 2008). Thus, alpine plants risk being unable to migrate in step with the changing climate (Grabherr et al. 1994; Huntley et al. 1995). Mountain topography further limits possibilities for migration, as conical peaks have an increasingly reduced surface area, and may become too steep or too frequently disturbed by debris falls to support certain plant communities, with alpine specialists subject to greater potential restriction than species with more extensive elevation ranges (Guisan and Theurillat 2000; Pauli et al. 2003). Indeed, survival is proving impossible for many species that already occupy the upper reaches of their mountaintops, with no further refuge; the first extinctions attributable to global warming have been alpine (Parmesan 2006).
It is doubtful whether species can evolve fast enough to keep pace with climate change (Parmesan 2006) and future survival of alpine species will thus probably depend on ability to disperse to new sites or to persist at current locations (Theurillat and Guisan 2001; Miller-Rushing and Primack 2004). Indeed, Theurillat and Guisan (2001) conclude that deeper understanding is needed of how alpine species may persist or disperse, particularly for keystone species or rare species with narrow distributions. Dispersal commonly relies on anemochory (Caccianiga et al. 2006), but many endemics exhibit alternative dispersal syndromes involving myrmecochory or ballistic autochory that are less capable of long-distance dispersal across geographic barriers. Furthermore, alpine species have inconsistent and unpredictable soil seed banks, particularly for the rarest species, and persistence is unlikely to rely on diaspores, depending more on the inherent capabilities of vegetative plants (Theurillat and Guisan 2001). For example, P. glaucescens Moretti, an endemic of the southern European Alps, is noted for its extensive phenotypic trait variability (Ravazzi and Ferlinghetti 1986), which could potentially facilitate the persistence of existing genotypes in warmer climates. However, it is not known if trait variability along altitudinal gradients is truly adaptive or simply represents a stress response towards the lower, warmer climatic range boundary.
Increased climatic stress at species’ range boundaries may result in a range of negative impacts on plant growth, reproduction and ultimately population densities (Jump and Woodward 2003). Additionally, range boundary effects, population fragmentation and restriction of population size are also known to be detrimental to gene flow, genetic variability and reproductive capability, and alter population demographics (Van Rossum et al. 2002; Van Rossum and Triest 2003; Lienert and Fischer 2003; Lehtilä et al. 2006). We hypothesise that variation in vegetative and reproductive traits for narrow alpine endemics reflects increasingly sub-optimal conditions towards the range boundary, rather than optimisation of function by well-adapted ecotypes. We also hypothesise that trait variation is correlated with climate and thus occurs along the entire altitudinal gradient, rather than strictly at the range boundary.
Investigating extremely rare and severely protected species poses an ethical and legal dilemma, as experimental studies, such as reciprocal transplantation experiments, destroy plants. Cultivating long-lived alpine species from seed could involve waiting decades for plants to mature and, in many cases, produce the rhizomes typical of fully established plants (e.g. P. glaucescens; Ravazzi and Ferlinghetti 1986). This is clearly impractical for species that may have individual life spans measured in hundreds or even thousands of years (Theurillat and Guisan 2001). However, it is precisely species such as this, which are more difficult to manipulate experimentally and that are highly endangered, for which we most keenly require information concerning responses to climate; information that can be used to gauge the urgency and utility of conservation activities. Körner (1999a, 2007) suggests that natural elevation/temperature gradients provide relatively artefact-free opportunities to study vegetation responses to climate change (i.e. space-for-time experiments, such as those used to study succession, in which spatial distance equates to temporal change) because the declining air temperature with increasing elevation is one of the strongest environmental selection pressures. Using environmental gradients has the advantage of maintaining plant roots in contact with a natural, undisturbed rhizosphere and also involves natural long-term plant responses, whereas short-term experiments are transient events with transient consequences that are unlikely to afford much predictive power (Körner 1999a). Huntley et al. (2004) also take the stance that natural distributions are more informative than an experimental approach. Thus, the present study aimed to investigate wild populations of a highly variable and well-studied endemic species, P. glaucescens (Ravazzi and Ferlinghetti 1986), throughout its entire elevational range/climate envelope. Our main hypothesis was that vegetative and reproductive traits are correlated with elevation, with lower/warmer elevation populations exhibiting less effective function and abundance. Compromises must always be made when studying protected species, and although correlation does not necessarily denote causality, this approach will at least provide a realistic picture of the natural variability in function of a narrow endemic alpine along a climate gradient.
