Abstract
Phosphorus concentrations in many south-east Asian tropical rain forest soils are very low. To determine the growth responses of seedlings of a light-demanding (Shorea leprosula) and a more shade-tolerant (Hopea nervosa) dipterocarp species to increasing P, we carried out a nursery fertilisation experiment. Responses of symbiotic ectomycorrhizal (EcM) fungi to the treatments were also determined. Seedlings were grown under high light (13 mol m−2 d−1) or moderate light (4 mol m−2 d−1) in shade-chambers and were fertilised with a solution containing 0, 1, 10 or 100 mg L−1 P. The growth of Hopea and Shorea showed different responses to the light and P fertilisation treatments with Hopea having greater growth under moderate light conditions and Shorea having greater growth under high light conditions. Shorea responded to P fertilisation by increasing its foliar P concentrations and growth rates, whereas Hopea did not take up additional P and did not improve its growth rates. There was no effect of either light or P fertilisation on total EcM colonisation or EcM diversity, but around half of the EcM morphotypes observed were affected by one of these two abiotic perturbations, most notably for Riessiella sp. which increased with P fertilisation suggesting it may not be a mutualistic fungus. These results show how niche partitioning in both dipterocarp seedlings and EcM fungi can be divided along contrasting axes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trees of the Dipterocarpaceae family dominate the lowland evergreen rain forests of south-east Asia (Whitmore 1984) where they grow in old, highly-leached soils which are low in major plant nutrients to an extent that they are often considered limiting to plant growth (Vitousek 1984; Tanner et al. 1998). The nutrient that is most likely to be limiting to growth is P, as in Bornean soils, it is often found at concentrations of less than 1 μg g−1 in its weak acid-extractable form (Green 1992; Brearley 2003; Brearley et al. 2004). This situation is also exacerbated by the acidic soils (pH of around 3.5–4.5) which cause binding of P with Al and Fe into insoluble Al and Fe phosphates.
Dipterocarps commonly reproduce at intervals of 3–8 years in mast-fruiting events, often associated with El Niño-Southern Oscillation climatic conditions, where huge numbers of seeds, from many species, are shed (Sakai 2002; Brearley et al. 2007). Their seeds are recalcitrant and germinate almost immediately on the forest floor. Seedlings grow slowly in the understorey until a gap is created in the forest canopy, increasing irradiance and releasing them from their light-limited state.
Over 100 species of dipterocarps have been recorded as forming ectomycorrhizal (EcM) associations (e.g. Singh 1966; Hong 1979; de Alwis and Abeyneyake 1980; Nuhamara et al. 1985; Alexander and Högberg 1986; Smits 1992; Pampolina et al. 1995; Brearley 2003). Colonisation by EcM fungi is considered important for the successful growth of dipterocarps, increasing nutrient uptake and improving growth (Lee and Alexander 1994; Yazid et al. 1994, 1996; Turjaman et al. 2006). Mycorrhizas are usually credited with enhancing plant P uptake as this is one of the least mobile nutrients within the soil and many species of EcM fungi can also make organic forms of N and P directly available to the host plant (Read and Perez-Moreno 2003).
Dipterocarps, therefore, require light, nutrients and EcMs for their successful regeneration but, as these factors can be intimately related, the interaction between them is not clear. Whilst a number of bioassays of nutrient limitation of dipterocarp seedlings have shown increased seedling growth in response to nutrient addition (Brearley 2003; Burslem et al. 1996; Yap and Moura-Costa 1994, 1996; Yap et al. 2000; Bungard et al. 2002), others have not (Brearley 2003; Turner et al. 1993; Burslem et al. 1995, 1996). These different results are likely to be due to the different species and soils assayed, but will also depend upon the experimental conditions and especially the irradiance under which the seedlings were grown. Seedlings would not be expected to respond in terms of growth to increased nutrients when low irradiance is the overriding limiting factor. Moreover, we are unaware of any nutrient addition experiments with dipterocarp species that have been carried out under more than one light regime. It is hypothesised that species with the highest relative growth rates, and hence the greatest nutrient demand, will be most responsive to nutrient addition. These species are also the ones which are most responsive to increased irradiance (Veenendaal et al. 1996; Coomes and Grubb 2000).
