Abstract
Purpose
Free calcium is the gold standard for diagnosis of calcium disorders, although calcium assessment is routinely performed by albumin-adjusted calcium. Our objective was to develop a novel-specific correction equation for free calcium employing serum total calcium and other analytes.
Methods
Retrospective single-center cohort study. A new equation for free calcium assessment was formulated from data of hospitalized patients (n = 3481, measurements = 7157) and tested in a validation cohort (n = 3218, measurements = 6911). All measurements were performed simultaneously from the same blood draw.
Results
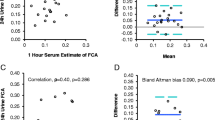
Total CO2 and phosphate, in addition to albumin, were the principal factors associated to calcium misdiagnosis. A novel laboratory-specific prediction equation was developed: free calcium (mmol/L) = 0.541 + (total calcium [mmol/L] *0.441) – (serum albumin [g/L] *0.0067) – (serum phosphate [mmol/L] *0.0425) – (CO2 [mmol/L] *0.003). This new equation substantially improved adjusted R2 to 0.67 (95% CI 0.78–0.82, p < 0.001; Kendall’s c-tau: 0.28, p < 0.001). Bland–Altman plots of estimated free calcium and free calcium showed a mean difference of − 0.0006 mmol/L (LOA + 0.126 to − 0.124). In validation cohort, the AUC–ROC curves for hypercalcemia and hypocalcemia diagnosis deploying the new equation were 0.88 (95% CI 0.86–0.89, p < 0.001) and 0.98 (95% CI 0.97–99, p < 0.001), respectively, which were superior to historical formulas for calcium. In univariate models, eGFR was associated with Ca-status misdiagnosis, yet this association disappeared when analysis was adjusted to phosphate and CO2.
Conclusions
The novel equation proposed for prediction of free calcium could be useful when free calcium is not available. The conventional formulas misclassify many patients, in particular when phosphate or bicarbonate disturbances are present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Direct measurement of free, hydrated divalent calcium cation in serum or plasma (commonly refer as ionized calcium) is considered the gold standard for diagnosis of calcium disorders [1]. Nevertheless, calcium-status assessment is routinely performed by albumin-adjusted calcium, since free calcium measurement is a time-consuming process with complex preanalytical requirements and uncertainties about cost-effectiveness [2].
Calcium circulates freely in blood (~ 50%), bound to plasma proteins (~ 40%, predominantly albumin) or complexed with anions such as phosphate, bicarbonate, citrate, and lactate (~ 10%) [3]. Hyperphosphatemia, acidosis, and other metabolic disarrangements modify calcium binding and increase the risk of calcium-status misdiagnosis when assessment is performed by albumin-adjusted calcium. The discrepancy between total/albumin-adjusted and free calcium has arisen events of “hidden hypercalcemia” (high free calcium with normal or low total calcium/albumin-adjusted calcium) and “hidden hypocalcemia” (low free calcium with normal or high total calcium/albumin-adjusted calcium). In hemodialysis patients, hidden hypercalcemia is associated with a higher mortality risk [4].
The primary aim of the present study was to develop a novel laboratory-specific correction equation for free calcium prediction according to estimate-regression coefficients of linear models taking into account other relevant explanatory variables such as acid–base status, phosphatemia, and kidney function calculated from data of hospitalized patients. The secondary aim was to determine laboratory factors related to discordant calcium-status diagnosis.
Materials and methods
This is a retrospective study from a single tertiary-care center in Mexico City. Two cohorts were selected to develop the new equation: an initial derivation cohort (January 1st, 2018–December 31st, 2018) and a validation cohort (January 1st, 2019–December 15th, 2019). Derivation and validation cohorts included 3481 and 3218 hospitalized patients aged ≥ 18 years to whom simultaneous measurements of free calcium and total serum calcium were performed from the same blood draw. These initially selected subjects were further screened for serum concentrations of albumin, phosphate, bicarbonate, creatinine, blood-urea nitrogen, pH (arterial or venous), lactate, total CO2, and magnesium, obtained in the simultaneous blood drawing. To ensure reliability, we excluded outlier free calcium measurements (n = 8) that were < 0.1 or > 99.9 percentile of observed free calcium (< 0.2 or > 9.98 mmol/L [< 0.8 or > 40.04 mg/dL]) in both cohorts. All outliers’ values excluded were clearly invalid measurements according to repeated calcium values on the same subject and other clinical characteristics. Calcium measurements from subjects with incomplete data (n = 125) and those processed after 2 h of puncture (n = 34) were also excluded.
