Abstract
Purpose
Evidence on the prognostic impact of malnutrition was focused on patients with advanced kidney disease. The relationships between malnutrition and all-cause and cardiovascular mortality in patients with different severity of chronic kidney disease (CKD) have not been adequately addressed. We aimed to reveal the prevalence of malnutrition and its prognostic value in patients with different severity of CKD undergoing coronary angiography (CAG).
Methods
This was a multicenter, longitudinal, and retrospective cohort study of 12,652 patients with non-dialysis dependent CKD (defined as estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) undergoing CAG from five tertiary hospitals between January 2007 and December 2020. The controlling nutritional status (CONUT) score was applied to assess nutritional status. Cox regression models and competing risk Fine and Gray models were used to examine the relationships between malnutrition, all-cause and cardiovascular mortality. Further stratified analysis was performed according to baseline CKD severity (mild, moderate and severe, defined by eGFR < 30, 30–44 and 45–59 ml/min/1.73 m2).
Results
During a median follow-up of 5.5 years (interquartile range: 3.2 to 8.6 years), 3801 patients (30.0%) died, and 2150 (17.0%) definitely died of cardiovascular disease. After controlling for confounders, patients had higher all-cause mortality (mild, moderate, and severe vs. absent: HR 1.27, 95 CI % [1.17–1.39]; HR 1.54, 95 CI % [1.39–1.71]; HR 2.22, 95 CI % [1.78–2.77], respectively; P for trend < 0.001) and cardiovascular mortality (mild, moderate and severe vs. absent: HR 1.35, 95 CI % [1.21–1.52]; HR 1.67, 95 CI % [1.45–1.92]; HR 2.10, 95 CI % [1.55–2.85], respectively; P for trend < 0.001) with the severity of malnutrition. In further stratified analysis, a similar prognostic impact of malnutrition was observed in patients with mild to moderate CKD, while mild malnutrition did not seem to have a consistent effect on severe CKD patients.
Conclusion
Malnutrition is common among patients with mild to severe CKD undergoing CAG and is strongly associated with increased risk of all-cause and cardiovascular mortality. Malnutrition seems to have a modestly stronger impact on mortality in patients with mild to moderate CKD.
This study was registered at Clinicaltrials.gov as NCT05050877.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) has become a worldwide health care burden with substantial morbidity and mortality [1, 2]. Cardiovascular disease (CVD) remains the leading cause of death in patients with CKD [3]. Traditional CVD risk factors are prevalent in patients with CKD but do not fully explain the high incidence of cardiovascular events or the increased mortality rates in these patients [4, 5].
Accumulating evidence suggests that malnutrition plays a crucial role in the increasing risk of cardiovascular mortality in patients with renal insufficiency [6,7,8,9]. Comprehensive recommendations on managing nutritional derangements for patients with renal insufficiency have already existed in current management guidelines [10]. However, previous evidence on the prognostic impact of malnutrition was limited by small sample sizes and predominantly focused on patients undergoing dialysis or end-stage renal disease [6,7,8]. Recently, a multicenter prospective study of 2791 patients with CKD suggested that only geriatric nutritional risk index (GNRI) in the 3rd quartile had a weak association with decreased risk of cardiovascular events after adjusting for confounders, which may be limited by over censored events [9]. To date, the prevalence of malnutrition and its association with cardiovascular mortality among patients with CKD have not been reported in a large cohort, and the relationship between malnutrition and mortality in patients with different severity of CKD has not been adequately addressed.
There was no consensus on nutritional screening tools for patients with CKD [10]. Recently, the controlled nutritional status (CONUT) score has been widely confirmed as a stronger nutritional evaluation indicator for predicting mortality risk in the cardio-renal community [6, 11,12,13]. Compared to other nutritional assessment tools, the CONUT score has the advantage that it is well-validated and easy to use.
