Abstract
Background and aims
Cognitive and renal impairment are pervasive among elderly frails, a high-risk, geriatric sub-population with peculiar clinical characteristics. In a series of frail individuals with non-advanced chronic kidney disease (CKD), we aimed at assessing the entity of functional, general health and cognitive impairment and the possible relationship between these types of dysfunction and the severity of renal impairment.
Methods
2229 geriatric subjects were screened for frailty and CKD. Severity of CKD was assessed by eGFR (CKD-EPI formula). Frailty was established by the Fried Index. Functional, general health and cognitive status were assessed by validated score measures.
Results
Final analysis included 271 frail CKD subjects (162 women, 109 men). Mean eGFR was 64.25 ± 25.04 mL/min/1.73 m2. Prevalence of mild-to-moderate CKD (stage 3–4) was 44%. Twenty-six percent of patients had severe cognitive impairment, while mild and moderate impairment was found in 7 and 67% of individuals, respectively. All subjects had poor functional and general health status. Cognitive capacities significantly decreased across CKD stages (p for trend < 0.0001). In fully adjusted multivariate analyses, cognitive status remained an independent predictor of eGFR (β = 0.465; p < 0.0001).
Conclusions
Mild-to-moderate CKD is highly pervasive among frail elderly individuals and the severity of renal dysfunction is independently correlated with that of cognitive impairment. Future studies are advocated to clarify whether the combination of kidney and mental dysfunction may portend a higher risk of worsen outcomes in this high-risk population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of elderly individuals is dramatically increasing worldwide, posing a challenge of sustainability for public health systems in view of the wealth of chronic diseases that associates with human senescence.
Kidney function is affected by ageing and chronic kidney disease (CKD) is pervasive in the elderly [1]. In the US population, the prevalence of renal impairment in persons older than 70 years resulted as high as 15% [2] and in the third National Health and Nutrition Examination Survey (NHANES III), 35% of the elderly population had stage 3 CKD [3].
Frail patients are high-risk elderly individuals showing particular characteristics that may include unintentional weight loss, self-reported exhaustion, low energy expenditure, slow gait speed and weak grip strength [4, 5].
There is now accruing evidence indicating that frailty is exceedingly prevalent among elderly subjects with overt CKD [6]. CKD patients show a three-time higher risk of frailty with respect to individuals with normal renal function [7] and the presence of frailty portends a higher risk of mortality in this population setting [8].
Similarly, CKD was recently indicated as an independent risk factor for cognitive impairment [9] and cognitive impairment, in turn, associates with poor prognosis in CKD individuals [10].
Although not directly included in the frailty assessment, the presence of a frank cognitive impairment is an ominous sign when found in old, frail populations as it conveys an incremental risk of worst outcomes [11].
Starting from a large geriatric population, we aimed at evaluating a series of frail elderly individuals with non-advanced CKD in order to establish (1) the presence/entity of functional, general health and cognitive impairment and (2) the possible, existing relationship between these types of dysfunction and the severity of CKD.
Methods
Patients’ selection
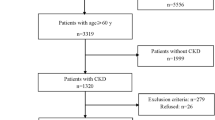
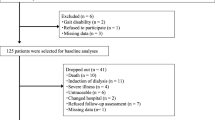
The source population included 2229 geriatric subjects over 65 years old recruited in the Centre for Cognitive Diseases and Dementias (Azienda Sanitaria Provinciale di Catanzaro, Italy) from January to September 2017. Individuals with a clinical diagnosis of CKD and frailty were invited to participate into the study. Subjects with already detected depression, acute cardiovascular, infectious or renal morbidity, hypothyroidism or active anti-neoplastic treatment or leucocytosis were excluded. The local Ethic Committee (ASP Catanzaro, Italy) approved the study in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration and all participants gave written informed consent.
Presence/severity of CKD was defined according to the National Kidney Foundation (NFK) classification [12] on the basis of the estimated glomerular filtration level (eGFR) as stage 1, GFR > 90 mL/min or stable presence of urinary abnormalities (e.g. haematuria, proteinuria); stage 2, GFR = 60–89 mL/min; stage 3A, GFR = 45–59 mL/min; stage 3B, GFR = 30–44 mL/min; stage 4, GFR = 15–29 mL/min. eGFR was assessed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [9], as suggested by the European Renal Best Practice Group guidelines for the management of older patients with CKD [13]. To minimize the effects of uraemia symptoms as a confounding factor, patients found to have an estimated glomerular filtration rate (GFR) < 15 mL/min (CKD stage 5) were excluded from the study. Frailty was established by the Fried Index on the basis of the presence of three or more of the following characteristics: unintentional weight loss, self-reported exhaustion, low energy expenditure, slow gait speed and weak grip strength [1, 2].