Materials and methods
Study species
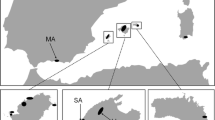
The Lombardy Primrose, P. glaucescens (Fig. 1), is a steno-endemic of the southern European Alps in Lombardy, Italy, and grows at rocky sites and on rock faces on a small number of peaks within this restricted geographic range (Fig. 2). P. glaucescens lacks anemochory (Cerabolini et al. 2003), and is unlikely to be able to disperse to the higher peaks to the north of the species’ current range (Ravazzi 1999; Theurillat and Guisan 2001). The precarious situation of P. glaucescens is reflected by its status as a severely protected species under the 1979 Bern Convention and inclusion in the Red List of Italian plants (Conti et al. 1997). Populations from higher elevation peaks were traditionally viewed as a separate subspecies, partly due to smaller leaves and a dwarf habit (Arietti and Crescini 1976). However, leaf size may be highly variable within each single population, and the subspecies are now thought to be extremes of a spectrum of phenotypic variability (Ravazzi and Ferlinghetti 1986). Indeed, molecular evidence indicates that populations of P. glaucescens from different mountaintops (i.e. at different elevations) are genetically distinct ecotypes, with genetic distance reflecting geographic distance (Ceriani et al. 2005). Thus, the phenotypic variability of the species as a whole encompasses both ecotypic variation and the variability between individuals within populations.
The location of P. glaucescens populations sampled in the present study, in the southern European Alps, north of Milan, Lombardy, Italy. Legends are the local names of peaks to which populations are restricted. The boundary delimits the home range according to Ravazzi (1999), although populations have not been reported from elevations below 500 m a.s.l. within this area. Additional low elevation populations reported at Valsolda and Grona by Antonietti (1986) were not rediscovered during the present study, and may now be extinct. Inset shows the study area location within Italy. Symbols represent each population, with lighter shades representing higher elevations (see Fig. 3)
Population characteristics
We visited accessible sites where P. glaucescens has previously been reported (Ravazzi 1999; Fig. 2) between May and September 2005. At each site, representing 14 populations, we placed six 1 m2 quadrats on areas of vegetation where P. glaucescens was most prevalent (sample size was limited by the rarity of the species—it was not necessarily possible to place greater numbers of quadrats due to the small size of many populations), with quadrats arranged perpendicular to the slope. The general location and elevation of quadrats was recorded with global positioning systems (GPS; eTrex Summit receivers; Garmin, Romsey, U.K.) with latitude and longitude measured to a precision of 7–13 m, depending on local reception (the eTrex Summit uses a barometer to measure elevation, which was calibrated at a reference point of known elevation prior to measurements). GPS data were uploaded to a PC and managed with GPS Trackmaker 12.2 (Odilon Ferriera, Belo Horizonte, Brazil), superimposed over a map of the territory (1:10,000 scale; Regione Lombardia 1998). We marked the precise locations of quadrats using iron rods placed at the corners of the quadrat and later detected with a metal detector. This allowed quadrats to be revisited throughout the season to ensure that flowers and fruits were counted/collected at the time of maximum abundance (fruits were collected in an equivalent state of maturity: i.e. immediately prior to dehiscence). We then measured the following population traits within each quadrat: the number of rosettes m−2, the number of rosettes in flower m−2, the number of rosettes with fruit m−2, number of flowers per rosette and aspect (measured with the electronic compass of the GPS receiver).