Accordingly, this study aimed to reveal the prevalence of malnutrition (based on the CONUT score) and its relationship with all-cause and cardiovascular mortality in patients with non-dialysis dependent CKD based on a larger multicenter longitudinal cohort study. Secondly, we aimed to investigate the prognostic impact of malnutrition on all-cause and cardiovascular mortality in different severity of CKD patients.
Methods
Study population
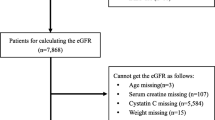
This was a multicenter, longitudinal and retrospective cohort study of 12,652 patients with non-dialysis dependent CKD undergoing coronary angiography (CAG) from five tertiary hospitals between January 2007 and December 2020 in CIN II (Cardiorenal Improvement II, ClinicalTrials.gov NCT05050877) study. A total of 145,267 patients undergoing CAG were initially evaluated, including patients admitted for an elective CAG examination and patients receiving an emergency CAG examination. Among them, 22,898 patients defined as CKD (estimated glomerular filtration rates [eGFR] < 60 mL/min/1.73 m2) were initially included in the study. The exclusion criteria were as follows: (a) patients receiving dialysis or those who were unclear to have received it or not (n = 7509); (b) patients missing follow-up data (n = 93); (c) patients with history of cancer (n = 318); (d) patients aged < 18 years (n = 0); and (e) patients lacking essential data to calculate the CONUT score (n = 2326) (Fig. 1). The study conformed to the principles outlined in the Declaration of Helsinki and was approved by the Guangdong Provincial People's Hospital Institutional Review Board. All participating sites received institutional review board approval from their own ethics committees. Written informed consent was waived due to the retrospective nature of the current study.
Baseline data collection
The patient data were extracted from the electronic clinical management records system, which included demographic characteristics, medical history, laboratory examinations, and medications at discharge. Baseline levels of serum creatinine (Scr), albumin, total cholesterol, lymphocyte count and other biomarkers were measured before CAG. The follow-up information was accessed by matching the survival information from local Centers for Disease Control and Prevention.
Malnutrition screening tools
The CONUT score was developed by Ulibarri et al. [14] in 2005 as a screening tool for the nutritional status of hospitalized patients. It takes into account serum albumin, total cholesterol, and total lymphocyte count. A score of 0 to 1 is considered normal; scores of 2 to 4, 5 to 8, and 9 to 12 reflect mild, moderate, and severe malnutrition, respectively (Supplemental Table S1).
Endpoint and clinical definition
The endpoints were all-cause death and cardiovascular death. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [15], and CKD was defined as eGFR < 60 mL/min/1.73 m2 [16]. Mild, moderate, and severe CKD were defined as subjects with an eGFR < 30, 30–44 and 45–59 ml/min/1.73 m2. Anemia was defined as a hematocrit < 39% (male) or < 36% (female). Congestive heart failure (CHF) was defined as New York Heart Association class > 2 or Killip class > 1 [17]. Acute myocardial infarction (AMI), diabetes mellitus (DM), and hypertension were defined according to the 10th Revision Codes of the International Classification of Diseases (ICD-10).
Statistical analysis
Demographic characteristics, complications, laboratory findings, and discharge medications were described for patients with CKD undergoing CAG in different groups stratified by nutritional status. For the baseline characteristics, continuous variables were summarized as mean and standard or median and quartiles deviation as appropriate. Categorical variables were described as frequencies and percentages. Student t-tests and non-parametric tests (Kruskal–Wallis) were used to compare normally and non-normally distributed variables, respectively. Differences in categorical variables were compared using the chi-square test. The distribution of patients with different renal functions was described by density curves for different nutrition stratified groups. Relationship between all-cause and cardiovascular mortality and malnutrition was analyzed by restricted cubic spline (RCS). Time-to-event data were presented graphically using Kaplan–Meier curves and compared with a log-rank test. The association between nutritional statuses and all-cause mortality was assessed by multivariate Cox regression models, adjusted for age, sex, percutaneous coronary intervention (PCI), comorbidities including DM, AMI, CHF, anemia, hypertension, and laboratory test including low density lipoprotein cholesterol (LDL-C). We generated cumulative incidence function (CIF) curves for competing risk of cardiovascular death. Gray’s tests were used to assess differences in cardiovascular mortality. For cause-specific mortality analyses, we further excluded individuals with unknown causes of death. The association between cardiovascular mortality and nutritional status was examined using competing risk Fine and Gray models, adjusted for the same confounders. Mortality due to other causes were treated as competing risks. We calculated linear P for trend and P for interaction. Further analysis was conducted by using stratification based on the baseline level of kidney function (eGFR < 30, 30–44 and 45–59 ml/min/1.73 m2) [18] and by evaluating effect modification through interaction terms for the respective categories. Data management and statistical analyses were performed by R version 4.0.3. We considered a two-sided P value < 0.05 statistically significant.