Assessment of cognitive functions
Cognitive functions were assessed by an Italian version of the Mini-Mental State Examination (MMSE): a brief 30-item test, which measures orientation to time and place, immediate recall, short-term memory, calculation, language and constructive ability [14]. Age and education were considered in single patients as determinants of cut-off points [15].
Assessment of functional status
Functional status was determined with a review of the two key divisions of functional ability: activities of daily living (ADL) and instrumental activities of daily living (IADL). ADL are self-care activities that a person performs daily (e.g. eating, dressing, bathing controlling bladder and bowel functions). IADL are activities that are needed to live independently (e.g. doing housework, preparing meals, taking medications properly, managing finances, using a telephone). ADL was assessed by the Katz ADL scale [16], while IADL by the Lawton IADL scale [17].
Clinical and laboratory data
Patients’ history was carefully recorded by interview and confirmed by checking patients’ record. General health status was assessed by the Cumulative Illness Rating Scale (CIRS) scoring system that can classify all medical conditions within 14 organ systems: cardiac, vascular, haematological, respiratory, ophthalmological–oto-rhino-laryngological, upper gastrointestinal, lower gastrointestinal, hepatic–pancreatic, renal, genitourinary, musculoskeletal–tegmental, neurological, endocrine–metabolic–breast and psychiatric [18].
Blood samples were taken in the morning before any food intake. Traditional biochemical parameters were measured at baseline in all subjects, following standard methods in the routine clinical laboratory. Total number of drugs was recorded and participants were divided into those with no polypharmacy (0–4 drugs), polypharmacy (5–9 drugs) and excessive polypharmacy (10+ drugs). Blood pressure was measured three times and the average value was considered for data analysis.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 20 and the Prism package (ver. 4.0; GraphPad Software, La Jolla, CA, USA) package. Continuous variables were expressed as means ± standard deviations or medians, as appropriate. Comparisons between continuous data were performed by two-sample t test or analysis of variance for continuous variables.
Pearson’s correlation coefficient was employed to test correlations between estimated GFR and other variables. Multiple regression analyses were performed by constructing a model including all univariate correlates of eGFR in order to assess independent relationships. Data were expressed as partial correlation coefficients (β) and p value. The level of significance was set at p values < 0.05.
Results
Population characteristics
Tables 1 and 2 summarize the main demographic, clinical and laboratory characteristics of the study participants. Final analysis included 271 frail elderly individuals (162 women, 109 men). Subjects had a mean age of 82.74 ± 6.77 (ranging from 68 to 98 years) and a mean ± SD estimated GFR (eGFR) of 64.25 ± 25.04 mL/min/1.73 m2 (ranging from 23.3 to 135.4 mL/min/1.73 m2). Average education level was below 8th grade. Overall, the MMSE yielded a mean score of 12.18 ± 3.65. Twenty-six percent of patients had a severe cognitive MMSE score under 10; 67% of patients had a moderate cognitive score between 10 and 20, while 7% had a MMSE score between 20 and 23. There was a significant decrease in MMSE values across CKD stages (p for trend < 0.0001), ranging from 16.63 ± 3.89 in subjects with CKD stage 1 to 10.16 ± 2.43 in patients with CKD stage 4 (Fig. 1). Functional status was also reduced with a mean ADL of 0.9 ± 2.02 and a mean IADL of 0.04 ± 0.19. General health status was also low with a mean CIRS of 3.83 ± 1.61. No significant differences in ADL, IADL, CIRS and total number of medications were found across the different CKD strata.
Clinical correlates of eGFR
In univariate analyses, eGFR was directly correlated with MMSE (R = 0.477 p < 0.0001) (Fig. 2) and no polypharmacy (0–4 drugs) (R = 0.125 p < 0.040), whereas a significant inverse correlation was found with CIRS (R = − 0.125 p < 0.04) and serum albumin (R = − 0.196 p < 0.001) (Table 3). Conversely, no significant correlations were found between eGFR and other clinical/functional parameters, including ADL, IADL, BMI and total number of drugs.
