Abstract
The genetic and fitness consequences of habitat fragmentation on the dry grassland species Anthericum liliago L. (Anthericaceae) were examined. We used random amplified polymorphic DNA (RAPD) markers to determine the distribution of genetic diversity within and among 10 German A. liliago populations, ranging in size from 116 to over 2 million ramets. The genetic diversity of an A. liliago population was highly positively correlated with its population size. The overall differentiation among populations (Phi-ST = 0.41, P < 0.0001, AMOVA) was considerably higher than expected for a species with a mixed breeding system. No strong correlation (P < 0.01) was detected between fitness parameters and population size and genetic diversity. The reproductive output (seeds per ramet) was only highly correlated (P < 0.001) with the proportion of flowering ramets in a population which could be caused by a more effective pollination in large populations which are more attractive to specialized pollinators. The specialized A. liliago pollinator Merodon rufus (Syrphidae) and high abundances of solitary bees could only be found in A. liliago populations with more than 10,000 individuals. Genetic differentiation among the investigated A. liliago populations may have been caused by limited seed and pollen dispersal and a mixed mating system permitting a high selfing rate. The differentiation among the small and isolated populations lacking main pollinators seems to be caused by genetic drift.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat fragmentation, the reduction of a continuous habitat into several smaller spatially isolated remnants, reduces the size and increases the spatial isolation of plant populations (Young et al. 1996). Small population size and isolation may have a number of negative effects on populations (e.g. Kery et al. 2000; Brys et al. 2004; Leimu et al. 2006). It is generally assumed that genetic diversity is reduced in small populations (e.g. Frankham 1996). Small and isolated populations may be subjected to genetic erosion and increasing genetic divergence among populations (Culley and Grubb 2003) through random genetic drift, increased levels of inbreeding and reduced gene flow (Llorens et al. 2004). Habitat fragmentation and isolation lead to changes in the quantity and quality of pollination (Kwak et al. 1998; Steffan-Dewenter and Tscharntke 1999). The modification of plant–pollinator interactions may have consequences for pollen limitation, reducing the number of insect visits, the size of pollen loads or pollen quality (Campbell and Husband 2007). In some cases, the loss of specialized pollinators will strongly select for self-pollination and reduced genetic variability in plants, resulting in a possible reduction in their evolutionary adaptability to environmental change (Allen-Wardell et al. 1998) and in their reproductive output, which is connected with an increasing extinction risk (Matthies et al. 2004).
In the agricultural landscapes of Europe, the ongoing process of habitat destruction and degradation due to the intensification and modernization of agriculture have reduced the distribution of many plant species (Jacquemyn et al. 2003) and increased the distances between the remaining populations. This is also the case for the plant species Anthericum liliago L. (Jackel 1999; Rosquist and Prentice 2002). In a former study of a small and isolated natural population of A. liliago (Halle, Kröllwitz), a low level of genetic polymorphism and a positive correlation between genetic and geographic distances in an area of a few m² were found (Peterson et al. 2002). These findings were interpreted as the result of restricted pollen and seed dispersal, and of an unusual ant pollination system connected with a high selfing rate.
In this study, we investigated 10 A. liliago populations (Fig. 1; study area c. 9,000 km²) in Saxony-Anhalt, Germany, on the border of the densely populated A. liliago southern range and the sparsely populated northern range, including some of the smallest and the largest known populations within this region. Firstly, we studied the genetic variation within and among these populations. Secondly, we looked for a possible relationship between genetic variability and population sizes. Thirdly, we investigated the influence of population size, pollinator community composition and genetic diversity on fitness parameters.
Geographic map of sampling localities of A. liliago populations (codes in Table 1) in Saxony-Anhalt, Germany
Material and methods
Species description
The plant A. liliago L. (Anthericaceae) is a self-compatible predominantly outcrossing (Rosquist 2001) herbaceous perennial found in dry grassland and dry shrubs, and rarely also in open oak or pine forests. The species has one or more leaf rosettes with one inflorescence each, ranging in height from 30 to 60 cm, sprouting from a short rhizome. The species has large flowers (35–40 mm in diameter) in a racemose inflorescence. Easily accessible floral nectaries are present. The plants have oviform seed capsules with relatively large orbicular seeds. The stem of A. liliago is sturdy and remains stiff and erect until the seeds are mature. At this time, the upper end of the erect seed capsules burst open and the seeds are released. The seeds are gravity-dispersed, and they do not have any adaptation for dispersal with animals or wind. Solitary bees (Apoidea) and syrphid flies (Syrphidae) have been described as the main pollinators (Rosquist 2001) although field studies have shown the presence of additional unspecialized flower visitors, e.g. ants (Formicidae) (Peterson et al. 2002). Anthericum liliago has a wide European distribution. Germany forms the northern limit of the continuous distribution range, the Danish and Swedish populations are isolated outposts.