All blood samples were drawn using uniform techniques, determined simultaneously on the same serum sample, and analyzed in a central laboratory typically within 10–15 min after obtaining the sample, according to our institutional protocol. Serum-free calcium was measured by the potentiometric method with ion-selective electrodes by ABL90 instruments, Radiometer Medical, standardized at pH 7.40 (Copenhagen, Denmark).
The reference range for free calcium was 1.07–1.3 mmol/L (4.3–5.2 mg/dL), limits: 0.2–9.98 mmol/L (0.8–40.04 mg/dL). The within-run and between-run coefficient variation (CV) were 1.9% and 1.9%, respectively, at a free calcium level of 0.75 mmol/L (3 mg/dL). The within-run and between-run CV were 1.4% and 1.6% at a free calcium level of 1.75 mmol/L (7 mg/dL). Hypercalcemia by free calcium levels was defined as > 1.30 mmol/L (> 5.3 mg/dL) [5]. Mild hypocalcemia was defined as 1–1.06 mmol/L (4.0–4.29 mg/dL), moderate hypocalcemia as 0.8–0.99 mmol/L (3.2–3.9 mg/dL), and severe hypocalcemia as a free calcium < 0.8 mmol/L (< 3.2 mg/dL) [6].
Total calcium was assayed using Arsenazo III reagent colorimetric method on fully automated modular instrument (reference range 2.15–2.57 mmol/L (8.6–10.3 mg/dL), Beckman Coulter, USA). The within-run and between-run CV was 2% and 2.5%, respectively, at a concentration 2.57 mmol/L (10 mg/dL). Hypocalcemia by total calcium and albumin-adjusted calcium were classified as mild 2.0–2.14 mmol/L (8.0–8.59 mg/dL), moderate 1.87–1.99 mmol/L (7.5–7.99 mg/dL), and severe < 1.87 mmol/L (7.5 mg/dL) [7]. Estimated glomerular filtration rate (eGFR) was performed by CKD-EPI formula [8]. The study was approved by the institutional review board (reference number 3204). Patients’ written informed consent was exempted because of the retrospective nature of study.
Means and standard deviations or medians and interquartile range are described for continuous variables, and frequency and percentages are reported for discrete variables. According to the STROBE guidelines, significance tests were avoided in descriptive tables. The normality of data was checked using Kolmogorov–Smirnov test. Logarithmic transformation was performed to variables with non-parametric distribution. Hidden hypocalcemia and hidden hypercalcemia were defined as a normal value of albumin-adjusted calcium/total calcium with a free calcium value < 1.07 or > 1.30 mmol/L (< 4.3 or > 5.2 mg/dL), respectively. Factors related to calcium-status misdiagnosis were established using logistic regression analysis. Concordances of calcium-status diagnosis were evaluated using kappa coefficient and C-Kendall’s Tau [9]. Agreement between free calcium and estimated free calcium with the new formula was compared using Bland–Altman limits of agreement (LOA).
Univariate analysis by linear regression was performed taking free calcium converted on z-scores as the dependent variable. Independent variables were tested and those with a p value ≤ 0.15 were selected to perform a further multiple-linear regression analysis (stepwise approach). Intraclass concordance coefficient values were calculated as a measure of reliability. We evaluated six models to develop a novel correction equation and assessed the predictive ability of the new formulas compared to other formulas in both cohorts by adjusted R2 with 95% CI based on all bootstrap samples. All models included parameters commonly measured in serum chemistry studies. Akaike’s information criterion (AIC) was calculated for each model and used as model fitness comparator for each model. The model with reduced number of predictors was obtained. Total CO2 values were employed in calculations instead of venous bicarbonate, to avoid blood gas determinations given that free calcium is commonly reported in blood gas analysis in which case the formula would not be necessary. Receiver-Operating Characteristic (ROC) curve analyses were performed to compare the area under curve (AUC) for diagnosis of calcium status (hypo- or hypercalcemia) between published formulas employed to correct calcium, including our new models. All models were succinctly electronically organized, and a free mobile application was deployed into iOS and Android platforms (Apple, Cupertino, CA, USA and the Google Play Store, Mountain View, CA, USA). Statistical analysis and graphics were performed with SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) and Graphpad prism 5 (San Diego, CA, USA), respectively. The results were considered statistically significant when p < 0.05.