All variables found to be significantly related to eGFR at univariate analyses were introduced in a multivariate model using eGFR as a dependent variable. In this multivariable model, eGFR remained significantly correlated only to MMSE (β = 0.465; p = 0.000) and serum albumin (β = − 0.173; p = 0.001) (Table 3).
Discussion
Findings from our study indicate that, in a large population of frail elderly with CKD, cognitive dysfunction reflects, in a strong and independent manner, the entity of renal function impairment. Conversely, no clear relationships emerged between the severity of kidney disease and that of functional and general health dysfunction.
Although both cognitive impairment and frailty were previously reported as separate risk factors for CKD, to the best of our knowledge this is one of the first reports in literature providing evidence of a direct link between reduced mental performance and reduced kidney function in a high-risk population of elderly frail individuals. This would support, again, the importance of a systematic assessment of renal and cognitive status as part of the routine clinical approach to elderly frail individuals.
Ageing is characterized by a progressive decline in renal function, as well as by a susceptibility to renal diseases even in elderly subjects with conserved kidney function [19,20,21,22].
In our cohort, roughly 44% of frail individuals were found to have mild-to-moderate CKD. Interestingly, this prevalence was notably higher than that reported (21.7%) in an elderly sub-population of the National Health and Nutrition Examination Survey (NHANES) [23]. Such a difference would support the role of frailty as an additional risk factor for CKD, hence justifying the need for paying careful attention to renal function in frail patients [13].
In parallel with the decline of renal function, also the deterioration of cognitive abilities is part of ageing process. Foster et al. identified a high number of patients with CKD stage 4–5 showing various degrees of cognitive impairment [9]. Unlike our study, however, these authors focused on a population with a more advanced CKD and uncovered a cognitive impairment in 60% of individuals using a different, although validated, screening tool, the Montreal Cognitive Assessment (MoCA). Conversely, similarly to our experience, Kurella Tamura et al. found a comparable prevalence of early mental dysfunction in (non-frail) CKD patients using the Modified Mini-Mental State Examination (3MS) [10].
Our results are important if we consider that cognitive and renal function decline may contribute to the clinical vulnerability of older persons, resulting as strong predictors of several adverse health-related outcomes. It has been demonstrated that CKD patients and older frail individuals with cognitive deterioration share an increased frequency and severity of symptoms such as tiring easily, weakness, lack of energy, difficulty sleeping, muscle cramps and easy bruising, as well as psychologic distress [24, 25]. It has also been shown that cognitive impairment is also associated with decreased adherence to medications and, hence, could contribute to faster progression to end-stage renal disease and to the need of chronic renal replacement therapy [26,27,28]. Nephro-geriatric medicine has recently opened a field of clinical research focusing efforts on the possibility to uncover early renal impairment and to explore also cognitive function to preserve quality of life [27].
Our study has limitations that deserve mentioning. Firstly, the cross-sectional nature of the study does not allow to draw definite conclusions on the exact causal relationship between mental and renal impairment—that is which one might be the villain or the victim. For instance, a systematic review showed that CKD affects per se various cognitive domains such as orientation, attention and language, finally hampering the ability of patients to take individual decisions regarding day life and healthcare [29]. On the other hand, however, pre-existing cognitive impairment in individuals developing CKD is known to have a clear impact on the patient well-being and also on the disease evolution [30, 31].
Secondly, as per the observational nature of the study, selection bias cannot be excluded. Although findings reported were mostly in agreement with the existing literature, this limitation could hamper the generalizability of observations to other geriatric cohorts. Lastly, the absence of a follow-up observation did not allow to analyse the long-term evolution of renal function and cognitive impairment, making difficult to ascertain while overtime changes in one dysfunction may affect the other, nor if the concomitant presence of both disorders may impact other patient-centred outcomes.
Furthermore, the use of MMSE alone was intended as a simple screening test for cognitive function in our population and not a solid psychometric tool for identify specific disorders alone. In conclusion, we demonstrated that mild-to-moderate CKD is highly pervasive among frail elderly individuals and the entity of renal dysfunction is independently correlated to that of cognitive impairment. Future, larger studies with a prospective design are needed to extend our observations to the whole frail CKD population and to clarify whether the combination of kidney and mental dysfunction may portend a higher risk of worsen outcomes.