Result
Baseline characteristics
Among the 12,652 patients with CKD undergoing CAG (the mean age was 69.0 ± 9.5 years, 31.5% were women) were included, 8692 (68.7%) had malnutrition, of which 48.7%, 18.5%, and 1.5% patients had mild, moderate, and severe malnutrition, respectively. Based on the CONUT score, patients in worse nutritional status groups were significantly older, were more likely to be male, and had a higher prevalence of AMI, CHF, PCI, anemia, hypertension, DM and stroke. They also were more likely to have a lower concentration of LDL-C, as well as a lower proportion of receiving statins and angiotensin converting enzyme inhibitors or angiotensin receptor blocker (ACEI or ARB). More data on the baseline characteristics of the study population are shown in Table 1.
Malnutrition and the baseline level of kidney function
The malnutrition prevalence of patients with mild to severe CKD ranges from 64.2% to 83.3%. The prevalence of moderate and severe malnutrition increases with higher severity of CKD from 14.6% and 0.7 to 32.4% and 4.7%, respectively. (Fig. 2A) From the perspective of malnutrition stratified (no malnutrition, mild, moderate and severe malnutrition), with the deterioration of nutritional status, the number of patients with poorer renal function increases (Fig. 2B).
Malnutrition and clinical outcomes
During a median follow-up of 5.5 years (interquartile range: 3.2 to 8.6 years), 3801 patients (30.0%) died, and 2150 (17.0%) definitely died of cardiovascular disease. The all-cause mortality of absent vs. mild vs. moderate vs. severe malnutrition was 22.5% vs. 30.1% vs. 40.2% vs. 62.2% and cardiovascular mortality of absent vs. mild vs. moderate vs. severe malnutrition: 12.0% vs. 17.1% vs. 23.7% vs. 35.1% in patients with CKD undergoing CAG (Table 1). RCS analysis suggested an approximately linear increase in the risk of all-cause and cardiovascular mortality as the CONUT score increased. (Fig. 3) Kaplan–Meier curves for all-cause mortality and cumulative incidence function curves for cardiovascular mortality according to groups of nutritional status and corresponding univariate analysis, are shown in Fig. 4A, B, respectively. After controlling for confounders, patients had higher all-cause mortality (mild, moderate and severe vs. absent: HR 1.27, 95 CI % [1.17–1.39]; HR 1.54, 95 CI % [1.39–1.71]; HR 2.22, 95 CI % [1.78–2.77], respectively; P for trend < 0.001) and cardiovascular mortality (mild, moderate and severe vs. absent: HR 1.35, 95 CI % [1.21–1.52]; HR 1.67, 95 CI % [1.45–1.92]; HR 2.10, 95 CI % [1.55–2.85], respectively; P for trend < 0.001) with the severity of malnutrition. (Table 2). RCS analyses were further conducted in patients with mild to severe CKD, we found the associations of the CONUT score and risk of all-cause and cardiovascular mortality tend to weaken as kidney function declines. We noted an interaction between mild to severe CKD and the CONUT score for cardiovascular mortality (P for interaction < 0.05), but not for all-cause mortality (Fig. 5). Kaplan–Meier curves for all-cause mortality and cumulative incidence function curves for cardiovascular mortality in patients with mild to severe CKD are showed in Supplemental Figure S1. In addition, a similar prognostic impact of different malnutrition severity on all-cause and cardiovascular mortality was observed in patients with mild to moderate CKD (eGFR 30 mL/min/1.73 m2). However, in patients with severe CKD (eGFR < 30 mL/min/1.73 m2), mild malnutrition did not seem to have a consistent effect on all-cause mortality and cardiovascular mortality (Fig. 6).