At higher irradiances, it is hypothesised that EcM colonisation will increase, as EcM fungi depend upon their host plants for carbohydrates derived from photosynthesis. Increased rates of carbon fixation under higher irradiances will lead to transport of more carbohydrates to the roots, allowing seedlings to support a greater EcM fungal biomass. To a large degree, mycorrhizal colonisation is also determined by soil fertility. As fertility increases, it has been observed that mycorrhizal colonisation decreases in a range of woody plant species (Beckjord et al. 1985; Bougher et al. 1990; Jones et al. 1990; Ingleby et al. 2001). Nutrient perturbations may also affect the community structure of EcM fungi where additions of N in boreo-temperate forests are generally detrimental to EcM fungi (Wallenda and Kottke 1998; Erland and Taylor 2002). As N is the limiting factor in the forests in the above reviews (Read 1991), its addition is expected to have the greatest effect on ecosystem functioning. In rain forest soils, where P is probably more limiting, we consider that this is the nutrient which will have the greatest control over ecosystem processes.
The aims of this paper are therefore three-fold: (i) to determine how increasing concentrations of P and increasing irradiance interact to influence dipterocarp seedling growth; (ii) to determine how these abiotic perturbations affect EcM colonisation and community structure; and (iii) to determine whether Shorea leprosula Miq., a light-demanding species, shows different responses to Hopea nervosa King, a more shade-tolerant species.
Methods
Study species
Dipterocarps are generally considered to be late-successional species, but within this ecological grouping there is a large amount of interspecific variation in responses to light. Shorea leprosula is one of the most abundant dipterocarp species in Borneo which grows to a large size; its light hardwood timber is used extensively. It is one of the more light-demanding dipterocarp species and has been used previously as a model species for ecophysiological studies (e.g. Zipperlen and Press 1996; Scholes et al. 1997; Leakey 2002). Hopea nervosa is a more shade-tolerant, medium hardwood species. It is locally common in northern Borneo and, although rarely used for timber as it is too small, large numbers have been used for reforestation in Sabah (S. W. Yap, pers. comm.).
Growth conditions and fertilisation treatments
One-year old seedlings of the two species were obtained from the INFAPRO nursery, Danum Valley, Sabah. They were planted in 1.2 l pots filled with a 3:1 mix of alluvial forest soil from Kabili-Sepilok Forest Reserve (fully homogenised and sieved to c. 0.5 cm) and river sand (soil plugs of around 400 cm3 in which the seedlings were maintained prior to the experiment were left intact). The seedlings were placed under shade cloth(s) under either high light (nominally 30% of full sunlight) or moderate light (nominally 10% of full sunlight) treatments in an extension to the nursery of the Sabah Forestry Department Forest Research Centre. Every 2 weeks, the seedlings were watered with 100 ml of a KH2PO4 solution containing 1, 10 or 100 mg L−1 P (0.0323, 0.323 or 3.229 mM KH2PO4) made up in rainwater; control seedlings received 100 ml of rainwater. There were three replicate shade-chambers for each light treatment, with each shade-chamber holding four individuals from each species*P combination; there were, therefore, 12 replicates of each species*light*P combination. The seedlings received natural rainfall and were given regular supplementary water on days when there was no rainfall until the soil in the pots reached field capacity. The pots containing the seedlings were re-randomised within the shade-chambers every month and grown for a total of 10 months.
Environmental conditions within the shade-chambers
The irradiance, temperature and relative humidity within the shade-chambers were recorded using a quantum sensor and data-logger (SKP 215 and DataHog2, Skye Instruments Ltd., Llandrindod Wells, Powys, Wales, UK). The values were recorded on six consecutive, typically sunny days near the end of the experiment; the data-logger recorded values every minute and stored 10-min means.
Growth rates
Initial measurements of total height and leaf number were taken. After 10 months, these measurements were re-taken. The seedlings were harvested, dried at 80°C for 48 h and then weighed to give the total seedling biomass (including roots). The total seedling biomass at the beginning of the experiment was calculated by measuring, harvesting and drying additional seedlings not used in the experiment as above and then weighing them to create regression equations (presented in Brearley 2003).
The relative height and biomass growth rates (RGR) and relative leaf production rate (RPR) were calculated according to Hunt (1990) as:
where M 0 and M 10 are the measurements at the beginning and end of the experiment, respectively.
Leaf mass per area (LMA) was measured by tracing one young, fully expanded leaf per plant and measuring the tracing on a leaf area meter (Mark 2, Delta-T Devices Ltd., Burwell, Cambridgeshire, UK). The leaf was then dried at 80°C for 48 h and weighed.
