Abstract
So far very few experiments have accounted for the combined effect of two phenomena co-occurring in stress gradients: local adaptation to stress and the increase in facilitation with increasing stress (predicted by the stress-gradient hypothesis, SGH). Mountain birch (Betula pubescens subsp. czerepanovii) facilitates conspecific seedlings in subarctic high stress sites and is capable of rapid evolutionary adaptation, being therefore a good model species for a study combining local ecotypes and SGH. A within-species experiment was conducted to test SGH in three stress gradients, detect potential local adaptations between low and high stress populations, and assess their effects on seedling-seedling interactions. Although no evidence for local adaptation was detected, high and low stress populations showed some differentiation, possibly explained by decreasing phenotypic plasticity in high stress conditions and/or neutral evolutionary mechanisms. Weak support for SGH was detected. While facilitation was unaffected by seedling origin, low stress populations showed better competitive ability.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The currently recognized importance of facilitation in plant communities under abiotic stress has resulted in the establishment of a wide array of field and laboratory experiments exploring both interspecific and intraspecific interactions (Bertness and Callaway 1994; Brooker et al. 2008; Callaway 2007 and references therein). However, with the exception of a study by Espeland and Rice (2007), these experiments have not addressed variation associated with evolutionary histories of populations inhabiting contrasting environments. Since Turesson’s pioneering works (Turesson 1922, 1925), local adaptations to stress have been documented in a wealth of studies across taxa (Futuyma 2001; Linhart and Grant 1996; Savolainen et al. 2007). However, the traits advantageous in conditions of high stress are often deleterious under low stress, resulting in a trade-off of decreasing performance in non-local environments, thus facilitating the formation of locally adapted ecotypes (Futuyma 2001; Kawecki and Ebert 2004). Evolutionary adaptations can have significant effects on plant-plant interactions, of which the decreasing competitive ability of stress adapted genotypes is a common example (Eränen 2008; Kawecki and Ebert 2004; Linhart and Grant 1996). Recently, differences in stress tolerance (and related optima in growth conditions) among species have been shown to affect also positive interactions (facilitation), with species susceptible to stress usually benefiting most from neighbouring plants (Choler et al. 2001; Liancourt et al. 2005). Adopting this field of thought to a within-species context, we may expect weaker facilitative responses on ecotypes adapted to stress (Espeland and Rice 2007).
The stress-gradient hypothesis (SGH), first phrased by Bertness and Callaway (1994), suggests that the relative importance of facilitation will increase with increasing stress and decreasing productivity. This can happen either via increasing facilitation (Bertness and Callaway 1994; Callaway et al. 2002) or decreasing competition in high stress habitats (Dormann and Brooker 2002; Grime 1973). SGH has received support from various environmental gradients (Brooker et al. 2008; Callaway 2007; Callaway et al. 2002; Gómez-Aparicio et al. 2008). However, several studies have failed to detect changes in plant-plant interactions as predicted by SGH (Armas and Pugnaire 2005; Casper 1996; Donovan and Richards 2000), generating considerable debate on the generality of the hypothesis (Brooker et al. 2008; Callaway 2007; Lortie and Callaway 2006; Maestre et al. 2005, 2006). Some part of the variation in outcomes of earlier experiments was recently demonstrated to result from the failure to impose stress on experimental plants (Lortie and Callaway 2006). We hypothesize that also among-population differentiation may have contributed to inconsistency in the results of earlier experiments.
Mountain birch (Betula pubescens subsp. czerepanovii (Orlova) Hämet-Ahti) is a good candidate for studying stress-related hypotheses. As a timberline species it can survive in highly stressful study sites, and is known to have evolved ecotypes in relation to altitude (Weih and Karlsson 1999) and heavy metal pollution (Eränen 2008; Eränen et al. unpublished). In previous studies in the highly stressful subarctic environments of the Kola Peninsula, NW Russia, we have shown that adult mountain birches facilitate conspecific seedlings and dwarf shrubs (Eränen and Kozlov 2007, 2008; Zvereva and Kozlov 2004). However, earlier experiments did not address effects of mountain birch ecotypes (originated from high vs. low stress conditions) on intraspecific interactions in contrasting environments.
Here we report the results of a four-year experiment performed in three different gradients of abiotic stress, one anthropogenic and two natural, in the Kola Peninsula, NW Russia. The effects of stress on mountain birch were verified with analyses of survival, growth and chlorophyll fluorescence in each study gradient. The questions we aimed to answer with this study were: (1) do birch seedlings planted at a 10 cm distance interact with each other? (2) Do these interactions (if any) differ between contrasting (low vs. high stress) environments? The importance of competition was expected to decrease and the role on facilitation was expected to increase with increasing stress, resulting in a stronger positive net effect. The experiment was replicated in three different gradients of abiotic stress to (3) check the generality of the putative changes in plant-plant interactions. Seedlings from both the high and low stress ends of the study gradients were included to (4) find out if the high stress populations are adapted to abiotic stress and have these adaptations resulted in trade-offs of reduced performance in pristine conditions. The seedlings were planted either with seedlings from their own growth environments, or seedlings from the opposite end of the gradient. This was done to (5) check whether the putative adaptations to stress affect competitive and facilitative interactions between mountain birch seedlings.