All-cause and cardiovascular mortality risk across malnutrition severity in patients with CKD undergoing CAG, Restricted cubic spline depicts the hazard ratio of malnutrition based on the CONUT score for all-cause (left) and cardiovascular mortality (right) in patients with CKD; The bar chart shows the frequency of the CONUT score from 1 to 12 (scores of 0 to 1 are considered normal and 2 to 4, 5 to 8, and 9 to 12 reflect mild, moderate, and severe malnutrition, respectively). Adjusted for the following factors (plot settings): age, sex, acute myocardial infarction, congestive heart failure, percutaneous coronary intervention, diabetes mellitus, hypertension, anemia, low density lipoprotein cholesterol, angiotensin converting enzyme inhibitors or angiotensin receptor blocker, beta blocker, calcium channel blockers and statins
Restricted cubic spline depicting the relationship of the Controlling Nutritional Status (CONUT) score with risk of all-cause and cardiovascular mortality in different severity of CKD patients undergoing CAG. Adjusted for the following factors (plot settings): age, sex, acute myocardial infarction, congestive heart failure, percutaneous coronary intervention, diabetes mellitus, hypertension, anemia, low density lipoprotein cholesterol, angiotensin converting enzyme inhibitors or angiotensin receptor blocker, beta blocker, calcium channel blockers and statins. P for interaction > 0.05 (on all-cause death) and P for interaction = 0.04 (on cardiovascular death)
Forest plot demonstrating the hazard ratio of malnutrition for all-cause and cardiovascular mortality in different severity of CKD patients undergoing CAG. The bar chart shows the population density of the CONUT score from 1 to 12. Adjusted for age, sex, acute myocardial infarction, congestive heart failure, percutaneous coronary intervention, diabetes mellitus, hypertension, anemia, low density lipoprotein cholesterol, angiotensin converting enzyme inhibitors or angiotensin receptor blocker, beta blocker, calcium channel blockers and statins
Discussion
To our knowledge, this was the largest multicenter, longitudinal cohort study to report the prevalence of malnutrition (based on the CONUT score) in patients with different severity of CKD undergoing CAG. The current study was also the first to examine the prognostic value of malnutrition for all-cause and cardiovascular mortality in this population. The findings of our study were that (1) malnutrition was common among patients with CKD undergoing CAG, affecting about 70 percent of patients regardless of the level of kidney function; (2) patients with severe malnutrition had a twofold increased risk of all-cause and cardiovascular mortality than those with normal nutritional status, and all-cause and cardiovascular mortality increased with higher severity of malnutrition; (3) stratified analysis by renal dysfunction status showed that malnutrition had a higher impact on all-cause and cardiovascular mortality in mild to moderate CKD patients.