Leaf and root nutrient concentrations
Three young, fully expanded leaves were removed from each individual seedling for foliar analyses. A sample of c. 75 mg containing sub-samples of all three leaves was digested in 2.5 ml of salicylic acid in sulphuric acid (33 g l−1) with a lithium sulphate/copper sulphate (10:1 ratio) catalyst at 375°C for 8 h. The digests were diluted with ultra-pure distilled water and then analysed for N and P using an auto-analyser (Tecator 5042 Detector and 5012 Analyser, Foss UK Ltd., Didcot, Oxfordshire, UK) using the ammonium molybdate–stannous chloride (Tecator Ltd. 1983) and gas diffusion (Tecator 1984) methods, respectively. The N and P concentrations in a sample of c. 75 mg of young fine (<2 mm diameter) roots were also determined in the same way as above.
Ectomycorrhizal colonisation
Initial percentage EcM colonisation (% EcM) of the fine roots of around 20 seedlings of each species was calculated as a percentage of the number of EcM root tips out of a total of c. 200 root tips. It was 96.5% ± SE 1.3 on Hopea and 92.7% ± SE 1.9 on Shorea. At the end of the experiment, % EcM of the fine roots was re-recorded on c. 200 root tips per seedling for all the seedlings. The EcM community was examined on eight seedlings per species*light*P combination divided randomly among the shade-chambers. EcM morphotypes were recognised from gross morphological features (e.g. branching patterns, colour, mantle texture, presence of emanating hyphae), the mantle and hyphal characteristics were examined microscopically using squashing (Ingleby et al. 1990) and scraping (Agerer 1991) techniques. Morphotype classifications were made with reference to the descriptions of Agerer et al. (1996–2006), Ingleby et al. (1990) and Jülich (1985).
The Shannon–Wiener diversity index (H′) was calculated for the EcM community on each seedling using the equation:
where s = number of morphotypes and p i = abundance of the ith morphotype expressed as a proportion of the total colonised root tips.
Soil analyses
At the end of the experiment, a soil sample was taken from every sixth pot, air-dried, ground to pass a 2 mm sieve and analysed for pH, total N, P and K and extractable P and K. Sample pH was measured by mixing 10 g of soil with 25 ml of distilled water; it was stirred and allowed to equilibrate for 1 h before being measured with a pH meter (Corning pH meter 140, Halstead, Essex, UK). Total nutrients were measured by digesting c. 0.25 g of soil in 5 ml of salicylic acid in sulphuric acid mix (plus catalyst, as described above for foliar nutrients), N and P were analysed as described above and K was analysed by atomic absorption spectrophotometry with an air-acetylene flame (Perkin-Elmer 2100 Atomic Absorption Spectrophotometer, Beaconsfield, Buckinghamshire, UK). Phosphorus and K were extracted with a Modified Kelowna (KM) extractant (Ashworth and Mrazek 1995). Five grams of soil was shaken with 50 ml of KM extractant on an over-arm shaker for 30 min; the solution was filtered and P and K were analysed as described above. In addition, P was extracted from c. 2 g of soil with 20 ml of 0.03 M NH4F plus 0.1 M HCl by shaking for 1 min in a test tube (the Bray II method). The solutions were then filtered and analysed for P on a spectrophotometer (Hitachi U-2000, Tokyo, Japan) using the molybdenum blue colour development method described in Anderson and Ingram (1993). All results are expressed on an oven-dry (105°C for 24 h) basis.
Statistical analyses
Seedling and EcM responses to the treatments were analysed separately for each species using a linear mixed effects model (Pinheiro and Bates 2000). Differences between shade-chambers were modelled by including intercept terms for each shade-chamber, treated as normally distributed random effects. The interaction between light and P fertilisation was never significant and was therefore removed from all analyses. Values were transformed prior to analysis by a Box–Cox transformation where appropriate. Soil chemical differences were analysed using one-way ANOVAs followed by Tukey’s tests.
Results
Environmental conditions within the shade-chambers
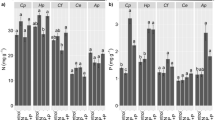
The high light conditions received 13.1 ± 1.1 mol m−2 d−1 and were nominally 30% of full sunlight. The moderate light conditions received 4.1 ± 0.3 mol m−2 d−1 and were nominally 10% of full sunlight (Fig. 1a). Between 12:00 h and 15:00 h, the temperature was up to 2°C higher in the high light shade-chambers and the relative humidity was up to 20% lower (Fig. 1b, c) although these values were small relative to the changes in the light environment.
Following P fertilisation, soil P and soil pH were increased, although these differences were only significant at the highest rate of P addition (Table 1). There was no effect of P fertilisation on soil K or total soil N (Table 1).