Results
In total, we analyzed 7157 and 6911 blood measurements from 3481 and 3218 subjects in the derivation and the validation cohort, respectively. In Table 1, demographic and clinical data, including age, sex, and hospitalization diagnosis, from medical records are presented. No clinically meaningful differences were observed between cohorts. In the derivation cohort, hypercalcemia and hypocalcemia according to venous free calcium were present in 3.8% (279) and 22% (1606) measurements, respectively. In the validation cohort, 5.5% (381) and 20% (1435) measurements were classified as hypercalcemia and hypocalcemia, respectively.
Total calcium and albumin-adjusted calcium misclassify calcium status frequently in both cohorts. In derivation cohort, 141 (141/279, 50%) and 60 (60/279, 21%) measurements were incorrectly diagnosed as non-hypercalcemic using total calcium and albumin-adjusted calcium, respectively. Likewise, 255 (255/1606, 16%) and 946 (946/1606, 59%) were incorrectly categorized as non-hypocalcemic using total calcium and albumin-adjusted calcium, respectively (Supplementary Tab. 1). The agreement of calcium status between free calcium and total calcium or albumin-adjusted calcium was fair (κ = 0.26 and κ = 0.36, respectively, both p < 0.001). Kendall’s c-tau correlations were 0.19 and 0.23 for total calcium and albumin-adjusted calcium, respectively. The Spearman correlations between free calcium vs. total calcium and free calcium vs. albumin-adjusted calcium were 0.66 and 0.67, respectively (p < 0.001); the adjusted R2 between free calcium vs. total calcium and free calcium vs. albumin-adjusted calcium were 0.48 (slope = 0.29, 95% CI 0.28–0.30) and 0.58 (slope = 0.44, 95% CI 0.43–0.46), respectively (Fig. 1A and B).
In derivation cohort, hidden hypercalcemia diagnosis occurred in 141 (2%) and 60 (0.8%) according to total calcium and albumin-adjusted calcium. The principal risk factor associated with hidden hypercalcemia diagnosis was CO2 value using total calcium or albumin-adjusted calcium for diagnosis (Supplementary Tab. 2). A CO2 value < 18 mmol/L had an OR of 4.0 (95% CI 2.5–6.3, p < 0.001) for hidden hypercalcemia diagnosis when albumin-adjusted calcium was employed. A CO2 value < 15 mmol/L increases the OR to 5.1 (95% CI 3.0–8.5, p < 0.001). Besides CO2, magnesium was the other factor independently related to hidden hypercalcemia diagnosis. A serum magnesium > 0.91 mmol/L (> 2.2 mg/dL) had an OR of 2.1 (95% CI 1.4–3.4, p < 0.001). A low serum phosphate (< 0.81 mmol/L [< 2.5 mg/dL]) was associated with a slight risk increase of hidden hypercalcemia diagnosis (OR 1.64, 95% CI 1.11–2.41, p: 0.013) only when total calcium was employed for calcium diagnosis. Hidden hypocalcemia was present in 255 (3.6%) and 946 (13.2%) measurements according to total calcium or albumin-adjusted calcium, respectively. When albumin-adjusted calcium was utilized for diagnosis, high phosphate levels and high CO2 were associated with hidden hypocalcemia (Supplementary Tab. 3). A serum phosphate concentration > 1.42 mmol/L (> 4.4 mg/dL) had an OR of 1.75 (95% CI 1.51–2.04, p < 0.001), and a phosphate > 1.78 mmol/L (> 5.5 mg/dL) increased the OR to 2.10 (95% CI 1.75–2.52).
In the derivation cohort, subjects (n = 1532, 44%) with eGFR < 60 mL/min/1.73m2 in the context of CKD and/or AKI were older and had a higher concentration of total calcium, phosphate, CO2, and pH, among others (Supplementary Tab. 4). These subjects had more measurements with misdiagnosis of calcium status compared with those with eGFR > 60 mL/min/1.73m2 (1819 (45%) vs. 1221 (18%) respectively, p = 0.001). A low eGFR was associated to hidden hypocalcemia (eGFR < 60 mL/min/1.73m2 had an OR 1.46, 95% CI 1.27–1.68; eGFR < 30 mL/min/1.73m2 had an OR 1.60, 95% CI 1.37–1.86; both with p < 0.001). Nevertheless, this low eGFR association did not have an independent association with calcium misdiagnosis given that these associations were modified according to phosphate levels and CO2 value in multivariate analysis (see below). In 358 (23%) subjects with eGFR < 60 mL/min/1.73m2 and normal values of albumin, phosphate, and CO2, only 45 (0.7%) measurements had an incorrect diagnosis of calcium status.