Vegetative functional traits
We quantified the following plant functional traits for 30 individuals per mountaintop site: maximum canopy height, maximum rosette diameter, number of leaves per rosette, leaf fresh weight (LFW), leaf dry weight (LDW), leaf area (LA), leaf thickness, specific leaf area (SLA) and leaf dry matter content (LMDC). All leaf traits were measured from the youngest fully expanded leaves of mature rosettes, thereby avoiding possible ontogenetic differences between populations. Leaves collected from the field were placed in labelled plastic bags in a refrigerated container at approximately 4°C, and transported immediately to the laboratory, where measurements followed the standardised methodologies detailed by Cornelissen et al. (2003); for the determination of LFW and LA (i.e. the mean surface area of fully expanded leaves) leaf material was stored at 4°C overnight to obtain full turgidity. LA was determined with a digital leaf area meter (Delta-T Image Analysis System; Delta-T Devices Co. Ltd., Burwell, Cambridgeshire, UK). LDW was then determined following drying for 24 h at 105°C, and parameters such as SLA (i.e. LA divided by LDW) were calculated. Leaf nitrogen content was then determined from dry leaf material with a CHN-analyzer (NA-2000 N-Protein; Fisons Instruments S.p.A., Rodano (MI), Italy).
Reproductive functional traits
The following traits were quantified from the fruits that we collected: number of seeds per fruit (and number of seeds per rosette), seed mass and seed variance (n = 10 for each population). Seed germination characteristics were determined following the non-sterile technique shown to be most effective for P. glaucescens by Cerabolini et al. (2004); seed samples were placed on three wetted Whatman No.3 filter paper discs in Petri dishes, incubated in a germination chamber (Snijders Scientific Economic Deluxe; Thermo-Lab, Codogno (LO), Italy) in the following environmental regime: 16/8 h light/dark cycle at 20°C. For each population, we allocated seeds to 10 replicate Petri dishes, each containing 10 seeds. Petri dishes were re-randomised every 2–3 days to minimise systematic effects due to position within the chamber, and water added weekly. Petri dishes were checked daily for germination, with seeds exhibiting radicle emergence recorded as ‘germinated’. For each trial, germination percentages were recorded at 45 days after sowing, along with the germination delay (days until the first germination record) and calculation of the half-germination time (days until 50% of the final germination percentage). We maintained non-germinated seeds for a further 56 days, to exclude the possibility of delayed germination.
Statistics
Regression analysis was performed using the statistical module of Sigmaplot 7 (SPSS Inc., Chicago, IL, USA).
Results
Populations of P. glaucescens were found between 500 and 2200 m a.s.l., mainly on northern, north-western and north-eastern slopes (Fig. 3). Populations on south-facing slopes occurred only above 1500 m a.s.l. on the highest peaks within the home range of the species (i.e. at Arera, Croce Domini, Presolana and Grigna; Fig. 3). Functional traits that were not correlated with elevation included maximum canopy height, the number of seeds per fruit, number of seeds per rosette, seed mass and seed variance (data not shown). Similarly, SLA exhibited no correlation with elevation, and was low for all populations, varying between 114.3 and 140.8 cm2 g−1, with a mean of 124.7 ± 2.04 cm2 g−1 (Fig. 4). (Confirmation that these values are low is provided by a study of 150 species within and around the P. glaucescens habitat at Monte Barro, which exhibit a mean SLA of 201.5 cm2 g−1, ranging between 60 and 570 cm2 g−1; Ceriani 2003). The investment of biomass in leaves (LDMC) was also consistent between sites, and did not reflect elevation (r 2 = 0.110, P = 0.179; Fig. 4).
The elevation and aspect of populations of P. glaucescens demonstrate that the species is restricted to high elevations (500–2,200 m a.s.l.) and mainly northern aspects. Site names are the local names of the individual mountain peaks on which populations occur (Presolana is the only mountain with a second population at lower elevation)
Plants from lower elevations had larger leaves, both in terms of dry weight and area. Indeed, leaf dry weight showed a negative correlation with elevation, diminishing from larger leaves (0.065 g) at low altitude to smaller leaves (0.019 g) at high altitude (r 2 = 0.396, P = 0.016). Leaf area showed a similar negative correlation, decreasing from 5.7 cm2 at low elevation to 2.2 cm2 on higher peaks (Fig. 5). Other functional traits that correlated negatively with elevation included rosette diameter (14.0 cm at low elevation to 7.1 cm at high elevation), and leaf C:N (ranging from 53.4 at low elevation, to 24.1 at high elevation) (Fig. 5). However, at lower elevations leaves exhibited lower nitrogen contents, germination was less successful and the species was less prevalent within the vegetation, compared with higher elevation populations (Fig. 5). Indeed, leaf nitrogen content correlated positively with elevation (LNC ranged from 0.9% at low elevation to 2.1% at high elevation), as did seed germination after 45 days (12.5–58.3%), and the number of rosettes m−2 (6.7–109.8; Fig. 5).