Materials and methods
Study sites
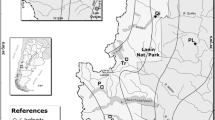
The potential adaptations to abiotic stress and the effects of stress on seedling-seedling interactions were studied in the high (H in codes of study sites) and low (L) stress ends of three stress gradients: one anthropogenic (pollution, P) gradient and two natural (elevation, E and seashore, S) environmental gradients in the Kola Peninsula, NW Russia (Table 1). There were a total of six study sites.
In the pollution gradient, the high stress site (PH) was located 7 km S and the control site (PL) 40 km S of the copper-nickel smelter in Monchegorsk (67°55′ N, 32°48′ E), central Kola Peninsula. In the vicinity of Monchegorsk 70 years of pollution impact has resulted in nearly total elimination of plant life, with sparsely growing stunted (1–2 m tall) mountain birches and willows (Salix spp.) dominating the previously healthy subarctic forest, now transformed into industrial barrens (Kozlov and Zvereva 2007; Kryuchkov 1993). Also field layer vegetation (Table 1) and uppermost soil layers in PH are all but destroyed (Kryuchkov 1993). Due to forest decline the barrens, along with pollution, suffer from climatic stress, mainly greater wind speeds (Table 1) and increased temperature fluctuations (Kozlov 2001; Kozlov and Haukioja 1998).
The natural stress gradients were located in the White Sea shore in southern Kola Peninsula near Olenitsa (66°28′ N, 35°12′ E), and in the Khibiny mountains in central Kola Peninsula in the south facing hill of the Lovchorr mountain (67°35′ N, 33°45′ E). The elevation gradient high stress site (EH) was located 100 m above the timberline, while the low stress site (EL) was located well below the timberline (Table 1). The seashore gradient high stress site (SH) was located some 50 m from the shoreline, while the low stress site (SL) was situated some 300 m inland. Both high stress sites (EH and SH) were open tundra-like habitats characterized by sparsely growing low-stature (1–2 m tall) trees (mainly mountain birch and willows). In contrast to the PH site, field layer vegetation in both EH and SH sites was abundant, not differing much from the respective low stress sites (Table 1). Mean wind speeds in the open high stress sites were up to 15 times higher than in the respective low stress sites (Table 1). The low stress sites (PL, EL and SL) were selected in healthy looking subarctic forests of high stature (>5 m tall) mountain birch, Siberian spruce (Picea abies subsp. obovata Ledeb.), and Scots pine (Pinus sylvestris L.) (PL only). For a more detailed description of study sites consult Eränen (2008), Eränen and Kozlov (2008) and Ruotsalainen et al. (2008).
Experimental design and measured variables
The study species, mountain birch, has been a foundation species in northern Fennoscandia since the last ice age (Aas and Faarlund 2001). Considering the vulnerability of the subarctic regions to the projected climate change (Anisimov et al. 2007), knowledge of ecological factors affecting mountain birch performance, e.g. competition, facilitation and local adaptation, are crucial for predicting the future of subarctic ecosystems (Wipf et al. 2006; Wolf et al. 2008).
Birch seeds were collected in October 2002 from five mother trees in each study site, 30 mother trees in total. The seeds were germinated in April 2003 and grown for 14 months in identical conditions (outdoors during summer and in a semi-enclosed space during hibernation) to minimize potential maternal effects. The seedlings were planted in early-mid June 2004, seedlings from each gradient in both sites of their respective gradients. Planting height was measured to serve as a covariate in further analyses. The amount of artificial soil inserted during planting was kept at a minimum, and potentially suppressing ground vegetation was cut up to 5 cm from the seedlings. Care was taken to distribute seedlings from different mother trees and of different sizes randomly to different treatments.
Five blocks were created in each end (low and high stress) of the pollution and seashore gradients, and three blocks were created in each end of the elevation gradient. Each block was subdivided into three sections consisting of two single growing seedlings (‘single’ from now on), a seedling pair (‘paired’ from now on) and a circle of eight seedlings (‘grouped’ from now on). The distance between seedlings in paired and grouped treatments was circa 10 cm. The putative competitive and facilitative interactions were expected to be stronger in groups of increasing size (single < paired < grouped; ‘group size’ from now on). In the first section we planted only seedlings from the high stress end of the respective study gradient, and in the second section we planted seedlings from the low stress end (‘stress at origin’ from now on). This was done to study potential adaptations to local growth environments. In the third section we used seedlings from both ends of the respective stress gradients. The pair included seedling from both origins, and the group of eight included four seedlings from low and four from high stress origins planted, so that each seedling was located between seedlings from the other end of the gradient. Single growing seedlings were not included in this section, because it was designed to study interactions. The different sections are from now on referred to as ‘competitor origin, local or non-local’. The aim was to study potential differences in competitive and facilitative abilities with respect to stress and stress at origin. Altogether there were 34 seedlings per block, with 884 seedlings in the entire experiment.