Field study
Field studies were undertaken between 2002 and 2006. The number of A. liliago ramets for each large population (>500 ramets) was determined in 12 areas of 4 m2 and extrapolated according to the populated area. Fitness parameters were determined from randomly selected plants in 2003 and 2005: flowering ramets (200 per population or all in HK and LB), number of flowers and seed set (100 randomly selected flowering ramets per population with the exception of the seed set in LB, SB and HF due to a loss of the fruits through herbivory; all flowering ramets in HK) and germination (all populations, with 139–1,708 seeds collected per population). Because it is difficult to break seed dormancy, the % of seed germination could only be successfully investigated with the seeds collected in 2003. According to former experiments under different storage and germination conditions, seeds were stored for 2 years at room temperature to overcome seed dormancy and then placed for germination at 4°C on moistened sheets of paper in the dark for about 2 months. The seed weight of the seeds of 2005 was determined after drying at room temperature over a period of 6 weeks. Observations of pollinators were done in 2003 and 2005, each during the main flowering period between May the 15th and June the 10th, at two sunny days for 4 h (between 11 a.m. and 3 p.m.). We measured the relative abundance and species composition of flower visiting insects. No difference in the pollinator abundance and community composition was found between both years.
RAPD analysis
Random amplified polymorphic DNA (RAPD) analysis (Williams et al. 1990) was conducted on 10 individuals from each of the 10 populations using 12 decamer oligonucleotide primers (primer sequences are listed in Peterson et al. 2002) using Ready-To-Go PCR-beads (Amersham Biosciences) in 20 μl reactions with 10 ng DNA as described in Peterson et al. (2002). The 12 primers gave consistently reproducible bands (total sample of 238 RAPD markers) from 180 to 2,000 bp. The reproducibility of the RAPD results was checked with 14 randomly chosen individuals for eight RAPD primers; 1,754 out of 1,771 fragments were reproducible (99.04%).
Statistical analysis
A data matrix was created from the photographs of the gels by assigning 1 to present bands and 0 to absent bands. We calculated genetic diversity within populations as Shannon’s indices of diversity to avoid uncertainty, associated with expected heterozygosity estimates, based on dominant marker systems (Lynch and Milligan 1994). Shannon’s index was calculated for each RAPD marker i, separately for each population, as in Bussel (1999). The average Shannon’s index H’ pop for a population of A. liliago was then calculated by averaging H’ pop (i) over all markers (Monaghan and Halloran 1996). Percentages of polymorphic loci were also calculated. Phi-ST coefficients of differentiation among populations were computed in ARLEQUIN ver. 2.000 (Schneider et al. 2000). This measurement of differentiation is based on squared Euclidean coefficients of variation between groups of haplotypes (Excoffier et al. 1992). We used individual patterns of presence/absence of RAPD markers as input data, following Huff et al. (1993) and estimated the partitioning of molecular variance within and among populations by AMOVA, as implemented in ARLEQUIN.
A matrix of geographic distances between populations was created using formulas given by Kirvan (1997) for converting coordinates in degrees of longitude/latitude to metric distances. The extent and significance of associations between matrices of the geographical distances and the Phi-ST coefficients was evaluated by Mantel tests (MXCOMP in NTSYS). We used Pearson correlation coefficients to check for associations between quantitative descriptors for population size, fitness parameters and genetic diversity (Shannon’s index), and Kendall’s tau test (SPSS rel. 14.00, Chicago: SPSS Inc.) for a three-level ordinal-scaled descriptor of flower visitation in relation to population size.