Development of new formula
We performed a multivariate linear regression analysis with Akaike’s information criterion to model formulas (Table 2). The univariate analysis found nine variables to predict free calcium: transformed age (log age: F = 9.26, β = 0.125, p = 0.002), sex (male F = 13.01, β = 0.001, p < 0.001), transformed pH (log pH: F = − 15.45, β = − 6.48, p:0.002), transformed venous pCO2 (log pCO2: F = 17.54, β = 0.30, p < 0.001), transformed albumin (log albumin F = 196.75, β = 0.71, p < 0.001), transformed serum creatinine (log creatinine F = 83.16, β = − 0.17, p < 0.001), transformed CO2 (log CO2: F = 0.56, β = 0.48, p < 0.001), transformed phosphate (log phosphate: F = 144.72, β = − 0.462, p > 0.001), and transformed magnesium (log magnesium: F = 6.28, β = 0.202, p:0.012). We identified only three independent variables to predict free calcium in addition to total calcium: serum albumin, phosphate, and CO2.
Based on these models, we developed the following equation converted into International System of Units with a substantially improved adjusted R2 (0.65):
Equation for free calcium converted to conventional units
We evaluated other models according to data availability, model 1 includes 4 variables, model 2 and 3, 2 variables, and model 4–6 only 1 variable (Table 2). The model with 4 variables substantially improves adjusted R2 (0.65, 95% CI 0.78–0.82, p < 0.001, slope = 0.99 95% CI 0.98 to 1.02, Fig. 1C) as compared to total calcium and albumin-adjusted calcium calculation which were less than 0.60. Bland–Altman plots of estimated free calcium by the formula with 4 variables showed a bias of − 0.0006 mmol/L (LOA + 0.126 to − 0.124) and performed better than other models with less variables (Fig. 2). For diagnosis of hypocalcemia and hypercalcemia, concordance index and AUC ROC curved for estimated free calcium with new formula were superior to total calcium or albumin-adjusted calcium (Fig. 3). The performance of the new formula was superior to detect hypocalcemia (free calcium < 1.07 mmol/L [< 4.3 mg/dL]) vs. hypercalcemia (free calcium > 1.3 mmol/L [> 5.2 mg/dL]) with an AUC ROC of 0.92 (95% CI 0.89–0.94) Vs 0.84 (95% CI 0.82–0.84), respectively. The number of hidden hypocalcemia measurements was inferior with the new formula compared to albumin-adjusted calcium (780 vs. 946, p < 0.001). Setting an estimated free calcium upper limit of 1.3 mmol/L (5.2 mg/dL), 21% (60/279) cases with hypercalcemia by free calcium were misclassified as normocalcemic according to the new formula. Even this number of miscues was inferior to albumin-adjusted calcium, the difference was not statistically significant (60 vs. 74, p: 0.22). Adjusting to a cut-off point of > 1.25 mmol/L (> 5.0 mg/dL), the number of hypercalcemia cases detected by the new formula increased from 219 to 244 (AUC: 0.86, sensitivity: 99%, specificity: 79%, p < 0.001).
Validation of the new formula
The new formula (4 variables) for estimated calcium consistently performed better than total calcium and albumin-adjusted calcium in the validation cohort. The model proposed in the validation cohort was well calibrated; the R2 estimated shrinkage factor was 0.001 in all models tested. The AUC–ROC for hypercalcemia and hypocalcemia diagnosis deploying the new equation were 0.88 (95% CI 0.86–0.89, p < 0.001) and 0.98 (95% CI 0.97–99, p < 0.001), respectively. Table 3 shows the agreement on classification by calcemic status, area under curve, and likelihood ratio for hypocalcemia and hypercalcemia diagnosis between the new model (4 variables) and 7 equations for adjust calcium found in previous publications (Supplementary Tab. 5 describes each mathematical formulation) [10,11,12,13,14,15,16]. Historical equations which included total proteins in their calculations were discarded due to the lack of total serum protein measurements in the derivation cohort [17,18,19]. The new formula was superior to other formulas for diagnosis of hypocalcemia when abnormal values of phosphate or CO2 values were present accordingly to 95% CI of AUC–ROC curves. For hypercalcemia diagnosis, the formula described by Obi et al. [16] and the new formula had a similar performance.