Seedling survival was monitored five times during the experiment: in August 2004, June and August 2005, August 2006 and June 2007. Seedlings were monitored in June and August because they represent roughly the beginning and end of the growth season in the Kola Peninsula. In addition to survival, performance indices were measured three times, between August 8th and 15th 2004, July 29th and August 5th 2005, and August 1st and 9th 2006. The fitness-related variables measured were seedling height (to the nearest 5 mm, up to the tip of the uppermost bud), the lengths of two largest leaves (to the nearest 1 mm, excluding the petiole) and chlorophyll fluorescence. Because leaf growth had already started before planting in field conditions in 2004, leaf length was not measured during that year. Average leaf lengths were calculated for each seedling at each time point for the statistical analyses. Chlorophyll fluorescence was measured using a portable plant stress meter (Biomonitor S.C.I. AB., Umeå, Sweden). The indices measured were the ratio of variable to maximum fluorescence (Fv/Fm) yielded under an artificial light treatment (200 μmol photons m−2 s−1) and the time needed for the leaf to reach half of its Fm (T1/2). The leaves were dark adapted with a lightweight cuvette for 15 min prior to measuring to ensure maximum level of fluorescence. Greater seedling survival, height, leaf length and Fv/Fm and smaller T1/2 values were considered to indicate less stress and higher fitness.
Quantitative estimates for phenotypic plasticity were calculated for both relative growth rate (the difference between height in 2006 and planting height divided by planting height) and leaf length (data of 2006) in each gradient and stress at seedling origin by using the relative distances plasticity index (RDPI), ranging from 0 (no plasticity) to 1 (maximum plasticity) (Valladares et al. 2006). Individual distances between seedlings growing in contrasting environments were calculated within families (mother trees) whenever possible (above 50% of cases), otherwise, when no individuals from the same family were available in the opposing environment, distances were calculated between seedlings from different mothers. Seedlings expressing abnormal growth (due to herbivory, human disturbance, etc.) and statistical outliers were omitted from the calculations.
Statistical analyses
All the measured performance variables (seedling height, leaf length, Fv/Fm and T1/2) were analysed with repeated analyses of covariance (ANCOVA) with measurement year as the repeated factor and planting height as the covariate. Block (nested within study site) and mother tree were used as random variables.
In the first analyses we aimed to find potential local adaptations and changes in plant-plant interactions in conditions of varying abiotic stress. Gradient, stress (high vs. low), stress at seedling origin (high vs. low) and group size (single vs. paired vs. grouped) were used as fixed explanatory variables. Also all their second level interactions were included. Because single growing seedling obviously had no within-species competitors, competitor origin was omitted from these analyses.
In the second analyses all performance variables were analysed with a repeated ANCOVA as above, while adding an interaction between stress, stress at origin and competitor origin (against local vs. non-local seedlings). Only grouped seedlings were analysed here, because single seedlings faced no within-species competition, and many of paired seedlings lost their potential competitors in the course of the experiment. Pairwise comparisons were done with least squares means in each analysis. At first all gradients were included in the analyses, but since the majority of variables showed no increase in stress in the PH site (see below), the pollution gradient was omitted from the final analyses.
One-way ANOVA was used to test population differences in plasticity (quantified with the relative distances plasticity index, RDPI) of each growth variable, with stress at origin as the fixed explanatory variable. The ANOVA was conducted separately for each gradient. All ANOVAs and ANCOVAs were performed with procedure MIXED in SAS Institute 9.1 (SAS Institute Inc., Cary, NC, USA) (Littell et al. 1996).
Survival analyses were conducted with a Cox regression (Cox 1972). The gradients were analysed separately to keep the amount of variables manageable. Stress, stress at origin, group size and their interactions were used as explanatory variables. The survival analyses were conducted with procedure PHREG in SAS Institute 9.1 (Allison 1995).
Results
Verifying stress effects
In the PH site seedlings were taller (136.4 ± 7.4 vs. 82.8 ± 7.4 mm, P < 0.0001), had longer leaves (25.3 ± 1.3 vs. 15.2 ± 1.2 mm, P < 0.0001) and had higher survival (P < 0.0001; Fig. 3a) than in the PL site; chlorophyll fluorescence indices did not differ between these sites (results in text given as least squares means ± SE). Seedling origin had no effect on mountain birch performance in either study site (PH or PL). Since three out of five variables showed effects opposite to those expected in a potentially stressful environment, the pollution gradient was discarded from the analyses of plant-plant interactions. In the other two gradients (elevation and seashore) the effects were as expected (Figs. 1 and 2, Table 2): stress adversely affected each performance variable except seedling height (16% shorter leaves, 13% lower Fv/Fm values and 50% higher T1/2 values). Also survival was significantly lower in the high stress sites (χ2 = 39.6, P < 0.0001, Hazard ratio = 0.666; Fig. 3b, c). With the majority of variables showing lower performance at higher levels of abiotic stress, both natural gradients were included in further analyses.