Results
Habitats, fragmentation and population sizes
Anthericum liliago inhabits different types of dry grassland vegetation in the investigated region: from species-poor atlantic Corynephorus grasslands on oligotrophic, highly siliceous soils, to sub-continental steppe grassland on relatively nutrient rich loess, or to xeric calcareous grassland dominated by saxicolous plant species originating from the Alps and the Mediterranean region (Table 1). Large differences between populations regarding the census number of ramets per population (from about 100 to over 2 million), the population areas (17.5 to more than 30,000 m2) or the density of plants within a population (0.17–83.5 ramets per m2; Table 1) were noticed. Generally, the population size depended on the size of the available habitat (Table 1). Habitats with relatively nutrient rich loess soils (E and HB) tend to have a higher population density (ramets per m2). The high population density in HW with nutrient poor sandy siliceous soils is probably caused by the extreme dry conditions witch inhibit most of the natural plant competitors. All small populations (HK, HF, SB, and LB) were found to be isolated (Table 1), i.e. no other A. liliago population is known in a radius of 1 km.
Pollinator community composition
The main pollinators (Table 1) of A. liliago were the syrphid Merodon rufus Meigen as well as solitary bees of the genera Andrena, Halictus, Lasioglossum and Osmia. The highly specialized A. liliago pollinator Merodon rufus was an abundant visitor of inflorescences of the studied plant in all very large populations (FZ, HB, R, and HW). It was rare (GL) or absent (E) in middle-sized populations, and was always absent in the small populations (HK, HF, LB, and SB). This syrphid species could only be detected on flowers of A. liliago and was never seen visiting flowers of other plants (several individuals per population were tracked). A high pollinator abundance, especially of solitary bees, was restricted to middle and large populations (>10,000 A. liliago ramets). Ants visited flowers in nearly all investigated populations, but they were abundant only in the two small populations HK and HF (Table 1). Low densities of other pollinators (Bombiliidae, Coleoptera, Diptera, Hymenoptera, and Lepidoptera) were found in all populations (data not shown).
Genetic variation and differentiation among populations
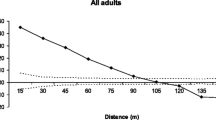
The values of Shannon’s index (I) of within-population diversity varied from 0.098 in population HK to 0.317 in population R (Table 1). Approximately the same pattern was found in the percentage of polymorphic RAPD loci within populations (Table 1). All Phi-ST values of differentiation among populations were significant (P < 0.05, detailed results not shown). A considerable part of total molecular variance could be attributed to the among-population component (41.04%, P < 0.0001, Table 2). We found a moderate correlation between genetic (pairwise Phi-ST values) and geographic distances between the populations (r = 0.36, P < 0.01 for all 10 populations; and a relatively strong correlation (r = 0.78, P < 0.001) for the seven largest populations; Fig. 2).
Correlation analyses of genetic diversity, population size, fitness parameters and pollinators
We found a very strong and significant correlation between the logarithm of census population size and Shannon’s index of gene diversity within populations (r = 0.912, P < 0.001; Fig. 3). The correlation is even greater within the range of population sizes between 103 and 106 ramets.
The fitness parameters (Table 3) were often inter-correlated (Table 4). We found a correlation between the mean number of seeds per seed capsule and the proportion of flowering ramets (r = 0.834, P < 0.01), the mean number of flowers per inflorescence (r = 0.867, P < 0.001), the germination rate (r = 0.871, P < 0.001), the seed weight (r = 0.672, P < 0.05), the mean number of seeds per ramet (r = 0.64, P < 0.05) and the population size (r = 0.614, P < 0.05). The population size was also significantly correlated with the mean seed weight (r = 0.708, P < 0.05). The mean number of seeds per ramet was strongly correlated with the proportion of flowering ramets in a population (r = 0.853, P < 0.001). The mean number of flowers per inflorescence was correlated with the percentage of germinated seeds (0.769, P < 0.01). Within-population Shannon’s diversity index was also correlated (Table 4) with the mean seed weight (r = 0.627, P < 0.05) and marginally associated with the percentage of flowering ramets (r = 0.508, P < 0.1).
There were significantly positive correlations between population size and flower visitation by Merodon rufus (τ = 0.747, P < 0.01), and solitary bees (Apoidea) (τ = 0.465, P < 0.05). Flower visitation of no other insect group (Bombiliidae, Coleoptera, Diptera, Hymenoptera, Lepidoptera and Formicidae) was significantly associated with population size (data not shown).