All models depicted in Table 2 were succinctly electronically organized, and a software was successfully deployed into iOS (Apple, Cupertino, CA) platform (App “True Calcium”, https://apps.apple.com/gt/app/true-calcium/id1479639808). The new mobile application allows rapid free calcium estimation, even if one or two data are missing.
Discussion/conclusion
In this large, observational study of more than 6500 subjects (derivation and validation cohorts), we developed and validated a new equation for estimation of free calcium in hospitalized patients. The new formula estimates free calcium concentration not only by adjusting for serum albumin concentration, but also according to phosphate and CO2 serum concentrations. Compared to other equations previously published, our formula consistently showed a greater correlations and concordance with calcium-status diagnosis, including severity of hypocalcemia and hypercalcemia. Our results are consistent with those published previously by Obi et al. [16], in which a calcium correction equation for total calcium derived from 242 American-hemodialysis patients incorporated phosphate and bicarbonate in its formulations. Our new model is easily accessible to an application of free access for physicians and could be automatically implemented in laboratory results. We believe that free calcium concentrations should always be measured directly. Nevertheless, this is not always feasible for many reasons, including laboratory performance, method availability, practicality of sample handling, lack of automated analysis, and many recommendations by guidelines fail to underline the necessity of this parameter [2].
Our results restate the fact that albumin-adjusted calcium is inaccurate. More than 50% and 25% of hypocalcemia and hypercalcemia measurements, respectively, in both cohorts were diagnosed as “false negatives” using albumin-adjusted calcium for diagnosis. Other studies have shown the poor association between total calcium and albumin-adjusted calcium [20,21,22].
The principal risk factor for hidden hypercalcemia was a low concentration of CO2. It is a well-known fact that acid–base disorder can lead to changes in calcium status, and the free calcium concentration may increase in metabolic acidosis due to less albumin binding without an alteration in the total serum calcium concentration [23]. A fall in pH of 1 unit will cause approximately a 0.4 mmol/L [1.6 mg/dL] rise in the free calcium levels [24]. In patients with stages 3–5 CKD, a low total CO2 concentration increases the risk for underestimation of free calcium levels [21]. In renal transplant recipients, the diagnosis of hidden hypercalcemia is missed in almost 80% which may be explained, at least partly, by the fact that none of the used formulas provided a calcium correction for low CO2 concentration [25]. Treatment with bicarbonate therapy or dialysis can lower the free calcium level and precipitate symptoms such as tetany in subjects with severe CKD, hypocalcemia and metabolic acidosis [26]. In our study, the high prevalence of metabolic acidosis (25%), defined as a venous HCO3 concentration < 22 mmol/L and a venous pH < 7.35, and hyperphosphatemia (32%) may explain why metabolic acidosis was a strong independent predictor of hidden hypercalcemia, while hyperphosphatemia was a predictor of hidden hypocalcemia.
It is well known that phosphate and other anions increase the ion-bound component of total calcium, although most of the previous calcium formulas have ignored these confounding factors. Our data are in line with the observations by Obi et al. who demonstrated an important effect of phosphate in free calcium concentrations from hemodialysis patients [4]. In vitro data suggest that the inverse relationship between free calcium and phosphate may increase by 50% when serum albumin concentrations is decreased from 40 to 20 g/L (the free calcium decreases 0.04 mmol/L per every mmol/L of phosphate for albumin at 40 g/L and 0.06 mmol/L per every mmol/L of phosphate for albumin at 20 g/L) [22]. An abrupt increase in serum phosphate levels may increase the risk of hypocalcemia [27]. There are reports of life-threatening cardiac events related to hypocalcemia following an abrupt increase in serum phosphate [28]. In a large cohort of 14 772 hospitalized patients, disturbances in calcium phosphate on admission were associated with in-hospital mortality, and highest risk was observed when total calcium-phosphate product were > 45 mg2/dL2 in both CKD and non-CKD patients [29]. In other cohort of hospitalized patients, changes in serum phosphate levels were significantly associated with in-hospital mortality independently of changes in glomerular filtration rate [30]. We believe that the new formula could improve the assessment of calcium changes in subjects with phosphate disturbances.