Survival of mountain birch seedlings in different conditions of abiotic stress in the (a) pollution, (b) elevation and (c) seashore gradients with respect to stress at origin (note: in Fig. 3a the high stress site seedlings from both stress origins have similar overlapping survival curves)
Treatment effects and population differences
Stress at origin had a significant effect on seedling height, but only in the elevation gradient (G × O in Table 2): low stress origin seedlings were 13% taller than high stress origin seedlings. Also, group size had a significant effect on seedling height (Table 2), but this effect was only significant in the elevation gradient (G × GS in Table 2): grouped seedlings (75.9 ± 1.7 mm) were shorter than seedlings planted single (85.8 ± 3.1 mm, P = 0.0012 vs. grouped) or in pairs (82 ± 2.6 mm, P = 0.0180 vs. grouped). The stress × stress at origin interaction was significant for leaf length and marginally significant for seedling height (Table 2). Low stress origin seedlings were taller and had longer leaves than high stress origin seedlings in the low stress sites (Fig. 1). Also the stress × group size interaction was significant for leaf length (Table 2); in low stress sites single seedlings had the largest leaves, and in high stress sites paired seedlings had the largest leaves (Fig. 2).
In the second analyses the competitor origin × stress at origin × stress interaction was significant for seedling height (P = 0.0190) and leaf length (P < 0.0001). Both variables showed superior performance for low stress origin seedlings in low stress sites when competing against high stress origin seedlings (height: 88 ± 2.8 vs. 79 ± 2.2 mm, P = 0.0028, leaf length: 19 ± 0.9 vs. 17.4 ± 0.8 mm, P = 0.0282, respectively, against high and low stress origin seedlings). In the high stress sites seedlings had longer leaves when competing against non-local seedlings, irrespective of stress at origin. High stress origin seedlings had longer leaves when competing against low stress origin seedlings (15.5 ± 1.2 vs. 11.5 ± 0.9 mm, P = 0.0004), and low stress origin seedlings had longer leaves when competing against high stress origin seedlings (11.9 ± 1 vs. 15.1 ± 1.1 mm, P = 0.0021).
Stress at origin had no effect on phenotypic plasticity in the seashore gradient, but in the elevation gradient leaf length showed 58% higher plasticity (according to the relative distances plasticity index, RDPI) for low stress origin seedlings than high stress origin seedlings (0.262 ± 0.030 vs. 0.166 ± 0.027, P = 0.0207).
In the survival analyses no effects (apart from stress) were significant in the elevation gradient (Fig. 3b). In the seashore gradient the interaction between stress and stress at origin was significant (χ2 = 6.06, P = 0.0138, Hazard ratio = 0.83). In the SH site stress at origin had no effect on survival (χ2 = 0.69, P = 0.40, Hazard ratio = 1.078), but in the SL site high stress origin seedlings were 35% more likely to die between monitoring rounds than low stress origin seedlings (χ2 = 6.17, P = 0.013, Hazard ratio = 0.739; Fig. 3c).
Discussion
Stressful environments and biotic stress
Since both elevation and seashore gradients showed decreasing performance of mountain birch seedlings with increasing stress, they (in agreement with Lortie and Callaway 2006) were considered suitable for studies on the impact of stress on plant-plant interactions.
Surprisingly, the harsh abiotic environment of the industrial barrens near Monchegorsk did not impose stress on mountain birch seedlings. This was surprising, especially as the barrens around the Severonikel smelter are a focal study area for pollution ecology, and have been a host to several stress-related studies where plant mortality and performance have been as expected (see for example papers by Eränen and Kozlov (2006, 2007), Kozlov and Zvereva (2007), Zvereva and Kozlov (2004) and references therein). The lack of a stress effect on birch seedlings in PH is likely due to a combination of several factors. First, pollution impact has destroyed practically all field layer vegetation (Table 1), creating an allelopathy free and competition free space in the high stress site (PH), and likely resulting in a substantial increase in seedling performance. Second, the emissions from the Severonikel smelter have been in dramatic decline during the past decade (from 232 600 t of SO2 in 1990 to 41 000 t in 2005: Berlyand 1991; Milyaev and Yasenskij 2006), hinting that stress from ambient SO2 and heavy metals during the current experiment (2004–2007) may have been much lower than during the previous experiments (conducted during the 1990 s and early 2000 s). Changes in seedling survival support the conclusion; survival of unprotected seedlings in PH in experiments conducted during 2000–2005 was circa 40–50% (Eränen and Kozlov 2006, 2007), whereas in the current experiment survival was close to 100%. Lack of field layer competition together with decreasing pollution impact may explain the surprising switch in birch performance in the pollution gradient. The performance of heavy metal adapted seedlings (see Eränen 2008) in the PH site was similar to that of control seedlings, providing further evidence on the declining effect of direct pollution around Monchegorsk. As the studied pollution gradient clearly no longer shows decreasing plant performance towards the high stress end, we disregard the gradient as unsuitable for SGH studies and concentrate on the two natural gradients. The disparity between biotic and abiotic measurements of stress support the argument that biotic measurements are needed to verify the actual stress experienced by plants (Lortie and Callaway 2006). However, this does not negate the results of earlier studies in the Severonikel pollution gradient, when environmental conditions have been different.