The only vegetative and reproductive traits that were significantly correlated with one another were leaf C:N and seed germination at 45 days (r 2 = 0.472, P = 0.014), and LNC and seed germination at 45 days (r 2 = 0.575, P = 0.007; data not shown), i.e. higher quality leaves were associated with more successful subsequent germination. Plants from all populations produced a single inflorescence supporting 1 to 4 flowers, with a mean of 19 seeds produced by each fruit (ranging from 0 to 43 seeds), but these traits were not correlated with elevation (data not shown). No functional traits were correlated with latitude or longitude (data not shown).
Discussion
We demonstrated a continuum of phenotypic diversity throughout the elevational/climatic range of P. glaucescens, characterised by larger leaf dimensions and a laxer habit towards lower elevations. However, the key leaf traits SLA and LDMC were not correlated with elevation, indicating that investment of biomass within leaf tissues was unchanged. SLA was consistently low, indicative of conservative leaf nutrient economy (Wright et al. 2004). Conservative resource dynamics involves the long-term investment of resources in dense tissues, allowing metabolism to stand by during sub-optimal periods for resource acquisition, i.e. stress-tolerance (Grime 2001; Pierce et al. 2005). Investment in denser tissues imposes greater internal diffusion resistances and slower inherent growth rates: a positive correlation between SLA and relative growth rate (RGR) is based on inevitable biophysical tradeoffs and is a widespread, general relationship throughout the plant kingdom (Poorter and Van der Werf 1998; Weiher et al. 1999; Ceriani et al. 2008). Thus, the low SLA exhibited by all populations is a strong indication that the entire species exhibits an essentially conservative, slow-growing survival strategy throughout its climate envelope, from the highest to the lowest elevations. Only species with a high degree of stress-tolerance exhibit low SLA (Grime 2001), and thus the survival strategy of P. glaucescens is based on the ability to resist sub-optimal periods for growth and development. This conforms to Körner’s (1999a) view that alpine species are stress adapted, rather than simply being stressed. As P. glaucescens is more prevalent on northern aspects of mountain peaks (Fig. 3), the particular stresses it is adapted to occur in colder situations. Thus, the local phenotypic variation we observed represents secondary variation around a core cold-adapted stress-tolerator survival strategy, and does not appear to confer an adaptive advantage.
Decreased local abundance towards the lower range boundary is likely to be due, in part, to stress imposed by warmer temperatures on a cold-adapted metabolism. Temperature induces stress by altering the form and function of membrane components and proteins. Optimisation of plant metabolism to low temperature involves regulating the degree of saturation of cell membrane phospholipids to maintain viable membrane fluidity and for the protection of the structure of metabolic machinery by cold shock proteins (Sung et al. 2003; Gimalov et al. 2004). Thus, a metabolism adapted to low temperature may perform poorly at warmer temperatures. However, our data indicate that in warmer climates P. glaucescens may also experience stress imposed indirectly by changes in growth form and the concomitant redistribution of limited resources throughout tissues. Increasing leaf size towards lower elevations was associated with decreasing leaf nitrogen contents—per unit mass (Fig. 5)—confirming that nitrogen was effectively diluted amongst more extensive tissues, decreasing availability to metabolism. Alpine plants generally tend to have lower leaf nitrogen contents and higher C:N towards lower elevations because the warmer situation imposes a longer growth season, and continued biomass production attenuates nutrient reserves (Körner 1999b). This may subsequently impact on the resources available for seed filling, and could explain the lower capacity for germination evident towards lower altitudes (Fig. 5). Indeed, seed mass, variance and seed number were consistent between populations, suggesting that seed quality, rather than quantity, was paramount. Pollination limitation is unlikely to have imposed problems with gene flow and fertility (as is evident for alpine/subalpine Primula farinosa towards lower elevations; Reisch et al. 2005) as flowering, fruiting and seed set were not correlated with elevation. Thus, it appears that P. glaucescens may essentially become nutrient-stressed towards its range boundary because there is less seasonal (climatic) constraint to growth, and edaphic limitations become more important. This ultimately limits regenerative ability, evident as a lower local abundance of plants (Fig. 5). Despite the importance of edaphic factors, it is unlikely that changing intensities of competition are critical for P. glaucescens, as the vegetation type was essentially the same at all mountaintop sites (i.e. Caricion austroalpinae Sutter 1963; Ravazzi 1992), with plants growing either in shallow soil amongst rocks or in cracks in rock faces. Neighbouring species, such as Carex austroalpina or the grass Sesleria varia, are also tough-leaved stress-tolerators that do not conform to Grime’s competitor strategy, lacking highly competitive traits such as tall canopies. Indeed, alpine species are essentially too busy resisting abiotic environmental pressures for competition to be paramount to survival (Callaway et al. 2002; Pauli et al. 2003; Caccianiga et al. 2006). Competition may become more important as lower altitude species arrive in future warmer climates, but is unlikely to explain the variability in functional traits recorded here.