The comprehensive, well-validated, and easy-to-use tool to identify patients at malnutrition risk is critically needed by the cardio-renal community. Previous studies reported that nutritional screening tools, such as the Mini-Nutritional Assessment (MNA) and subjective global assessment (SGA), could identify patients with renal insufficiency at risk of malnutrition [19, 20]. However, carrying out assessments in a large sample is difficult because of subjective evaluations. For patients with renal insufficiency who underwent CAG, cardiologists usually determine their nutritional status based only on serum albumin levels. However, serum albumin provides limited information about the complex nature of the underlying nutritional problem in the setting of renal insufficiency [21, 22]. Moreover, high cholesterol levels have been preconceived by cardiologists as a poor cardiovascular prognostic factor, even though the cholesterol paradox has been found when patients with poor renal function are malnourished [23]. Compared with other nutritional assessment tools, the CONUT score consisting of total cholesterol, serum albumin, and lymphocyte count has the advantage that it is a well-validated and easy-to-use nutritional assessment tool which comprehensively considers all conditions described above. Recently, Roubín et al. have explored the prognostic consequences of malnutrition in patients with acute coronary syndrome based on the CONUT score, the Nutritional Risk Index (NRI), and the Prognostic Nutritional Index (PNI), and they recommend the use of the CONUT score [11]. Yang et al. have evaluated the effect of objective nutritional indexes, (i.e., the CONUT score, PNI and GNRI) on all-cause mortality and new-onset major adverse cardiovascular events (MACE) in peritoneal dialysis patients, and they suggest that the CONUT score and PNI may provide more useful predictive values than GNRI [6]. However, clinical cardiologists are unaware that screening for malnutrition based solely on albumin misses some high-risk patients. Accordingly, the first lesson that derives from our study is that clinical cardiologists need to use comprehensive nutritional assessment tools to screen CKD patients undergoing CAG for malnutrition. The CONUT score is a comprehensive, well-validated and easy-to-use tool to identify patients at risk of malnutrition.
Notably, malnutrition is common among patients with mild to severe CKD. More relevantly, people with poorer kidney function are more likely to have poor nutritional status. Recently, Guligowska et al. reported a similar result that decreased eGFR was associated with increased risk of malnutrition, which was assessed by albumin level or MNA in elderly subjects aged ≥ 75 years [24] Few large cohort studies have reported the prevalence of malnutrition in CKD patients. A meta-analysis of the global prevalence of malnutrition (defined by subjective global assessment or malnutrition-inflammation score) in kidney disease, which was from contemporary studies between 2000 and 2014, reported that malnutrition prevalence of patients with CKD stages 3–5 ranges from 11 to 54% in five studies including 1776 patients. In addition, the 25th–75th percentiles range in malnutrition prevalence of patients on maintenance dialysis was from 28 to 54% in ninety studies including 16,434 patients [25]. Evidence on the prevalence of malnutrition was limited by small sample sizes and predominantly focused on patients undergoing dialysis. Our study is a multicenter cohort study including more than 12,000 patients with CKD undergoing CAG and may provide more solid evidence on the prevalence of malnutrition in patients with CKD. In our study, the malnutrition prevalence of patients with mild to severe CKD ranged from 64.2 to 83.3%, and the prevalence of moderate to severe malnutrition ranged from 15.3 to 37.1%. Given the high prevalence rate, clinical cardiologists should pay attention to this condition and screening routinely for malnutrition in patients with CKD hospitalized in cardiology department.
Another highlight of this study is that higher severity of malnutrition is strongly associated with increased all-cause and cardiovascular mortality in patients with CKD undergoing CAG. Evidence on the prognostic impact of malnutrition was limited by small sample sizes and predominantly focused on patients undergoing dialysis or end-stage renal disease. Previous studies showed that malnutrition assessed by MNA or SGA was an independent and strong risk factor for all-cause mortality in dialysis patients [26, 27]. As with subjective nutritional indexes, objective nutritional indexes have similar prognostic effects. A retrospective, single-center study of 252 Chinese has demonstrated that malnutrition using the CONUT score is associated with increased all-cause mortality in patients undergoing peritoneal dialysis [28]. Similarly, Panichi et al. have shown that malnutrition assessed by the GNRI is a significant predictor of mortality in patients receiving hemodialysis. However, cardiovascular events did not differ among the GNRI quartiles in the study of Panichi et al. [29]. Recently, Xiong