Seedling-seedling interactions
The seedling-seedling interactions detected in this study were weak and like in a previous study (Eränen and Kozlov, 2008), predominantly neutral or negative. Only leaf length data gave some support for SGH, with paired seedlings outperforming the other group sizes in high stress sites (Fig. 2). It may be that seedling-seedling facilitation increases the performance of paired seedlings compared to single seedlings, but in groups of eight competition dominates even in high stress sites, resulting in weaker performance. In the low stress sites competition dominated, with single growing seedling outperforming paired and grouped seedlings (Fig. 2), in line with the hypothesis that competition intensity decreases with increasing stress (Bertness and Callaway 1994; Dormann and Brooker 2002; Grime 1973). The dominance of low over high stress origin seedlings in low (but not high) stress sites also hints on greater importance of competition in low stress environments, substantiating the results of a previous study (Eränen and Kozlov, 2008).
The dominance of competition in seedling-seedling interactions can be explained by the similar size and developmental status of the experimental plants. It is logical that individuals of similar size and same species should share similar resource requirements, resulting in competition for both light and nutrients. The weak seedling-seedling facilitation is likely a result of too large planting distance (Dickie et al. 2005; Eränen and Kozlov 2007) relative to the size attained by the seedlings by the end of the experiment. Due to their small size, the shelter provided by individual (or even groups of) mountain birch seedlings may have been too minor (either in effect or space) to mitigate the abiotic stress. In previous studies the strongest positive net effect of adult mountain birches was detected at a planting distance of 25 cm, i.e. less than one-fourth of benefactor height (Eränen and Kozlov 2007). In the current experiment planting distance and mean seedling height at the end of the experiment were about the same (10 cm). It may thus take several more years before positive interactions between the seedlings manifest as more visible differences in performance.
Population differentiation among levels of abiotic stress
Both growth variables (height and leaf length) showed a significant interaction between stress and stress at origin (Fig. 1), indicating differentiation between birch populations from different environments. Also survival showed a similar effect for seashore gradient seedlings (Fig. 3c). However, the stress × stress at origin interactions were, in each case, result of selection against high stress origin seedlings in low stress environments (Figs. 1 and 3). This response is similar to trade-offs often detected in relation to stress adaptations (Eränen 2008; Futuyma 2001; Savolainen et al. 2007), but is in itself not proof enough of local adaptation (Kawecki and Ebert 2004). The lack of a home-site advantage is in contrast with a large body of experiments where plants from different elevations have shown local adaptations to their home environments (Byars et al. 2007; Ohsawa and Ide 2008; Savolainen et al. 2007 and references therein). The result to a certain extent disagrees also with outcomes of previous experiments by our group (partially involving seeds from the same mother trees), which have shown that mountain birch is capable of very rapidly adapting to heavy metal stress (Eränen 2008; Eränen et al. unpublished). Factors favouring generalization (as opposed to adaptation) in the studied natural gradients include weak selection pressure (compared to the selection history in the pollution gradient), strong pollen-driven gene flow as well as possibly great year-to-year variation in abiotic conditions. An important thing to note, however, is the strong positive effect of nurse trees on mountain birch seedlings, especially in exposed habitats (Eränen and Kozlov 2008). Refuges created (on ‘niches constructed’) by adult conspecifics might override the need to adapt to wind-induced stress (Laland et al. 1999; Odling-Smee et al. 1996), effectively opposing forces driving divergent selection, while nurse trees cannot create similar refuges against pollution stress (with increasing pollution loads often detected under canopies, see Lukina and Nikonov (1999) and Ginocchio et al. (2004)). However, because selection pressure can vary with time and between life-history stages (Geber and Griffen 2003), it is possible that the high stress origin seedlings might be selected for in high stress sites during other, more stressful, years or during later life-history stages.