Seedling growth rates
The relative biomass and height-growth rates and RPR of Hopea tended to be greater under moderate light conditions, significantly so for leaf production but were not affected by P fertilisation (Fig. 2a–c, Table 2a). The relative biomass growth rate of Shorea was significantly greater under high-light conditions and tended to be greater following P fertilisation (Fig. 2d, Table 2b). Shorea relative leaf production rate was significantly greater following P fertilisation whilst its relative height growth rate was not affected by any treatment (Fig. 2e, Table 2b). The LMA of both species was lower under moderate light conditions, but was not affected by P fertilisation (Table 3).
Seedling foliar and root nutrient concentrations
For Hopea, foliar N and P were not affected by the different light conditions and there was no consistent change following P fertilisation (Table 4a). Root N was lower following P fertilisation although root P was unaffected (Table 4a).
For Shorea, foliar N was higher under moderate light conditions and decreased with the addition of P. Foliar P was unaffected by the light conditions, but increased with the addition of P (Table 4b). Root N was higher under the moderate light conditions (Table 4b).
Ectomycorrhizal colonisation
Total EcM colonisation was significantly greater on Hopea than on Shorea (75.1% ± SE 1.5 vs. 65.3% ± SE 2.0; t 165 = 3.98, p < 0.001), but was not affected by the light conditions or by P fertilisation in both species (Hopea: Light: F 1,4 = 0.009, p = 0.93; P: F 3,75 = 0.85, p = 0.47. Shorea: Light: F 1,4 = 0.04, p = 0.85; P: F 3,74 = 1.10, p = 0.36). EcM diversity, as assessed by the Shannon–Wiener diversity index, was also unaffected by the light conditions or by P fertilisation in both species (Hopea: Light: F 1,4 = 2.79, p = 0.17; P: F 3,55 = 1.66, p = 0.19. Shorea: Light: F 1,4 = 0.01, p = 0.94; P: F 3,55 = 1.39, p = 0.26).
There were, however, changes in the abundance of individual EcM morphotypes in response to both light and P fertilisation. Basidiomycete sp. 2 was only found under high-light conditions on the roots of both species (Fig. 3, Table 5) whereas Cenococcum geophilum Fr. was more common under moderate light conditions on the roots of Hopea (Fig. 3a, Table 5a). Basidiomycete sp. 1 was reduced following P fertilisation on the roots of Hopea (Fig. 3a, Table 5a) whilst, in contrast, Riessiella sp. increased on both species following P fertilisation, most notably on Hopea (Fig. 3, Table 5).
Discussion
Seedlings showed contrasting responses to the treatments
In this study, Hopea showed greater growth under moderate light conditions (c. 4 mol m−2 d−1), whereas Shorea showed greater growth under high light conditions (c. 13 mol m−2 d−1). The moderate light fluxes are comparable to those found within a single treefall gap and the high light fluxes compare with those that are typically observed following logging, where a large clearing is created (Veneklaas and Poorter 1998; Clearwater et al. 1999). The growth responses observed by these two species are consistent with our current knowledge of the light requirements and autecology of these and other dipterocarp species (Zipperlen and Press 1996; Scholes et al. 1997; Leakey 2002). It is likely that, under the moderate light conditions, the growth of Shorea was limited by the low light fluxes; conversely under the high light conditions, Hopea seedlings may have suffered from photoinhibition. Shorea responded to P fertilisation by increasing its foliar P and growth rates whereas Hopea was unresponsive to P fertilisation. There are a number of other studies which have shown that dipterocarp seedlings can increase their growth when given additional nutrients (Yap and Moura-Costa 1994, 1996; Yap et al. 2000; Bungard et al. 2002; Brearley 2003) and our results are consistent with the hypothesis of a more light-demanding species (Shorea) showing a greater response to additional nutrients (Veenendaal et al. 1996; Bungard et al. 2002). Furthermore, seedlings are most likely to respond to nutrient addition when they are grown under high light conditions where irradiance is not limiting growth. Perhaps the most relevant study here, is that of Bungard et al. (2002) who found the growth of a light-demanding dipterocarp, Shorea johorensis Foxw., increased following fertilisation, but a more shade-tolerant species, Dryobalanops lanceolata Burck, was not affected.
It has been proposed that there is a trade-off between growth under high light versus low light conditions due to the fact that, in deep shade, the maximum photosynthetic rate of the leaves of shade plants exceeds those of sun plants, whilst under higher irradiances the reverse pattern is found (Boardman 1977; Givnish 1988); our results are consistent with this view. However, more recent results have shown positive correlations between rankings of the relative growth rates of species under high and low light conditions suggesting that there is a trade-off between growth under high light conditions survival versus under low light conditions (Kitayama 1994; Bloor and Grubb 2003). It must be noted that the low light fluxes in the two studies cited above were about 10-fold lower than the moderate light fluxes in this study and therefore valid comparisons are difficult to make.