Almost a quarter of hospitalized patients have an abnormal calcium concentration according to reference values of free calcium, usually mild hypocalcemia. It is possible that the use of this new equation increases the number of mild asymptomatic hypocalcemia cases, something already observed when universal measurement of free calcium is employed [6]. There is controversy over whether these cases require medical treatment, in regard to evidence that links low free calcium and protective/adaptative phenomena in ill patients [31]. Further studies are needed to expand the understanding of the importance of this high frequency of hypocalcemia status.
Hypercalcemia diagnosis occurred at a low frequency (< 5% in both cohorts). Nevertheless, hidden mild hypercalcemia cases occurred between 25 and 39%, even with the new formula. When we decrease the cut-off value for hypercalcemia diagnosis to 5.0 mg/dL [1.25 mmol/L] with the new estimation, the rate of hidden hypercalcemia significantly decreases. It is possible, that with the new formula, a high borderline value (> 1.3 mmol/L [> 4.9 mg/dL]) should raise our suspicion about the presence of hypercalcemia. Ideally, this value should be confirmed by a direct free calcium measurement, to detect promptly hypercalcemia, a modifiable risk factor for mortality and vascular calcification, especially in patients with CKD [32].
In this paper, our model was validated for application in heterogeneous clinical practice environments, including patients with varying degrees of renal dysfunction. Kidney diseases have been proposed as a risk factor for calcium misdiagnosis [33, 34]. The concentration of free calcium is dependent of many of biochemical parameters modified by kidney disease such as acidosis, hyperphosphatemia, or hypoalbuminemia, among others [35]. Unfortunately, most of the formulas to adjust calcium are associated with substantial inaccuracy and erroneous diagnosis, especially in chronic kidney disease patients [21, 36, 37]. For example, some authors suggest do not employ albumin-adjusted calcium formula, especially in patients with albumin below 30 g/L (3.0 g/dL) and impaired renal function [38]. A high proportion (~ 30%) of end-stage renal disease (ESRD) patients are incorrectly categorized as normocalcemic by either total calcium or albumin-adjusted calcium. In 1536 patients with eGFR less than 60 mL/min/1.73m2, the diagnostic accuracy of total calcium outperformed all formulas [39]. In our study, we identified that only the subpopulation with abnormal phosphate, CO2, and/or magnesium concentrations had an increased risk of calcium misdiagnosis when conventional formulas were employed. Patients with kidney failure had more risk of calcium misdiagnosis, but in the multivariate analysis, this association was confounded by the phosphate and CO2 levels, the two biochemical parameters associated with hidden hypocalcemia and hidden hypercalcemia, respectively. From a practical point of view, the analysis shows that in patients with kidney dysfunction but with normal levels of albumin, phosphate, or CO2, the calcium status can be determined with total calcium measurements without the use of calcium correction formulas. Validation in larger cohorts with more CKD patients, including more subjects with end-stage renal disease, is warranted.
This study has limitations. While all samples are strictly processed according to an institutional protocol, the large size of samples processed daily in the hospital may be subject to bias: there is a risk of errors in sample collection for anaerobic conditions to avoid pH change caused by loss of carbon dioxide, time to centrifugation, and temperature changes [23]. Fasting and circadian rhythm could be other factors which modify measurements [40, 41]. Nevertheless, all samples in this protocol were processed in significantly less time than 6 h after venous puncture [42] and the difference in repeated samples from those that were repeated was small. Other limitations of our study are: (i) we cannot extrapolate our formula to measurements performed in other laboratories, especially due to the effects of the altitude of Mexico City (2250 m[7382 Ft] on gasometrical values and CO2 concentrations [41], (ii) the number of hypercalcemia cases was low, which could modify the models obtained by linear regression, (iii) we did not perform measures of total proteins, globulins, or other explanatory variables that could modify the binding of calcium properties, (iv) we did not have data on whether the type of kidney disease was acute or chronic due to the study design, and (v) bicarbonate and total CO2 concentrations were used interchangeably, which could lead to bias. Nevertheless, the results of equation were similar when bicarbonate was used instead of CO2 in the linear regression.
In conclusion, we calculated and validated a practical correction equation for free calcium in general population hospitalized in one center. The novel equation proposed for prediction of free calcium is superior to the albumin-adjusted calcium equation and could be useful when free calcium is not available. The conventional formulas currently used in practice are inaccurate and misclassify many patients, in particular when phosphate or bicarbonate disturbances are present.
Availability of data and materials
Code availability
Not applicable.