Also the possibility that high stress origin seedlings are indeed not selected for in the high stress sites, and only selected against in the low stress sites, needs to be considered. Intuitively, such a response should be maladaptive, and disappear during the course of evolution. However, there are some mechanisms that could cause such an unexpected result. First, high phenotypic plasticity, which is the capacity of a single genotype to produce different phenotypes in different environments (Kingsolver et al. 2002; Via et al. 1995), can be detrimental in conditions of severe directional selection, when any deviation from the optimal phenotype is selected against (Emery et al. 1994; Heschel et al. 2004; Taylor and Aarssen 1988). This can result in a population of very low phenotypic variation, creating a selective disadvantage in non-native environments. RDPI showed lower plasticity for leaf length in progenies of mountain birches from the high elevation site (EH). This could indicate that the putative “trade-off” in the elevation gradient is due to the elimination of plastic genotypes in the high stress site. Finally, adaptively neutral mechanisms, i.e. population bottlenecks and genetic drift, can change the genetic composition of populations especially in cases of high random mortality (Futuyma 2001; Kawecki and Ebert 2004; Turelli et al. 2001). It is possible that in the seashore gradient, with 80% mortality during the experiment in the SH site, neutral evolutionary mechanisms have resulted in random mutations causing population differentiation despite the possibly balancing effects of gene flow.
Effects of population differentiation on seedling-seedling interactions
No interactions between group size and seedling origin were detected. This finding indicates that population differences do not affect the competitive ability or facilitative response of mountain birch seedlings as such, conflicting with the results by Espeland and Rice (2007). That stress at origin had no effect on the facilitative responses of the seedlings is no surprise, considering the weak facilitative effects detected in the current study. Though seedlings of different origin did not vary in their response to competition level, competitor origin had some effect. Low stress origin seedling in low stress conditions were higher and had longer leaves when interacting with high stress origin seedlings. This might indicate a trade-off of decreased competitive ability of high stress origin seedlings (Eränen 2008; Kawecki and Ebert 2004; Linhart and Grant 1996). However, the high stress origin seedlings themselves showed no differences in performance with respect to competitors in the low stress sites. The result must therefore be interpreted with caution. In high stress sites both growth variables, height and leaf length, indicated better performance for seedlings when competing against non-local seedlings. However, barring competitor origin, no differences were detected between seedlings of different origin. This could indicate that there are no population differences in competitive ability as such in high stress sites, but that there is some difference in resource acquisition, resulting in lower levels of competition against non-local seedlings.
Conclusions
In summary, interactions between mountain birch seedlings yielded only weak support for the stress-gradient hypothesis. That only marginal increase in facilitation was detected with increasing stress can be explained by the small size and related weak shelter effect of the studied seedlings. Although no evidence for local adaptation was detected, populations from high and low stress environments showed some degree of differentiation, possibly explained by decreasing phenotypic plasticity in high stress conditions and/or neutral evolutionary mechanisms, i.e. population bottlenecks and genetic drift. These populations also differed in competitive ability and possibly resource acquisition, but facilitation remained unaffected by seedling origin.
The results suggest that in spite of the capacity of mountain birch for rapid evolution (Eränen 2008; Eränen et al. unpublished), long lasting stress does not necessarily result in adaptation. Also, even though sheltering and facilitation by mountain birch may be useful tools in reforestation (Eränen and Kozlov 2006, 2007), it is important to properly select benefactor size and planting distance; planting nurse trees that are too small and/or distant may result in competitive net effects, thus hampering restoration efforts.
References
Aas B, Faarlund T (2001) The holocene history of the Nordic mountain birch belt. In: Wielgolaski FE (ed) Nordic mountain birch ecosystems. The Parthenon Publishing Group, New York, USA, pp 5–22
Allison PD (1995) Survival analysis using the SAS system: A practical guide. SAS Institute Inc., Cary, NC, USA
Anisimov OA, Vaughan DG, Callaghan TV, et al. (2007) Polar regions (Arctic and Antarctic). In: Parry ML, Canziani OF, Palutikof JP et al. (eds) Climate change 2007: impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, UK, pp 653–685