There were notable effects on the ectomycorrhizal community in response to the treatments
The extent of mycorrhiza colonisation is affected, to a certain degree, by the nutritional status of the host plant which will, in turn, be affected by soil fertility. The total % EcM colonisation was not reduced following the addition of P in this study, as had previously been observed in many studies with both arbuscular mycorrhizal (Cade-Menun and Berch 1997; Ingleby et al. 2001) and EcM (Beckjord et al. 1985; Bougher et al. 1990; Jones et al. 1990; Baum and Makeschin 2000) tree species. Given the number of previous studies that had observed a reduction in mycorrhizal colonisation following P fertilisation, our results emphasise the obligate nature of the EcM symbiosis for dipterocarp seedlings. It is also possible that the low levels of P addition (<100 mg L−1 P) in our study were not sufficient to have a large impact on the total % EcM. Suhardi et al. (1993) and Suhardi (2000) also found that the addition of rock phosphate had no effect on the total % EcM of Shorea leprosula or S. bracteolata Dyer. However, different tree and fungal species combinations will respond individually to different levels of fertilisation (Bougher et al. 1990; Jones et al. 1990; Mason et al. 2000), as shown by the different responses of fungal species presented here.
It has been hypothesised that, under low light conditions, there will be lower EcM colonisation due to lower carbohydrate availability to support EcM fungal tissue. A number of other studies with dipterocarp species have found that, this is indeed the case, with seedlings grown in the understorey having a lower level of EcM colonisation than those in canopy gaps (Becker 1983; Ingleby et al. 1998). It appears that the highest levels of mycorrhizal colonisation occur under the light levels where the greatest growth, and hence carbon fixation, of the host plant is found (Prajadinata and Santoso 1993; Béreau et al. 2000). However, many EcM fungi are capable of a certain amount of saprotrophic growth and may not, therefore, be totally reliant upon plant carbohydrates leading to the lack of effect of the light conditions on EcM colonisation in this present study. In our study, the soil nutrient levels were very low, so, regardless of the light environment, EcMs may have remained beneficial due to their importance in nutrient uptake.
Only one species (Basidiomycete sp. 1) showed the classic mycorrhizal response to fertilisation of a decreasing frequency on the seedling roots. The fact that Riessiella sp. increased in abundance following P fertilisation is of considerable interest as this is a common dipterocarp EcM type, along with the closely related Riessia species, which has been found in a number of other studies, on the roots of Hopea and Shorea seedlings (Jülich 1985; Lee and Alexander 1996; Ingleby et al. 1998). These two EcM species do not possess a Hartig net (Smits 1994; Lee et al. 1997) suggesting that they may not function as a mutualistic EcM in which the plant is assisted with nutrient uptake. That Riessiella sp. increases under conditions of increased soil fertility in this study, where seedlings might be less reliant upon EcMs, further suggests that, it may have less mutualistic tendencies, or might even be parasitic (Johnson 1993; Johnson et al. 1997). The increased frequency of Basidiomycete sp. 2 and reduced frequency of Cenococcum geophilum under high light conditions suggests that these changes are in response to an environmental factor caused by changes in the light conditions, such as increased soil temperature or reduced humidity. This is because if the responses were seedling mediated, it would be expected that the two EcM morphotypes would show different responses on the roots of each of the two dipterocarp species as the two species showed contrasting growth patterns under the two light conditions.
It is notable that a large proportion of the EcM species found (Cenococcum geophilum, Inocybe spp., Riessiella spp. and Thelephorales spp.) are early colonisers. This may be due to the homogenisation of the soil before planting or also due to the input of airborne fungal spores. These two factors may also help explain why larger differences were not found in the EcM community. Of course, any explanation for the relative abundance of each fungal species will have to consider interactions (competitive or facilitative) between species but, given the poor knowledge of the ecology of these tropical EcM species, this would be purely speculative.
Conclusions
The seedling regeneration niche (sensu Grubb 1977) may be defined by a number of environmental gradients and, as such, we have shown that the regeneration niche for Shorea leprosula is defined by both irradiance and soil nutrient status. In contrast, the niche of Hopea nervosa, being non-responsive to P addition, is only defined by irradiance. Our study has also described, in detail, individual EcM fungal responses to these abiotic perturbations and has shown how the fungal, as well as the seedling, niche may be affected by irradiance and soil fertility. It remains to be determined, however, how seedling performance may be affected by the relative diversity and abundance of each individual EcM species on its roots and this would be a fruitful area for future research.