Discussion
Genetic differentiation among A. liliago populations
Theoretically, reduced population sizes and increased spatial isolation of populations can lead to erosion of genetic variation and increased inter-population genetic divergence (Young et al. 1996). These predicted effects were fully confirmed in our study of A. liliago. The pronounced genetic differentiation among the populations of A. liliago corresponds well with the strong isolation of their habitats in the intensively managed agricultural landscape of Middle Europe. The genetic differentiation we found among populations is correlated with the geographical distance separating them. This spatial pattern could be caused by fragmentation of a formerly larger population followed by genetic drift within the remaining relic of populations. It may also indicate that the current gene flow connects populations closer to each other more efficiently, possibly via gene flow among intermediated non-investigated populations, than populations divided by greater distances (see also Hensen et al. 2005). A strong population structure and significant relationship between genetic and geographic distances among populations was also found for Grevillea caleyi (Llorens et al. 2004) and Euphrasia stricta (Kolseth et al. 2005).
Differentiation among the highly isolated populations may be best explained by genetic drift. The area between the populations is likely to be unfavourable for extensive pollinator movement. The most agile pollinators of A. liliago, solitary bees, have foraging distances less than 1 km (Gathmann and Tscharnke 2002). In addition to the restricted pollinator movement, a lack of seed dispersal may have contributed to the high degree of differentiation found in A. liliago populations (see also Peterson et al. 2002). Although we did not investigate the possible effects of selection for specific loci or traits in our sample, these effects are unlikely to be restricted only to very small populations and to contribute considerably to their differentiation. Similar results on genetic differentiation among populations have been reported for a number of rare plant species and attributed to the absence of interpopulation gene flow, e.g. for populations of Gentianella germanica (Fischer and Matthies 1998), Arnica montana (Luijten et al. 2000) and Viola pubescens (Culley and Grubb 2003).
The size range and the degree of isolation of populations in our study are representative for the entire distributional range of A. liliago in Saxony-Anhalt, Germany. In Scandinavia, A. liliago is restricted to dry open grasslands, a type of habitat that has declined dramatically during the last 150 years (Rosquist and Prentice 2002). Similar to the situation in Germany, the Scandinavian populations are fragmented and highly disjunctive (Rosquist and Prentice 2002). Results of an allozyme study of tetraploid A. liliago populations by Rosquist and Prentice (2002), where the majority of the total diversity was explained by the within-population component (89%), are in contrast with our RAPD analysis where a considerable proportion of total diversity was attributed to the among-population component. This could be caused by a stronger degree of fragmentation of the German range of this species due to a higher human population density in Central Europe in the 20th century. Another explanation could be a higher availability of open vegetation in Scandinavia. Differences could also be explained by a difference in ploidy levels. It is possible that polyploidy buffers the effects of genetic drift, so that it takes much more time for polyploids to develop a strong differentiation between populations, than for diploids. In Germany, both tetraploids and diploids are reported (see Rosquist and Prentice 2002), but no information regarding the ploidy levels of the investigated A. lilago populations was available.
The overall differentiation among populations (Phi-ST = 0.41) is considerably higher than the corresponding mean estimate for species with mixed breeding system subjected to RAPD analyses (Phi-ST = 0.27; Nybom and Bartish 2000). This suggests a high rate of selfing. The value from our study, however, is in good congruence with the corresponding mean estimates of population differentiation for species with gravity-dispersed seeds (Phi-ST = 0.44), species with regional geographic range (Phi-ST = 0.43) and short-lived perennial species (Phi-ST = 0.39) as summarized by Nybom and Bartish (2000).
Correlation of genetic variability and population sizes
We established a very strong and significantly positive correlation between population size and gene diversity (r = 0.91). Therefore, it can be assumed that, apart from population size, the genetic diversity in our sample was only weakly influenced by a number of important demographic and genetic factors. Although our results should be considered as preliminary, and more data is necessary to clarify the details of genetic structure for populations of A. liliago in the area of our study, we can nevertheless assume a certain degree of demographic stability and genetic equilibrium for populations from our sample, at least for those with census sizes of more than 103 ramets. A positive correlation between genetic variation and population size, using RAPD, was also demonstrated for Gentianella germanica (Fischer and Matthies 1998), Senecio vulgaris (Müller-Schärer and Fischer 2001), Sesleria albicans (Reisch et al. 2003) and Dictamnus albus (Hensen and Oberprieler 2005). In a meta-analysis of the relationships between plant population size, genetic variation and fitness reported in studies published between 1987 and 2005, the mean correlation between population size and genetic variation was found to be significantly positive (Leimu et al. 2006).