Global anthropogenic climate change is an insidious and pervasive pressure operating at scales that are difficult to regulate, with the restriction of suitable habitat the principal risk to alpine species (Huntley et al. 1995). In situ conservation of many alpine species is unlikely to succeed in the long term without a successful international effort to reduce greenhouse gas emissions. Indeed, local intervention is unlikely to resolve a global problem, and even setting aside protected areas may not be a viable conservation strategy if climate envelopes migrate beyond static protected zones (Miller-Rushing and Primack 2004). Ex situ conservation may seem futile if suitable habitat disappears, but could facilitate the relocation of species to higher peaks that are currently outside their range and dispersal capabilities, but which may be suitable in the future. Assisted migration raises ethical concerns (Miller-Rushing and Primack 2004) and should be conducted responsibly with regard to the vegetation at transplant sites. However, as differing migration rates throw species together that have not previously co-existed, altering communities and causing extinctions (Gottfried et al. 1999), it could be argued that favouring endemic or endangered species is an exercise in damage-limitation in the face of a more extensive and pernicious manipulation of the environment. In the case of P. glaucescens, specialist techniques for seed storage, germination and cultivation are not required (Cerabolini et al. 2004), and thus ex situ conservation is likely to be both practical and economical.
Conclusions
Primula glaucescens exhibits superficial variability around an underlying conservative, cold-adapted survival strategy, and is unlikely to be able to migrate or adapt in response to rapid climate warming. Traits exhibited towards lower elevations (larger leaves, lower N contents, higher C:N and larger rosettes) are typical of alpine species growing in warmer situations with a longer growth period, and suggest that climate warming, by increasing the length of the growth season, may exacerbate the effect of edaphic stressors, ultimately leading to low nutrient availability during seed filling and reduced capacity for regeneration. This species is traditionally regarded as highly variable (for an alpine species) but in reality the underlying strategy of stress-tolerance and conservative leaf economy remains essentially unchanged throughout the climate envelope of the entire species. This suggests that other alpine specialists that cannot rely on dispersal to escape climatic changes will be faced with similar, or worse, challenges in the future. Assisted migration provides hope that endangered alpine endemics may be conserved, but this must be balanced against the risk of further anthropogenic disturbance.