et al. have performed a multicenter prospective study which showed that malnutrition defined by GNRI was associated with renal progression, cardiovascular events, and all-cause mortality in a cohort of 2791 patients with CKD [9]. However, the relationship between GNRI and cardiovascular events trended to be non-significant after a fully adjusted multivariate analysis. Moreover, for patients with different severity of CKD, the associations between malnutrition, all-cause and cardiovascular mortality have been scarcely reported. We further demonstrated that higher severity of malnutrition assessed by the CONUT was significantly associated with an increased risk of cardiovascular mortality. Malnutrition, inflammation, and atherosclerosis (MIA) syndrome are possible explanations for the association between malnutrition and higher cardiovascular mortality risk in patients with CKD [30]. Chronic inflammation is a common condition of CKD. Inflammation can induce additional catabolism and anorexia in CKD patients, leading to protein-energy wasting (PEW), impaired nutritional status and higher risk of cardiovascular diseases and mortality [31]. In a 5-year follow-up of 310 Swedish patients, mortality was greater in patients with a C-reaction protein (CrP) > 10 mg/l and a worse nutritional status [32]. Moreover, we also explored the relationships between malnutrition, all-cause and cardiovascular mortality in patients with different severity of CKD, and found that malnutrition had a higher impact on all-cause and cardiovascular mortality in patients with mild to moderate CKD, while mild malnutrition did not seem to have a consistent effect on all-cause mortality and cardiovascular mortality in patients with severe CKD. Thereinto, no significant difference in the risk of cardiovascular mortality was observed between no malnutrition and severe malnutrition in patients with moderate CKD (eGFR 30–44 mL/min/1.73 m2). We speculated that the statistical power was diminished in that the number of cardiovascular deaths was limited in severely malnourished patients with moderate CKD. The likely explanation is that patients with more advanced stages of CKD were more likely to be weaker and their conditions were poorer, thereby associated with a relatively high risk of mortality at baseline. Thus, the contribution of malnutrition to mortality is diminished in this setting.
The results from our study could have the following clinical implications. The CONUT score is an objective nutritional index which uses only three inexpensive laboratory values routinely measured in the cardiology department and is very easy to calculate, as well as can be widely used to identify mortality risk in CKD patients undergoing CAG. A recent longitudinal study of advanced CKD inpatients at nutritional risk found that individualized nutrition education had long-term improvements in nutritional status, reduced hospital admissions, and improved survival [33]. Our findings suggest that individualized nutrition education may also improve long-term prognosis in patients with CKD, and may even be more beneficial in patients with mild to moderate CKD. Further investigation is needed to prospectively evaluate the efficacy of nutritional interventions on outcomes in patients with mild to moderate CKD.
Study limitations
This is a retrospective observational study with the subsequent disadvantages secondary to its nature, so our inferences did not reflect direct causality. Besides, we only adopted the CONUT score as the assessment tool for malnutrition. Due to the retrospective nature of the study, certain baseline variants had relatively a high missing rate, such as body mass index and waist to hip circumference, which could help us apply alternative and comprehensive nutritional assessment tools to understand the nutritional status and verify our findings in this population. Furthermore, nephrotic syndrome is a possible cause for patients with hypoalbuminemia. Due to proteinuria was not routinely tested in this population, patients with nephrotic syndrome could not be easily recognized and excluded in this study. Similarly, chronic inflammation is prevalent and linked with impaired nutritional status and in CKD patients, but it could not be properly recognized and adjusted during the analysis, because inflammation markers such as serum C-reaction protein (CrP) were not routinely tested in this population. Lastly, we only evaluated preoperative nutritional indexes and did not record changes during the follow-up period, and the effect of nutritional support was also neglected. Well-designed and well-executed future studies are needed to examine the predictive validity of the CONUT score in general CKD patients.
Conclusions
Malnutrition is common among patients with CKD undergoing CAG regardless of the level of kidney function. Higher severity of malnutrition is strongly associated with higher all-cause mortality and cardiovascular mortality in patients with CKD undergoing CAG, and this correlation appears to be stronger in patients with mild to moderate CKD.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding authors.