References
Baird GS (2011) Ionized calcium. Clin Chim Acta 412:696–701. https://doi.org/10.1016/j.cca.2011.01.004
Glendenning P (2013) It is time to start ordering ionized calcium more frequently: preanalytical factors can be controlled and postanalytical data justify measurement. Ann Clin Biochem 50:191–193
Payne B (2019) Albumin-adjusted calcium and ionized calcium. Clin Chem 65:705–706
Obi Y, Mehrotra R, Rivara MB et al (2016) Hidden hypercalcemia and mortality risk in incident hemodialysis patients. J Clin Endocrinol Metab 101:2440–2449
Carroll MF, Schade DS (2003) A practical approach to hypercalcemia. Am Fam Physician 67:1959–1966
Dickerson RN (2006) Hypocalcemia during critical illness. Hosp Pharm 41:993–999
Cooper MS, Gittoes NJL (2008) Diagnosis and management of hypocalcaemia. BMJ 336:1298–1302
Levey AS, Stevens LA (2010) Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55:622–627
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159
Butler S, Payne R, Gunn I et al (1984) Correlation between serum ionised calcium and serum albumin concentrations in two hospital populations. Br Med J 289:948–950
James MT, Zhang J, Lyon AW, Hemmelgarn BR (2008) Derivation and internal validation of an equation for albumin-adjusted calcium. BMC Clin Pathol 8:1–6
Orrelll D (1971) Albumin as an aid to the interpretation of serum calcium. Clin Chim Acta 35:483–489
Anon (1977) Correcting the calcium. Br Med J 5:598
Siggaard-Andersen O, Thode J, Fogh-Andersen N (1983) Nomograms for calculating the concentration of ionized calcium of human blood plasma from total calcium, total protein and/or albumin, and pH. Scand J Clin Lab Invest Suppl 165:57–64
Berry EM, Gupta MM, Turner SJ, Burns RR (1973) Variation in plasma calcium with induced changes in plasma specific gravity, total protein, and albumin. Br Med J 4:640
Obi Y, Nguyen D, Streja E et al (2017) Development and validation of a novel laboratory-specific correction equation for total serum calcium and its association with mortality among hemodialysis patients. J Bone Miner Res 32:549–559
McLean F, Hastings A (1935) The state of calcium in the fluids of the body. J Biol Chem 108:285–321
Zeisler E (1954) Determination of diffusible serum calcium. Am J Clin Pathol 24:588–593
Hanna E, Nicholas H, Chamberlin J (1964) Nomogram for estimating diffusible serum calcium. Clin Chem 10:235–240
Björkman MP, Sorva AJ, Tilvis RS (2009) Calculated serum calcium is an insufficient surrogate for measured ionized calcium. Arch Gerontol Geriatr 49:348–350
Gauci C, Moranne O, Fouqueray B et al (2008) Pitfalls of measuring total blood calcium in patients with CKD. J Am Soc Nephrol 19:1592–1598
Ferrari P, Singer R, Agarwal A et al (2009) Serum phosphate is an important determinant of corrected serum calcium in end-stage kidney disease. Nephrology 14:383–388
Boink ABTJ, Buckley BM, Christiansen TF et al (1992) Recommendation on sampling, transport, and storage for the determination of the concentration of ionized calcium in whole blood, plasma, and serum. J Int Fed Clin Chem 4:149–150
Wang S, McDonnell EH, Sedor FA, Toffaletti JG (2002) pH effects on measurements of ionized calcium and ionized magnesium in blood. Arch Pathol Lab Med 126:947–950
Evenepoel P, Bammens B, Claes K et al (2010) Measuring total blood calcium displays a low sensitivity for the diagnosis of hypercalcemia in incident renal trasplant recipients. Clin J Am Soc Nephrol 5:2085–2092
Kaye M, Somerville P, Lowe G et al (1997) Hypocalcemic tetany and metabolic alkalosis in a dialysis patient: an unusual event. Am J Kidney Dis 30:440–444
Jabbar M, Sardar A (2018) Phosphate-induced hypocalcemia may have a role to play in a patient of recurrent cardiac arrest with severe hypophosphatemia. Saudi Crit Care 2:12
Nemer W, Teba L, Schiebel F, Lazzell V (1988) Cardiac arrest after acute hyperphosphatemia. South Med J 81:1068–1069
Thongprayoon C, Cheungpasitporn W, Mao MA, Erickson SB (2020) Calcium-phosphate product and its impact on mortality in hospitalized patients. Nephrology 25:22–28
Thongprayoon C, Cheungpasitporn W, Hansrivijit P et al (2020) Impact of serum phosphate changes on in-hospital mortality. BMC Nephrol 21:1–7
Aberegg SK (2016) Ionized calcium in the ICU should it be measured and corrected? Chest 149:846–855