References
Agerer R (1991) Characterisation of ectomycorrhiza. In: Norris JR, Read DJ, Varma AK (eds) Methods in microbiology, vol 23. Academic Press, London, pp 25–73
Agerer R, Danielson RM, Egli S et al (eds) (1996–2006) Descriptions of ectomycorrhizae. Einhorn-Verlag, Schwäbisch Gmünd
Alexander IJ, Högberg P (1986) Ectomycorrhizas of tropical angiospermous trees. New Phytol 102:541–549
Anderson JM, Ingram JSI (eds) (1993) Tropical soil biology and fertility: a handbook of methods, 2nd edn. CAB International, Wallingford
Ashworth J, Mrazek K (1995) ‘‘Modified Kelowna’’ test for available phosphorus and potassium in soil. Commun Soil Sci Plan 26:731–739
Baum C, Makeschin F (2000) Effects of nitrogen and phosphorus fertilization on mycorrhizal formation of two poplar clones (Populus trichocarpa and P. tremula × tremuloides). J Plant Nutr Soil Sci 163:491–497
Becker P (1983) Ectomycorrhizas on Shorea (Dipterocarpaceae) seedlings in a lowland Malaysian rainforest. Malay For 46:146–170
Beckjord PR, Melhuish JH Jr, McIntosh MS (1985) Effect of nitrogen and phosphorus fertilization on growth and formation of ectomycorrhizae of Quercus alba and Q. rubra seedlings by Pisolithus tinctorius and Scleroderma auranteum. Can J Bot 63:1677–1680
Béreau M, Barigah TS, Louisanna E et al (2000) Effects of endomycorrhizal development and light regimes on the growth of Dicorynia guianensis Amshoff seedlings. Ann Forest Sci 57:725–733
Bloor JMG, Grubb PJ (2003) Growth and mortality in high and low light: trends among 15 shade-tolerant topical rain forest tree species. J Ecol 91:77–85
Boardman NK (1977) Comparative photosynthesis of sun and shade plants. An Rev Plant Physiol 28:355–377
Bougher NL, Grove TS, Malajczuk N (1990) Growth and phosphorus acquisition of karri (Eucalyptus diversicolor F. Muell.) seedlings inoculated with ectomycorrhizal fungi in relation to phosphorus supply. New Phytol 114:77–85
Brearley FQ (2003) The role of ectomycorrhizas in the regeneration of dipterocarp seedlings. PhD Thesis, University of Sheffield
Brearley FQ, Prajadinata S, Kidd PS et al (2004) Structure and floristics of an old secondary rain forest in Central Kalimantan, Indonesia, and a comparison with adjacent primary forest. For Ecol Manage 195:385–397
Brearley FQ, Proctor J, Suriantata et al (2007) Reproductive phenology over a ten-year period in a lowland evergreen rain forest of central Borneo. J Ecol 95:828–839
Bungard RA, Zipperlen SA, Press MC et al (2002) The influence of nutrients on growth and photosynthesis of seedlings of two rainforest dipterocarp species. Funct Plant Biol 29:505–515
Burslem DFRP, Grubb PJ, Turner IM (1995) Responses to nutrient addition among shade-tolerant tree seedlings of lowland tropical rain forest in Singapore. J Ecol 83:113–122
Burslem DFRP, Grubb PJ, Turner IM (1996) Responses to simulated drought and elevated nutrient supply among shade-tolerant tree seedlings of lowland tropical forest in Singapore. Biotropica 28:636–648
Cade-Menun BJ, Berch SM (1997) Response of mycorrhizal western red cedar to organic phosphorus sources and benomyl. Can J Bot 75:1226–1235
Clearwater MJ, Nifinluri T, van Gardingen PR (1999) Growth responses of wild Shorea seedlings to high light intensity. In: Sist P, Sabogal C, Byron Y (eds) Management of secondary and logged-over forest in Indonesia. Centre for International Forestry Research, Bogor
Coomes DA, Grubb PJ (2000) Impacts of root competition on forests and woodlands: a theoretical framework and review of experiments. Ecol Monogr 70:171–207
de Alwis DP, Abeynayake K (1980) A survey of mycorrhiza in some forest trees in Sri Lanka. In: Mikola P (ed) Tropical mycorrhiza research. Clarendon Press, Oxford
Erland S, Taylor AFS (2002) Diversity of ecto-mycorrhizal fungal communities in relation to the abiotic environment. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology, Ecological Studies 157, pp 163–200
Givnish TJ (1988) Adaptation to sun and shade: a whole plant perspective. Aust J Plant Physiol 15:63–92