References
Cockwell P, Fisher LA (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395(10225):709–733
Shlipak MG, Tummalapalli SL, Boulware LE, Grams ME, Ix JH, Jha V, Kengne AP, Madero M, Mihaylova B, Tangri N et al (2021) The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int 99(1):34–47
Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y et al (2020) US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 75(1 Suppl 1):A6-a7
Longenecker JC, Coresh J, Powe NR, Levey AS, Fink NE, Martin A, Klag MJ (2002) Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol 13(7):1918–1927
Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ (2007) The framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 50(3):217–224
Yang Y, Zhou H, Zhang P, Chao W, Zou Y, Yang M (2020) Evaluation of objective nutritional indexes as predictors of worse outcomes in peritoneal dialysis patients. Nutrition 79–80:110963
Xiong J, Wang M, Zhang Y, Nie L, He T, Wang Y, Huang Y, Feng B, Zhang J, Zhao J (2018) Association of geriatric nutritional risk index with mortality in hemodialysis patients: a meta-analysis of cohort studies. Kidney Blood Press Res 43(6):1878–1889
Kiuchi A, Ohashi Y, Tai R, Aoki T, Mizuiri S, Ogura T, Aikawa A, Sakai K. (2016) Association between Low Dietary Protein Intake and Geriatric Nutrition Risk Index in Patients with Chronic Kidney Disease: A Retrospective Single-Center Cohort Study. Nutrients 8 (10).
Xiong J, Wang M, Wang J, Yang K, Shi Y, Zhang J, Zhang B, Zhang L, Zhao J (2020) Geriatric nutrition risk index is associated with renal progression, cardiovascular events and all-cause mortality in chronic kidney disease. J Nephrol 33(4):783–793
Fiaccadori E, Sabatino A, Barazzoni R, Carrero JJ, Cupisti A, De Waele E, Jonckheer J, Singer P, Cuerda C (2021) ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin Nutr 40(4):1644–1668
Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, Barreiro Pardal C, Lizancos Castro A, Parada JA, Pérez DD, Blanco Prieto S, Rossello X, Ibanez B et al (2020) Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol 76(7):828–840
Seo M, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Kawai T, Abe M et al (2019) Prognostic significance of serum cholinesterase in patients with acute decompensated heart failure: a prospective comparative study with other nutritional indices. Am J Clin Nutr 110(2):330–339
Sze S, Pellicori P, Zhang J, Weston J, Clark AL (2021) The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am J Clin Nutr 113(3):695–705
Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G (2005) CONUT: a tool for controlling nutritional status first validation in a hospital population. Nutr Hosp 20(1):38–45
Aguiar-Souto P, Ferrante G, Del Furia F, Barlis P, Khurana R, Di Mario C (2010) Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol 139(1):68–74
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130(6):461–470
Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW et al (2004) A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 44(7):1393–1399
Bansal N, Hyre Anderson A, Yang W, Christenson RH, deFilippi CR, Deo R, Dries DL, Go AS, He J, Kusek JW et al (2015) High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the chronic renal insufficiency cohort (CRIC) study. J Am Soc Nephrol 26(4):946–956
Tsai AC, Wang JY, Chang TL, Li TY (2013) A comparison of the full mini nutritional assessment, short-form mini nutritional assessment, and subjective global assessment to predict the risk of protein-energy malnutrition in patients on peritoneal dialysis: a cross-sectional study. Int J Nurs Stud 50(1):83–89
Khor BH, Tiong HC, Tan SC, Abdul Rahman R, Abdul Gafor AH (2020) Protein-energy wasting assessment and clinical outcomes in patients with acute kidney injury: a systematic review with meta-analysis. Nutrients 12(9):2809
Jeejeebhoy KN (1998) Nutritional assessment. Gastroenterol Clin North Am 27(2):347–369
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1):16–31
Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ (2004) Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 291(4):451–459