References
Antonietti A (1986) Segnalazioni floristiche in Valsolda. Atti dell’Istituto di Botanica e del Laboratorio Crittogamico dell’Università di Pavia 5:53–55 (in Italian)
Arietti N, Crescini A (1976) Gli endemismi della flora Insubrica: la Primula longobarda Porta e sua posizione tassonomica nel quadro della subsect. Arthritica Schott. Natura Bresciana 13:3–32 (in Italian)
Caccianiga M, Luzzaro A, Pierce S, Ceriani RM, Cerabolini B (2006) The functional basis of a primary succession resolved by CSR classification. Oikos 112:10–20. doi:10.1111/j.0030-1299.2006.14107.x
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ et al (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848. doi:10.1038/nature00812
Cerabolini B, Ceriani RM, Caccianiga M, De Andreis R, Raimondi B (2003) Seed size, shape and persistence in soil: a test on Italian flora from Alps to Mediterranean coast. Seed Sci Res 13:75–85. doi:10.1079/SSR2002126
Cerabolini B, De Andreis R, Ceriani RM, Pierce S, Raimondi B (2004) Seed germination and conservation of endangered species from the Italian Alps: Physoplexis comosa and Primula glaucescens. Biol Conserv 117:351–356. doi:10.1016/j.biocon.2003.12.011
Ceriani RM (2003) Ecological, morpho-functional and regenerative characteristics of plant species from Prealpine grassland. PhD Thesis, University of Insubria, Italy
Ceriani RM, Perini D, De Andreis R, Brusa G, Cerabolini B (2005) Confronto della variabilità genetica tra popolazioni della specie endemica Primula glaucescens Moretti a fini conservazionistici. Informatore Botanico Ital 37(1A):196–197 (in Italian)
Ceriani RM, Pierce S, Cerabolini B (2008) Are morpho-functional traits reliable indicators of inherent relative growth rate for prealpine calcareous grassland species? Plant Biosyst 142(1):60–65. doi:10.1080/11263500701872374
Conti F, Manzi A, Pedrotti F (1997) Liste Rosse regionali delle piante d’Italia. WWF and Società Botanica Italiana, Camerino, Italy (in Italian)
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE et al (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380. doi:10.1071/BT02124
Franco AMA, Hill JK, Kitschke C, Collingham YC, Roy DB, Fox R et al (2006) Impacts of climate warming and habitat loss on extinctions at species’ low-altitude range boundaries. Glob Chang Biol 12:1545–1553. doi:10.1111/j.1365-2486.2006.01180.x
Gimalov FR, Baymiev AK, Matniyazov RT, Chemeris AV, Vakhitov VA (2004) Initial stages of low-temperature induction of cabbage cold shock protein gene csp5. Biochemistry (Mosc) 69(5):575–579. doi:10.1023/B:BIRY.0000029857.05522.d5
Gottfried M, Pauli H, Reiter K, Grabherr G (1999) A fine-scaled predictive model for changes in species distributions patterns of high mountain plants induced by climate warming. Divers Distrib 5(6):241–252. doi:10.1046/j.1472-4642.1999.00058.x
Grabherr G, Gottfried M, Pauli H (1994) Climate effects on mountain plants. Nature 369:448. doi:10.1038/369448a0
Grime JP (2001) Plant strategies, vegetation processes and ecosystem properties, 2nd edn. Wiley, Chichester, UK
Guisan A, Theurillat J-P (2000) Assessing alpine plant vulnerability to climate change: a modelling perspective. Integr Assess 1:307–320. doi:10.1023/A:1018912114948
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Glob Chang Biol 12:450–455. doi:10.1111/j.1365-2486.2006.01116.x
Huntley B, Berry PM, Cramer W, McDonald AP (1995) Modelling present and potential future ranges of some European higher plants using climate response surfaces. J Biogeogr 22:967–1001. doi:10.2307/2845830
Huntley B, Green RE, Collingham YC, Hill JK, Willis SG, Bartlein PJ et al (2004) The performance of models relating species geographical distributions to climate is independent of trophic level. Ecol Lett 7:417–426. doi:10.1111/j.1461-0248.2004.00598.x
Jump A, Woodward FI (2003) Seed production and population density decline approaching the range-edge of Cirsium species. New Phytol 160:349–358. doi:10.1046/j.1469-8137.2003.00873.x
Körner C (1999a) Alpine plants: stressed or adapted? In: Press MC, Scholes JD, Barker MG (eds) Physiological plant ecology. Blackwell Science, Oxford, pp 297–311
Körner C (1999b) Alpine plant life: functional plant ecology of high mountain ecosystems. Springer, Berlin
Körner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22(11):569–574. doi:10.1016/j.tree.2007.09.006