Green JJ (1992) Fine root dynamics in a Bornean rain forest. PhD Thesis, University of Stirling
Grubb PJ (1977) The maintenace of species-richness in plant communities: the importance of the regeneration niche. Biol Rev Camb Philos Soc 52:107–145
Hong LT (1979) A note on dipterocarp mycorrhizal fungi. Malay For 42:280–283
Hunt R (1990) Basic growth analysis. Unwin Hyman, London
Ingleby K, Mason PA, Last FT et al (1990) Identification of ectomycorrhizas, ITE Research Publication 5. HMSO, London
Ingleby K, Munro RC, Noor M et al (1998) Ectomycorrhizal populations and growth of Shorea parvifolia (Dipterocarpaceae) seedlings regenerating under three different forest canopies following logging. For Ecol Manage 111:171–179
Ingleby K, Fahmer A, Wilson J et al (2001) Interactions between mycorrhizal colonisation, nodulation and growth of Calliandra calothyrsus seedlings supplied with different concentrations of phosphorus solution. Symbiosis 30:15–28
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–585
Jones MD, Durall DM, Tinker PB (1990) Phosphorus relationships and production of extramatrical hyphae by two types of willow ectomycorrhizas at different soil phosphorus levels. New Phytol 115:259–267
Jülich W (1985) Fungi associated with Dipterocarpaceae in Southeast Asia. 1. The genera Riessia and Riessiella. Int J Mycol Lichenol 2:123–140
Kitayama K (1994) Relative importance of photosynthetic traits and allocation patterns of correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419–428
Leakey ADB (2002) Photosynthetic and growth responses of tropical rain forest dipterocarp seedlings to flecked irradiance. Ph.D. Thesis, University of Sheffield
Lee SS, Alexander IJ (1994) The response of seedlings of two dipterocarp species to nutrient additions and ectomycorrhizal infection. Plant Soil 163:299–306
Lee SS, Alexander IJ (1996) The dynamics of ectomycorrhizal infection of Shorea leprosula seedlings in Malaysian rain forests. New Phytol 132:297–305
Lee SS, Alexander IJ, Watling R (1997) Ectomycorrhizas and putative ectomycorrhizal fungi of Shorea leprosula Miq. (Dipterocarpaceae). Mycorrhiza 7:63–81
Mason PA, Ingleby K, Munro RC et al (2000) The effect of reduced phosphorus concentration on mycorrhizal development and growth of Eucalyptus globulus Labill. seedlings inoculated with 10 different fungi. For Ecol Manage 128:249–258
Nuhamara ST, Hadi S, Bimaatmadja EI (1985) Suspected ectomycorrhizal fungi commonly associated with dipterocarp species. In: Molina R (ed) Proceedings of the 6th North American conference on mycorrhizae. Forest Research Laboratory, Corvallis, Oregon
Pampolina NM, de la Cruz RE, Garcia MU (1995) Ectomycorrhizal roots and fungi of Philippine dipterocarps. In: Brundrett MC, Dell B, Malajczuk N et al (eds) Mycorrhizas for plantation forestry in Asia, ACIAR Proceedings No. 62. Australian Centre for International Agricultural Research, Canberra, pp 47–50
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Prajadinata S, Santoso E (1993) The influence of light intensity on mycorrhizal development on Shorea spp. seedlings. In: Soerianegara I, Supriyanto (eds) Proceedings of second Asian conference on mycorrhiza. BIOTROP Special Publication 42, Bogor, pp 101–106
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol 157:475–492
Sakai S (2002) General flowering in lowland mixed dipterocarp forests of South-east Asia. Biol J Linn Soc 75:233–247
Scholes JD, Press MC, Zipperlen SW (1997) Differences in light energy utilisation and dissipation between dipterocarp rain forest tree seedlings. Oecologia 109:41–48
Singh KG (1966) Ectotrophic mycorrhiza in equatorial rain forests. Malay For 29:13–18
Smits WTM (1992) Mycorrhizal studies in dipterocarp forests in Indonesia. In: Read DJ, Lewis DH, Fitter AH et al (eds) Mycorrhizas in ecosystems. CAB International, Wallingford
Smits WTM (1994) Dipterocarpaceae: mycorrhizae and regeneration. The Tropenbos Foundation, Wageningen
Suhardi (2000) Treatment to develop mycorrhiza formation on dipterocarp seedlings. In: Guhardja E, Fatawi M, Sutisna M et al (eds) Rainforest ecosystems of East Kalimantan: El Niño, drought, fire and human impacts, Ecological Studies 140. Springer, Tokyo, pp 245–250