References
Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C (2014) The aging kidney revisited: a systematic review. Ageing Res Rev 14:65–80
Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW et al (2007) Prevalence of chronic kidney disease in the United States. JAMA 298:2038–2047
Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS (2003) Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41:1–12
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A 56:M146–M156
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB et al (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ 173:489–495
Dalrymple LS, Katz R, Rifkin DE, Siscovick D, Newman AB et al (2013) Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol 8:2091–2099
Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D et al (2004) The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis 43:861–867
Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV et al (2012) A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 60:912–921
Foster R, Walker S, Brar R, Hiebert B, Komenda P et al (2016) Cognitive impairment in advanced chronic kidney disease: the Canadian Frailty Observation and Interventions Trial. Am J Nephrol 44:473–480
Kurella Tamura M, Yaffe K, Hsu CY, Yang J, Sozio S et al (2016) Cognitive impairment and progression of CKD. Am J Kidney Dis 68:77–83
Vermeiren S, Vella-Azzopardi R, Beckwee D, Habbig AK, Scafoglieri A et al. (2016) Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 17:1163 e1161–1163 e1117
National Kidney F (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:S1-266
Farrington K, Covic A, Aucella F, Clyne N, de Vos L et al (2016) Clinical practice guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR < 45 mL/min/1.73 m2). Nephrol Dial Transplant 31:ii1–ii66
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Magni E, Binetti G, Bianchetti A, Rozzini R, Trabucchi M (1996) Mini-Mental State Examination: a normative study in Italian elderly population. Eur J Neurol 3:198–202
Katz S, Downs TD, Cash HR, Grotz RC (1970) Progress in development of the index of ADL. Gerontologist 10:20–30
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Parmelee PA, Thuras PD, Katz IR, Lawton MP (1995) Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc 43:130–137
Esposito C, Plati A, Mazzullo T, Fasoli G, De Mauri A et al (2007) Renal function and functional reserve in healthy elderly individuals. J Nephrol 20:617–625
Coppolino G, Presta P, Saturno L, Fuiano G (2013) Acute kidney injury in patients undergoing cardiac surgery. J Nephrol 26:32–40
Leporini C, Pisano A, Russo E, D’Arrigo G, de Sarro G, et al (2016) Effect of pentoxifylline on renal outcomes in chronic kidney disease patients: a systematic review and meta-analysis. Pharmacol Res 107:315–332
Buemi M, Coppolino G, Bolignano D, Sturiale A, Campo S et al (2009) Arrhythmias and hemodialysis: role of potassium and new diagnostic tools. Ren Fail 31:75–80
Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL et al (2016) Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165:473–481
Coppolino G, Campo S, Crasci E, Aloisi C, Giacobbe MS et al (2008) Neurobiological model and quality of life in discovering personality of the uremic patient. J Nephrol 21(Suppl 13):S139–S145
Buemi M, Caccamo C, Floccari F, Coppolino G, Tripodo D et al (2003) Correlation between quality of life assessment and a personality neurobiologic model in dialyzed patients. J Nephrol 16:895–902
Hayes TL, Larimer N, Adami A, Kaye JA (2009) Medication adherence in healthy elders: small cognitive changes make a big difference. J Aging Health 21:567–580
Buemi M, Lacquaniti A, Bolignano D, Donato V, Fazio MR et al (2008) Dialysis and the elderly: an underestimated problem. Kidney Blood Press Res 31:330–336
Bolignano D, Coppolino G, Romeo A, Lacquaniti A, Buemi M (2010) Neutrophil gelatinase-associated lipocalin levels in chronic haemodialysis patients. Nephrology 15:23–26
Berger I, Wu S, Masson P, Kelly PJ, Duthie FA et al (2016) Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med 14:206
Kurella Tamura M, Muntner P, Wadley V, Cushman M, Zakai NA et al (2011) Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis 58:756–763
Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW et al (2010) Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 58:338–345
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Coppolino, G., Bolignano, D., Gareri, P. et al. Kidney function and cognitive decline in frail elderly: two faces of the same coin?. Int Urol Nephrol 50, 1505–1510 (2018). https://doi.org/10.1007/s11255-018-1900-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1900-3