Plant fitness and pollinator community composition
We found no strong correlation between reproductive output, measured by the mean number of seeds per ramet, and genetic variation and population size, respectively. In addition, Jackel (1999) also found no positive correlation between the reproductive output (fruit and seed set) and population size of 5 A. liliago populations ranging in size from 35 to 5,000 flowering ramets. In contrast to our results, a positive correlation of genetic variation and fruit and seed set was found in Gentianella germanica (Fischer and Matthies 1998) and Cochlearia bavarica (Paschke et al. 2002). Population size had a significantly positive effect on reproductive success in Primula vulgaris (Brys et al. 2004), Primula veris and Gentiana lutea (Kery et al. 2000), Arnica montana (Luijten et al. 2000) and Aquilegia canadensis (Mavraganis and Eckert 2001). Leimu et al. (2006) found that the mean correlation between plant population size and fitness was generally significantly positive in self-incompatible plant species, but not significant in self-compatible plant species.
Our detailed analysis of fitness parameters in the sampled populations of A. liliago revealed numerous other interrelated associations. Some of these could be a result of greater pollination success in larger populations. Although no strong correlations were found between the reproductive output and population size or genetic diversity, the number of flowering individuals and number of flowers per inflorescence seem to influence the relationships between population size and seed set success. This may suggest disrupted pollination when the abundance of flowering individuals and flowers is reduced. Noteworthy, flower visits by the specialized pollinator species Merodon rufus (Jentzsch and Köberlein 2000) and by solitary bees were relatively frequent and significantly associated with population size. Following the trends of increased isolation and decreased numbers of ramets in populations of A. liliago, both abundance and species richness of flower visiting solitary bees decreased and Merodon rufus is completely absent in small populations. In small populations mainly unspecialized pollinators like ants can be found (see also Peterson et al. 2002). Our results therefore strongly indicate that the pollinator community composition, which depends on the population size of A. liliago affects the pollination success and is associated with the reproductive output.
References
Allen-Wardell G, Bernhardt P, Bitner R et al (1998) The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv Biol 12:8–17
Brys R, Jacquemyn H, Endels P et al (2004) Reduced reproductive success in small populations of the self-incompatible Primula vulgaris. J Ecol 92:5–14
Bussel JD (1999) The distribution of random amplified polymorphic DNA (RAPD) diversity amongst populations of Isotoma petraea (Lobeliaceae). Mol Ecol 8:775–789
Campbell LG, Husband BC (2007) Small populations are mate-poor but pollinator-rich in a rare, self-incompatible plant, Hymenxys herbaceae (asteraceae). New Phytol 174:915–925
Culley TM, Grubb TC (2003) Genetic effects of habitat fragmentation in Viola pubescens (Violaceae), a perennial herb with chasmogamous and cleistogamous flowers. Mol Ecol 12:2919–2930
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Fischer M, Matthies D (1998) RAPD variation in relation to population size and plant fitness in the rare Gentianella germanica (Gentianaceae). Am J Bot 8:811–819
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv Biol 10:1500–1508
Gathmann A, Tscharnke T (2002) Foraging ranges of solitary bees. J Animal Ecol 71:757–764
Hensen I, Oberprieler C (2005) Effects of population size on genetic diversity and seed production in the rare Dictamnus albus (Rutaceae) in central Germany. Conserv Genet 6:63–73
Hensen I, Oberprieler C, Wesche K (2005) Genetic structure, population size, and seed production of Pulsatilla vulgaris Mill. (Ranunculaceae) in Central Germany. Flora 200:3–14
Huff DR, Peakall R, Smouse PE (1993) RAPD variation within and among natural populations of outcrossing buffalograss (Buchloe dactyloides (Nutt.) Engelm.). Theor Appl Genet 86:927–934
Jackel AK (1999) Strategien der Pflanzenarten einer fragmentierten Trockenrasengesellschaft. J. Cramer, Berlin, Stuttgart