Suhardi, Darmawan A, Faridah E (1993) Effect of shading, fertilizer and mulching with alang-alang to the early growth and mycorrhiza formation of Shorea bracteolata in Bukit Suharto. In: Anon (ed) BIO-REFOR: Proceedings of Tsukuba-Workshop. BIO-REFOR, IUFRO-SPDC, Tsukuba Science City
Tanner EVJ, Vitousek PM, Cuevas E (1998) Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology 79:10–22
Tecator (1984) Determination of ammonium nitrogen in water by flow injection analysis of gas diffusion. Application Note ASN 50-02/84. Didcot
Tecator Ltd (1983) Determination of orthophosphate based on the stannous chloride method using flow injection analysis. Application Note AN 60/83. Didcot
Turjaman M, Tamai Y, Segah H et al (2006) Increase in early growth and nutrient uptake of Shorea seminis seedlings inoculated with two ectomycorrhizal fungi. J Trop For Sci 18:243–249
Turner IM, Brown ND, Newton AC (1993) The effect of fertilizer addition on dipterocarp seedling growth and mycorrhizal infection. For Ecol Manage 57:329–337
Veenendaal EM, Swaine MD, Lecha RT et al (1996) Responses of west African forest tree seedlings to irradiance and soil fertility. Funct Ecol 10:501–511
Veneklaas EJ, Poorter L (1998) Growth and carbon partitioning of tropical tree seedlings in contrasting light environments. In: Lambers H, Poorter H, van Vuuren MMI (eds) Inherent variation in plant growth: physiological mechanisms and ecological consequences. Backhuys Publishing, Leiden
Vitousek PM (1984) Litterfall, nutrient cycling and nutrient limitation in tropical forests. Ecology 65:285–298
Wallenda T, Kottke I (1998) Nitrogen deposition and ectomycorrhizas. New Phytol 139:169–187
Whitmore TC (1984) Tropical rain forests of the Far East, 2nd edn. Clarendon Press, Oxford
Yap SW, Moura-Costa PH (1994) Potting media for Hopea nervosa, INFAPRO Technical Report No 1. INFAPRO, Lahad Datu
Yap SW, Moura-Costa PH (1996) Effects of nitrogen fertilization and soil texture on growth and morphology of Dryobalanops lanceolata seedlings. In: Appanah S, Khoo AC (eds) Proceedings of the fifth round table conference on dipterocarps. Forest Research Institute of Malaysia, Kepong
Yap SW, Simmons E, Moura-Costa PH (2000) Growth and development responses of Dryobalanops lanceolata Burck and Shorea johorensis Foxw. seedlings to different combinations of nitrogen, phosphorus and potassium concentrations. In: Bista MS, Joshi RB, Amatya SM et al (eds) Proceedings of the 8th international workshop of BIO-REFOR, Kathmandu, Nepal. BIO-REFOR, IUFRO-SPDC, Tokyo
Yazid SM, Lee SS, Lapeyrie FF (1994) Growth stimulation of Hopea spp. (Dipterocarpaceae) seedlings following mycorrhizal inoculation with an exotic strain of Pisolithus tinctorius. For Ecol Manage 67:339–343
Yazid SM, Lee SS, Lapeyrie FF (1996) Mycorrhizal inoculation of Hopea odorata (Dipterocarpaceae) in the nursery. J Trop For Sci 9:276–278
Zipperlen SW, Press MC (1996) Photosynthesis in relation to growth and seedling ecology of two dipterocarp rain forest tree species. J Ecol 84:863–876
Acknowledgements
We thank the British Ecological Society for financial support through their Overseas Research Programme and the Economic Planning Unit of the Prime Minister’s Department of the Government of Malaysia for permission for FQB to work in Sabah. We are grateful to Dainold Yudat and Daulin Yudat for regular help with watering and fertilising the seedlings, Andy Fairburn, Lee Yun Len, Noreen Majalap and Bob Keen for assistance with the nutrient analyses and Robert Bagchi who provided valuable statistical advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brearley, F.Q., Scholes, J.D., Press, M.C. et al. How does light and phosphorus fertilisation affect the growth and ectomycorrhizal community of two contrasting dipterocarp species?. Plant Ecol 192, 237–249 (2007). https://doi.org/10.1007/s11258-007-9325-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-007-9325-6