Armas C, Pugnaire FI (2005) Plant interactions govern population dynamics in a semi-arid plant community. J Ecol 93:978–989. doi:10.1111/j.1365-2745.2005.01033.x
Berlyand ME (1991) Annual report of ambient air pollution in cities and industrial centres of Soviet Union. Volume “emission of pollutants”. Voeikov Main Geophysical Observatory, St. Petersburg, Russia (in Russian)
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193. doi:10.1016/0169-5347(94)90088-4
Brooker RW, Maestre FT, Callaway RM et al (2008) Facilitation in plant communities: The past, the present, and the future. J Ecol 96:18–34
Byars SG, Papst W, Hoffmann AA (2007) Local adaptation and cogradient selection in the alpine plant, Poa hiemata, along a narrow altitudinal gradient. Evolution Int J Org Evolution 61:2925–2941. doi:10.1111/j.1558-5646.2007.00248.x
Callaway RM (2007) Positive interactions and interdependence in plant communities. Springer, Dordrecht, Netherlands
Callaway RM, Brooker RW, Choler P et al (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848. doi:10.1038/nature00812
Casper BB (1996) Demographic consequences of drought in the herbaceous perennial Cryptantha flava: Effects of density, associations with shrubs, and plant size. Oecologia 106:144–152. doi:10.1007/BF00328593
Choler P, Michalet R, Callaway RM (2001) Facilitation and competition on gradients in alpine plant communities. Ecology 82:3295–3308
Cox DR (1972) Regression models and life-tables. J R Stat Soc B Stat Methodol 34:187–220
Dickie IA, Schnitzer SA, Reich PB et al (2005) Spatially disjunct effects of co-occurring competition and facilitation. Ecol Lett 8:1191–1200. doi:10.1111/j.1461-0248.2005.00822.x
Donovan LA, Richards JH (2000) Juvenile shrubs show differences in stress tolerance, but no competition or facilitation, along a stress gradient. J Ecol 88:1–16. doi:10.1046/j.1365-2745.2000.00411.x
Dormann CF, Brooker RW (2002) Facilitation and competition in the high Arctic: The importance of the experimental approach. Acta Oecol-Int J Ecol 23:297–301. doi:10.1016/S1146-609X(02)01158-X
Emery RJN, Chinnappa CC, Chmielewski JG (1994) Specialization, plant strategies, and phenotypic plasticity in populations of Stellaria longipes along an elevational gradient. Int J Plant Sci 155:203–219. doi:10.1086/297160
Eränen JK (2008) Rapid evolution towards heavy metal resistance by mountain birch around two subarctic copper-nickel smelters. J Evol Biol 21:492–501. doi:10.1111/j.1420-9101.2007.01491.x
Eränen JK, Kozlov MV (2006) Physical sheltering and liming improve survival and performance of mountain birch seedlings: A 5-year study in a heavily polluted industrial barren. Restor Ecol 14:77–86. doi:10.1111/j.1526-100X.2006.00107.x
Eränen JK, Kozlov MV (2007) Competition and facilitation in industrial barrens: Variation in performance of mountain birch seedlings with distance from nurse plants. Chemosphere 67:1088–1095. doi:10.1016/j.chemosphere.2006.11.048
Eränen JK, Kozlov MV (2008) Increasing intraspecific facilitation in exposed environments: consistent results from mountain birch populations in two subarctic stress gradients. Oikos. doi:10.1111/j.2008.0030-1299.16772.x
Espeland EK, Rice KJ (2007) Facilitation across stress gradients: The importance of local adaptation. Ecology 88:2404–2409. doi:10.1890/06-1217.1
Futuyma DJ (2001) Ecological specialization and generalization. In: Fox CW, Roff DA, Fairbairn DJ (eds) Evolutionary ecology–Concepts and case studies. Oxford University Press, New York, USA, pp 177–189
Geber MA, Griffen LR (2003) Inheritance and natural selection on functional traits. Int J Plant Sci 164:S21–S42. doi:10.1086/368233
Ginocchio R, Carvallo G, Toro I et al (2004) Micro-spatial variation of soil metal pollution and plant recruitment near a copper smelter in Central Chile. Environ Pollut 127:343–352. doi:10.1016/j.envpol.2003.08.020
Gómez-Aparicio L, Zamora R, Castro J et al (2008) Facilitation of tree saplings by nurse plants: Microhabitat amelioration or protection against herbivores? J Veg Sci 19:161–172
Grime JP (1973) Competitive exclusion in herbaceous vegetation. Nature 242:344–347. doi:10.1038/242344a0
Heschel MS, Sultan SE, Glover S et al (2004) Population differentiation and plastic responses to drought stress in the generalist annual Polygonum persicaria. Int J Plant Sci 165:817–824. doi:10.1086/421477
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241. doi:10.1111/j.1461-0248.2004.00684.x
Kingsolver JG, Pfennig DW, Servedio MR (2002) Migration, local adaptation and the evolution of plasticity. Trends Ecol Evol 17:540–541. doi:10.1016/S0169-5347(02)02641-1
Kozlov MV (2001) Snowpack changes around a nickel-copper smelter at Monchegorsk, northwestern Russia. Can J Res 31:1684–1690. doi:10.1139/cjfr-31-10-1684
Kozlov MV, Haukioja E (1998) Microclimate changes along a strong pollution gradient in northern boreal forest zone. In: Uso JL, Brebbia CA, Power H (eds) Ecosystems and sustainable development. Computational Mechanics Publications, Southampton, UK, pp 603–614