Jacquemyn H, Van Rossum F, Brys R et al (2003) Effects of agricultural land use and fragmentation on genetics, demography and population persistence of the rare Primula vulgaris, and implications for conservation. Belg J Bot 136:5–22
Jentzsch M, Köberlein T (2000) Zur Schwebfliegen-Fauna des Naturschutzgebietes "Hasenwinkel” im Landkreis Mansfelder Land mit Bemerkungen zur Biologie von Merodon rufus MEIGEN 1838 und Eumerus strigatus (FALLEN, 1817) (Dipt., Syrphidae). Entomolog Nachr Ber 44:189–192
Kery M, Matthies D, Spillmann HH (2000) Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea. J Ecol 88:17–30
Kirvan AP (1997) Latitude/Longitude, NCGIA Core Curriculum in GI Science. http://www.ncgia.ucsb.edu/giscc/units/u014/u014html. Accessed 29 Nov 2005
Kolseth AK, Lonn M, Svensson BM (2005) Genetic structure in two meadow varieties of Euphrasia stricta on the Baltic Island of Gotland (Sweden) and implications for conservation. Folia Geobot 40:163–176
Kwak MM, Velterop O, van Andel J (1998) Pollen and gene flow in fragmented habitats. Appl Veg Sci 1:37–54
Leimu R, Mutikainen P, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation? J Ecol 94:942–952
Llorens TM, Ayre DJ, Whelan RJ (2004) Evidence for ancient genetic subdivision among recently fragmented populations of the endangered shrub Grevillea caleyi (Proteaceae). Heredity 92:519–526
Luijten SH, Dierick A, Gerard J et al (2000) Population size, genetic variation, and reproductive success in a rapidly declining, self-incompatible perennial (Arnica montana) in the Netherlands. Conserv Biol 14:1776–1787
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
Matthies D, Brauer I, Maibom W et al (2004) Population size and the risk of local extinction: empirical evidence from rare plants. Oikos 105:481–488
Mavraganis K, Eckert CG (2001) Effects of population size and isolation on reproductive output in Aquilegia canadensis (Ranunculaceae). Oikos 95:300–310
Monaghan BG, Halloran GM (1996) RAPD variation within and between natural populations of morama [Tylosema esculentum (Burchell) Schreiber] in Southern Africa. South African J Bot 62:287–291
Müller-Schärer H, Fischer M (2001) Genetic structure of the annual weed Senecio vulgaris in relation to habitat type and population size. Mol Ecol 10:17–28
Nybom H, Bartish IV (2000) Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspectives Plant Ecol Evol Syst 3:93–114
Paschke M, Abs C, Schmid B (2002) Relationship between population size, allozyme variation, and plant performance in the narrow endemic Cochlearia bavarica. Conserv Genet 3:131–144
Peterson A, Bartish IV, Peterson J (2002) Genetic structure detected in a small population of the endangered plant Anthericum liliago (Anthericaceae) by RAPD analysis. Ecography 25:677–684
Reisch C, Poschlod P, Wingender R (2003) Genetic differentiation among populations of Sesleria albicans Kit. ex Schultes (Poaceae) from ecologically different habitats in central Europe. Heredity 91:519–527
Rennwald E (ed) (2000) Verzeichnis und Rote Liste der Pflanzengesellschaften Deutschlands. Schriftenreihe für Vegetationskunde 35. Bonn-Bad Godesberg
Rosquist G (2001) Reproductive biology in diploid Anthericum ramosum and tetraploid A. liliago (Anthericaceae). Oikos 92:143–152
Rosquist G, Prentice HC (2002) Genetic variation in Scandinavian Anthericum liliago (Anthericaceae): allopolyploidy, hybridization and immigration history. Plant Syst Evol 236:55–72
Schneider S, Roessli D, Excoffier L (2000) ARLEQUIN: a software for population genetics data analysis.Version 2.000. University of Geneva, Geneva, Switzerland
Steffan-Dewenter I, Tscharntke T (1999) Effects of habitat isolation on pollinator communities and seed set. Oecologia 12:432–440
Williams JGK, Kubelik AR, Livak KJ et al (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl Acids Res 18:6531–6535
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418
Acknowledgements
We are grateful to Martin Trost for creation of Fig. 1, Matthias Jentzsch and Eckart Stolle for the determination of M. rufus and Tobias Meitzel for the determination of solitary bees.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peterson, A., Bartish, I.V. & Peterson, J. Effects of population size on genetic diversity, fitness and pollinator community composition in fragmented populations of Anthericum liliago L.. Plant Ecol 198, 101–110 (2008). https://doi.org/10.1007/s11258-007-9388-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-007-9388-4