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group (2017) KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 7:1–59
Seng J, Tan Y, Lim R et al (2018) Prevalence and risk factors for hypercalcemia among non-dialysis patients with chronic kidney disease-mineral and bone disorder. Int Urol Nephrol 50:1871–1877
Çeltik A, Şen S, Yılmaz M (2016) The effect of hypercalcemia on allograft calcification after kidney transplantation. Int Urol Nephrol 48:1919–1925
Gøransson LG, Skadberg Ø, Bergrem H (2005) Albumin-corrected or ionized calcium in renal failure? What to measure? Nephrol Dial Transplant 20:2126–2129
Clase CM, Norman GL, Beecroft ML, Churchill DN (2000) Albumin-corrected calcium and ionized calcium in stable haemodialysis patients. Nephrol Dial Transplant 15:1841–1846
Gouri A, Dekaken A (2012) A comparison of corrected serum calcium levels to ionized calcium levels in haemodialysis patients. Ann Biol Clin (Paris) 70:210–212
Smith JD, Wilson S, Schneider HG (2018) Misclassification of calcium status based on albumin-adjusted calcium: Studies in a tertiary hospital setting. Clin Chem 64:1713–1722
Lian I, Asberg A (2018) Should total calcium be adjusted for albumin? A retrospective observational study of laboratory data from central Norway. BMJ Open 8:e017703
Nielsen HK, Laurberg P, Brixen K, Mosekilde L (1991) Relations between diurnal variations in serum osteocalcin, cortisol, parathyroid hormone, and ionized calcium in normal individuals. Acta Endocrinol (Copenh) 124:391–398
Ramirez-Sandoval JC, Castilla-Peón MF, Gotés-Palazuelos J et al (2016) Bicarbonate values for healthy residents living in cities above 1500 meters of altitude: a theoretical model and systematic review. High Alt Med Biol 17:85–92
Nikolakakis N, De Francisco A, Rodger R et al (1985) Effect of storage on measurement of ionized calcium in serum of uremic patients. Clin Chem 31:287–289
Acknowledgements
The authors greatly appreciate all of the hospital staff for their efforts in treating patients and thank all patients involved in this study.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the study. JCRS, PDC, FGV, SLT, SPB, PEG, MMY, and AARA performed the clinical study including subjects, and analyzed data. JCRS, RF, MMY, and RCR contributed reagents and analysis tools. JCRS, AARA, RF, MMY, and RCR wrote the article. All authors revised the article.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interests, no support from any organization for the submitted work, and no financial relationship with any organizations that might have an interest in the submitted work. No pharmaceutical or biotechnology company, foundation, or any other source participated in the design, monitoring, data collection, and analysis. Dr. Juan Carlos Ramírez Sandoval has received honoraria from Takeda Mexico (speakers’ bureau), Roche Mexico (speakers’ bureau), Amgen Mexico (speakers’ bureau), Valdecasas Mexico (research funding), and Mercurio S.A. de C.V (advisory). Dr. Ricardo Correa Rotter is a member of the Review Board of Blood Purification, has received honoraria from AbbVie, AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim, and has lectured for Amgen, Takeda, AstraZeneca, and Roche. Dr. Diener-Cabieses Pablo, Gutiérrez-Valle Fabián, Ley-Tapia Sofía, Pastrana-Brandes Santiago, Galindo Pablo E., Reynerio Fagundo, Moreno-Yañez M., and Reza-Albarrán Alfredo Adolfo have no conflicts of interest to declare.
Ethics approval
The study was approved by the institutional review board (reference number 3204). Patients’ written informed consent was exempted because of the retrospective nature of study.
Consent to participate
Not applicable.
Consent for publication
All authors give our consent for the submission and publication of the paper in International Urology and Nephrology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramirez-Sandoval, J.C., Diener-Cabieses, P., Gutiérrez-Valle, F. et al. Validation of an equation for free calcium estimation: accuracy improves after adjustment for phosphate and CO2. Int Urol Nephrol 54, 2625–2635 (2022). https://doi.org/10.1007/s11255-022-03170-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03170-z