Kozlov MV, Zvereva EL (2007) Industrial barrens: Extreme habitats created by non-ferrous metallurgy. Rev Environ Sci Biotechnol 6:231–259. doi:10.1007/s11157-006-9117-9
Kryuchkov VV (1993) Extreme anthropogenic loads and the northern ecosystem condition. Ecol Appl 3:622–630. doi:10.2307/1942095
Laland KN, Odling-Smee FJ, Feldman MW (1999) Evolutionary consequences of niche construction and their implications for ecology. Proc Natl Acad Sci USA 96:10242–10247. doi:10.1073/pnas.96.18.10242
Liancourt P, Callaway RM, Michalet R (2005) Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology 86:1611–1618. doi:10.1890/04-1398
Linhart YB, Grant MC (1996) Evolutionary significance of local genetic differentiation in plants. Annu Rev Ecol Syst 27:237–277. doi:10.1146/annurev.ecolsys.27.1.237
Littell RC, Milliken GA, Stroup WW et al (1996) SAS system for mixed models. SAS Institute Inc., Cary, North Carolina, USA
Lortie CJ, Callaway RM (2006) Re-analysis of meta-analysis: Support for the stress-gradient hypothesis. J Ecol 94:7–16. doi:10.1111/j.1365-2745.2005.01066.x
Lukina NV, Nikonov VV (1999) Pollution-induced changes in soils subjected to intense air pollution. In: Nikonov VV, Koptsik GN (eds) Acidic deposition and forest soils. Kola Science Centre, Apatity, Russia, pp 79–126 (in Russian)
Maestre FT, Valladares F, Reynolds JF (2005) Is the change of plant-plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J Ecol 93:748–757. doi:10.1111/j.1365-2745.2005.01017.x
Maestre FT, Valladares F, Reynolds JF (2006) The stress-gradient hypothesis does not fit all relationships between plant-plant interactions and abiotic stress: further insights from arid environments. J Ecol 94:17–22. doi:10.1111/j.1365-2745.2005.01089.x
Milyaev VB, Yasenskij AN (2006) Annual report on emissions of pollutants into the atmosphere in cities and regions of the Russian Federation in 2005. Institute of Ambient Air Protection, St. Petersburg, Russia (in Russian)
Odling-Smee FJ, Laland KN, Feldman MW (1996) Niche construction. Am Nat 147:641–648. doi:10.1086/285870
Ohsawa T, Ide Y (2008) Global patterns of genetic variation in plant species along vertical and horizontal gradients on mountains. Glob Ecol Biogeogr 17:152–163. doi:10.1111/j.1466-8238.2007.00357.x
Ruotsalainen AL, Markkola AM, Kozlov MV (2008) Mycorrhizal colonisation of mountain birch (Betula pubescens ssp. czerepanovii) along three environmental gradients: does life in harsh environments alter plant-fungal relationships? Environ Monit Assess. doi:10.1007/s10661-007-0152-y
Savolainen O, Pyhäjärvi T, Knurr T (2007) Gene flow and local adaptation in trees. Annu Rev Ecol Evol Syst 38:595–619
Taylor DR, Aarssen LW (1988) An interpretation of phenotypic plasticity in Agropyron repens (Graminae). Am J Bot 75:401–413. doi:10.2307/2443987
Turelli M, Barton NH, Coyne JA (2001) Theory and speciation. Trends Ecol Evol 16:330–343. doi: 10.1016/S0169-5347(01)02177-2
Turesson G (1922) The species and the variety as ecological units. Hereditas 3:100–113
Turesson G (1925) The plant species in relation to habitat and climate. Hereditas 6:147–236
Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116. doi:10.1111/j.1365-2745.2006.01176.x
Via S, Gomulkiewicz R, De Jong G et al (1995) Adaptive phenotypic plasticity - Consensus and controversy. Trends Ecol Evol 10:212–217. doi:10.1016/S0169-5347(00)89061-8
Weih M, Karlsson PS (1999) Growth response of altitudinal ecotypes of mountain birch to temperature and fertilisation. Oecologia 119:16–23. doi:10.1007/s004420050756
Wipf S, Rixen C, Mulder CPH (2006) Advanced snowmelt causes shift towards positive neighbour interactions in a subarctic tundra community. Glob Change Biol 12:1496–1506. doi:10.1111/j.1365-2486.2006.01185.x
Wolf A, Kozlov MV, Callaghan TV (2008) Impact of non-outbreak insect damage on vegetation in northern Europe will be greater than expected during the changing climate. Clim Change 87:91–106. doi:10.1007/s10584-007-9340-6
Zvereva EL, Kozlov MV (2004) Facilitative effects of top-canopy plants on four dwarf shrub species in habitats severely disturbed by pollution. J Ecol 92:288–296. doi:10.1111/j.0022-0477.2004.00854.x
Acknowledgments
We wish to thank V. Zverev, A. Vassiliev, L. Zvereva and D. Kozlov for their help during fieldwork. Thanks are also due to A. Koryak for initiating the growth of the experimental seedlings and for pre-experiment seedling maintenance. Two anonymous referees receive our gratitude for their useful comments. The Polar-Alpine Botanical Garden and Institute and the botanical garden of the Saint Petersburg Forest Technical Academy provided the space and resources needed for seed and seedling storage. Funding was provided by the Academy of Finland (projects nr 201991 and 211734) and the Finnish Cultural Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eränen, J.K., Kozlov, M.V. Interactions between mountain birch seedlings from differentiated populations in contrasting environments of subarctic Russia. Plant Ecol 200, 167–177 (2009). https://doi.org/10.1007/s11258-008-9441-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-008-9441-y