Lehtilä K, Syrjänen K, Leimu R, Garcia MB, Ehrlén J (2006) Habitat change and demography of Primula veris: modification of management targets. Conserv Biol 20(3):833–843. doi:10.1111/j.1523-1739.2006.00368.x
Lienert J, Fischer M (2003) Habitat fragmentation affects the common wetland specialist Primula farinosa in north-east Switzerland. J Ecol 91:587–599. doi:10.1046/j.1365-2745.2003.00793.x
Miller-Rushing AJ, Primack RB (2004) Climate change and plant conservation. Plant Talk 35:34–38
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669. doi:10.1146/annurev.ecolsys.37.091305.110100
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. doi:10.1038/nature01286
Parolo G, Rossi G (2008) Upward migration of vascular plants following a climate warming trend in the Alps. Basic Appl Ecol 9:100–107. doi:10.1016/j.baae.2007.01.005
Pauli H, Gottfried M, Grabherr G (2003) Effects of climate change on the alpine and nival vegetation of the Alps. J Mt Ecol 7(Suppl):9–12
Pierce S, Vianelli A, Cerabolini B (2005) From ancient genes to modern communities: the cellular stress response and the evolution of plant strategies. Funct Ecol 19(5):763–776. doi:10.1111/j.1365-2435.2005.01028.x
Pignatti S (1982) Flora d’Italia. Edagricole, Bologna (In Italian)
Poorter H, Van der Werf A (1998) Is inherent variation in RGR determined by LAR at low irradiance and by NAR at high irradiance? A review of herbaceous species. In: Lambers H et al (eds) Inherent variation in plant growth. Physiological mechanisms and ecological consequences. Backhuys Publishers, Leiden, Netherlands, pp 309–336
Ravazzi C (1992) Lineamenti fisionomici, ecologia e fattori edafici della vegetazione di alcuni massicci calcareo-dolomitici della prealpi lombarde. Praterie naturali e seminaturali. Natura Bresciana 27:11–42 (in Italian)
Ravazzi C (1999) Distribuzione ed ecologia di due Primule endemiche delle Prealpi calcaree meridionali, Primula glaucescens e P. spectabilis, e considerazioni sulla loro corogenesi. Arch Geobotanico 3:125–148 (in Italian)
Ravazzi C, Ferlinghetti R (1986) Analisi dei caratteri geoambientali e tassonomici di una nuova stazione disgiunta di Primula gr. glaucescens nelle alpi Orobie. Rivista del Museo Civico di Scienze Naturali “Enrico Caffi” di Bergamo 10: 79–99 (in Italian)
Reisch C, Anke A, Röhl M (2005) Molecular variation within and between ten populations of Primula farinosa (Primulaceae) along an altitudinal gradient in the northern Alps. Basic Appl Ecol 6:35–45. doi:10.1016/j.baae.2004.09.004
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60. doi:10.1038/nature01333
Sung DY, Kaplan F, Lee KJ, Guy CL (2003) Acquired tolerance to temperature extremes. Trends Plant Sci 8(4):179–187. doi:10.1016/S1360-1385(03)00047-5
Theurillat J-P, Guisan A (2001) Potential impact of climate change on vegetation in the European Alps: a review. Clim Change 50:77–109. doi:10.1023/A:1010632015572
Van Rossum F, Triest L (2003) Spatial genetic structure and reproductive success in fragmented and continuous populations of Primula vulgaris. Folia Geobot 38:239–254. doi:10.1007/BF02803196
Van Rossum F, Echchgadda G, Szabadi I, Triest L (2002) Commonness and long-term survival in fragmented habitats: Primula elatior as a case study. Conserv Biol 16(5):1286–1295. doi:10.1046/j.1523-1739.2002.01162.x
Weiher E, Van Der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O (1999) Challenging theophrastus: a common core list of plant traits for functional ecology. J Veg Sci 10:609–620. doi:10.2307/3237076
Wright IJ, Reich PB, Westoby M, Ackerley DD, Baruch Z et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827. doi:10.1038/nature02403
Acknowledgements
We thank Francesco Bedin for assistance in the field, Dr. Guido Brusa (University of Insubria) for help with the germination tests and Prof. Brian Huntley (University of Durham, UK) for comments on a draft of the manuscript. This study was supported by the Centro Flora Autoctona (Native Flora Centre) of Lombardy (Galbiate, Lecco, Italy), via the University of Insubria.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nomenclature: Pignatti (1982).
Rights and permissions
About this article
Cite this article
Ceriani, R.M., Pierce, S. & Cerabolini, B. The survival strategy of the alpine endemic Primula glaucescens is fundamentally unchanged throughout its climate envelope despite superficial phenotypic variability. Plant Ecol 204, 1–10 (2009). https://doi.org/10.1007/s11258-008-9559-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-008-9559-y