Guligowska A, Corsonello A, Pigłowska M, Roller-Wirnsberger R, Wirnsberger G, Ärnlöv J, Carlsson AC, Tap L, Mattace-Raso F, Formiga F et al (2020) Association between kidney function, nutritional status and anthropometric measures in older people: the screening for CKD among older people across Europe (SCOPE) study. BMC Geriatr 20(Suppl 1):366
Carrero JJ, Thomas F, Nagy K, Arogundade F, Avesani CM, Chan M, Chmielewski M, Cordeiro AC, Espinosa-Cuevas A, Fiaccadori E et al (2018) Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J Ren Nutr 28(6):380–392
de Mutsert R, Grootendorst DC, Boeschoten EW, Brandts H, van Manen JG, Krediet RT, Dekker FW (2009) Subjective global assessment of nutritional status is strongly associated with mortality in chronic dialysis patients. Am J Clin Nutr 89(3):787–793
Brzosko S, Hryszko T, Kłopotowski M, Myśliwiec M (2013) Validation of mini nutritional assessment scale in peritoneal dialysis patients. Arch Med Sci 9(4):669–676
Zhou H, Chao W, Cui L, Li M, Zou Y, Yang M (2020) Controlling Nutritional Status (CONUT) score as immune-nutritional predictor of outcomes in patients undergoing peritoneal dialysis. Clin Nutr 39(8):2564–2570
Panichi V, Cupisti A, Rosati A, Di Giorgio A, Scatena A, Menconi O, Bozzoli L, Bottai A (2014) Geriatric nutritional risk index is a strong predictor of mortality in hemodialysis patients: data from the Riscavid cohort. J Nephrol 27(2):193–201
Maraj M, Kuśnierz-Cabala B, Dumnicka P, Gala-Błądzińska A, Gawlik K, Pawlica-Gosiewska D, Ząbek-Adamska A, Mazur-Laskowska M, Ceranowicz P, Kuźniewski M (2018) Malnutrition, inflammation, atherosclerosis syndrome (mia) and diet recommendations among end-stage renal disease patients treated with maintenance hemodialysis. Nutrients 10(1):69
Fouque D, Pelletier S, Mafra D, Chauveau P (2011) Nutrition and chronic kidney disease. Kidney Int 80(4):348–357
Avesani C, Carrero J, Axelsson J, Qureshi AR, Lindholm B, Stenvinkel P (2006) Inflammation and wasting in chronic kidney disease: partners in crime. Kidney Int 70:S8–S13
Pérez-Torres A, González García ME, Ossorio-González M, Álvarez García L, Bajo MA, Del Peso G, Castillo Plaza A, Selgas R. (2021) The Effect of Nutritional Interventions on Long-Term Patient Survival in Advanced Chronic Kidney Disease. Nutrients13 (2) 621
Funding
This work was supported by grants from Beijing Lisheng Cardiovascular Health Foundation (LHJJ20141751); Guangdong Provincial science and technology project (2020B1111170011, KJ022021049); Guangdong Provincial People's Hospital Foundation (DFJH201919), and National Science Foundation of China (81670339, 81970311). The work was not funded by any industry sponsors.
Author information
Authors and Affiliations
Contributions
FG, ZDH, JYC: research idea and study design. ZDH, JJL, YK, YHL, YBH, JHC, SSX and XMY: data acquisition. JL and SQC: data analysis/interpretation. ZDH and ZWZ: statistical analysis. YL and JYC: supervision and mentorship. DQH, AW, XMY and YL: writing guidance. All authors contributed important intellectual content during manuscript drafting or revision and accept accountability for the overall work by ensuring that questions on the accuracy or integrity of any portion of the work are appropriately investigated and resolved, and read and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics statement
All traceable personal identifiers were removed from the analytic dataset to protect patients’ privacy. The study protocol was approved by Guangdong Provincial People’s Hospital ethics committee and the study was performed according to the declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, F., Huang, Z., Liang, J. et al. Association of malnutrition with all-cause and cardiovascular mortality in patients with mild to severe chronic kidney disease undergoing coronary angiography: a large multicenter longitudinal study. Int Urol Nephrol 55, 3225–3236 (2023). https://doi.org/10.1007/s11255-023-03566-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03566-5