Abstract
Background
Both elevated serum uric acid and serum asymmetric dimethylarginine (ADMA) are risk factors for cardiovascular disease. We hypothesized that combined elevation of uric acid and ADMA amplifies the risk of all-cause mortality and/or cardiovascular events (CVE) in patients with chronic kidney disease (CKD).
Methods
A total of 259 patients with CKD stages 1–5 were followed up in a time-to-event analysis for all-cause mortality and fatal and non-fatal CVE (including death, stroke, and myocardial infarction). Baseline measurements included serum uric acid and ADMA and endothelial function [ultrasound determined flow-mediated dilatation (FMD)].
Results
As a measure of endothelial function, log FMD value was positively associated with log eGFR, but negatively associated with log ADMA and log uric acid levels. During follow-up (median 38 months), 24 (9.3 %) deaths, 90 (34.7 %) CVE, and 95 (36.7 %) deaths and CVE (composite outcome) occurred. In the univariate Cox analysis, patients with both serum uric acid and ADMA levels above the median had an increased risk of all-cause mortality, CVE, and the composite outcome (HR 5.06, 95 % CI 2.01–12.76; HR 4.75, 95 % CI 2.98–7.59; and HR 4.13, 95 % CI 2.66–6.43, respectively). However, after adjustment for renal-specific risk factors (glomerular filtration rate, proteinuria, and hsCRP), this association was maintained only for CVE and the composite outcome. The addition of both biomarkers into a model with traditional and renal-specific risk factors did not increase the prediction abilities of the model for none of the three outcomes.
Conclusion
Elevated serum uric acid and ADMA levels are associated with an increased cardiovascular risk, but their combination does not improve risk prediction. The effects are not additive, possibly because uric acid may lie in the causal pathway by which ADMA acts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uric acid is generated during the metabolism of nucleotides and adenosine triphosphate (ATP) and represents the end product of purine metabolism in humans [1]. While uric acid may act as an antioxidant in extracellular settings [2, 3], an elevated serum uric acid is an important risk factor for cardiovascular disease [4]. Elevated serum uric acid levels are associated with a variety of adverse pathological and cellular processes, including inflammation, endothelial dysfunction, vasoconstriction, and hypertension, and, in turn, increased risk of renal failure and cardiovascular events (CVE): coronary artery disease, heart failure, or stroke [1, 5].
Uric acid plays an important role with respect to the nitric oxide system. Entry of uric acid into endothelial cells is associated with a reduction in nitric oxide (NO) bioavailability via blocking uptake of l-arginine [6], increased l-arginine degradation via arginase [7], and by scavenging of NO from uric acid-generated oxidants [8] or by uric acid itself [9]. Consequently, uric acid has been reported to inhibit the NO-dependent dilatation of isolated aortic rings in rats [10]. Reciprocally, lowering uric acid with xanthine oxidase inhibitors is strongly associated with improvement in endothelial function [11].
Asymmetric dimethylarginine (ADMA), an endogenous NO synthase inhibitor, plays a role in endothelial dysfunction and is likely involved in the pathogenesis of atherosclerosis [12, 13]. Elevated serum ADMA is associated with metabolic syndrome, endothelial dysfunction, and cardiovascular diseases such as hypertension and atherosclerosis [14, 15]. Elevated serum ADMA levels have also been reported in adolescents with hyperuricemia and in women with hyperuricemia and cardiovascular disease, suggesting a potential relationship between these molecules [13, 16]. Furthermore, one recent study showed that genetic polymorphisms associated with hyperuricemia synergize with ADMA levels as a risk factor for CKD [17].
Given the observation that uric acid and ADMA are both associated with decreased NO bioavailability and increased cardiovascular disease and mortality [18, 19], we hypothesized that elevation of both uric acid and ADMA would increase synergistically the risk prediction of all-cause mortality and/or development of CVE in a cohort of patients with stages 1–5 chronic kidney disease (CKD).
Materials and methods
Patients and study design
This prospective observational study included 259 patients attending the Nephrology Unit of the Gulhane School of Medicine Medical Center between January 2011 and December 2011. All subjects were previously diagnosed as having CKD according to National Kidney Foundation K/DOQI guidelines [20] and were followed up in our unit for at least 3 months in order to exclude acute kidney injury. Stages of CKD were determined using estimated glomerular filtration rates (eGFR) according to the Modification of Diet in Renal Disease (MDRD) equation [21]. None of the patients in stage 5 CKD were on dialysis. Some enrolled subjects were included in our previous study [22]. Patients with chronic infection or malignancy as well as those unwilling to enroll were not included in the study.
Arterial blood pressure was determined in the morning by a physician based on three consecutive measurements, each after a 15-min resting period, with the mean values calculated for systolic and diastolic pressure. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, or the current use of antihypertensive medications.
All included patients were followed up for time-to-event analysis, until occurrence of death and fatal or non-fatal CVE. Information on all-cause mortality and CVE including death, stroke, and myocardial infarction was obtained from the Gulhane School of Medicine Medical Center registries by investigators unaware of baseline parameters. If information could not be obtained, the patient was assumed to be lost to follow-up starting from the date of the last actual visit. The Gulhane School of Medicine Ethics Committee approved the study protocol, and all patients were included in the study after signing informed consent forms.
Biochemical analyses
All blood samples were obtained from patients in the morning, after 12 h of fasting, for measurement of serum creatinine, albumin, hsCRP, uric acid, calcium, phosphate, parathyroid hormone, fasting plasma glucose, total serum cholesterol, triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol. Serum ADMA was measured by high-performance liquid chromatography, as described by Yilmaz et al. [23].
Assessment of endothelial function
Endothelium-dependent vasodilatation (flow-mediated dilatation (FMD)) of the brachial artery was assessed noninvasively, using high-resolution ultrasound as described by Celermajer [24] using criteria established by the International Brachial Artery Reactivity Task Force [25]. Measurements were taken by a single observer using an ATL 5000 ultrasound system (Advanced Technology Laboratories Inc., Bothell, WA., USA) with a 12-Mhz probe. The maximum FMD diameter was calculated as the average of the three consecutive maximum diameter measurements. The FMD was calculated as the percentage change in diameter compared with baseline resting diameters. The intra-observer coefficient of variation for FMD was 5.9 %.
Statistical analysis
All calculations were made using SPSS for Windows, version 19.0.1, Chicago, IL, and R (version 3.2.0)—package for statistical analysis (Foundation for Statistical Computing, Vienna, Austria).
Data are expressed as mean ± SD or as percentage frequency, as appropriate. Between-group comparisons were made for the categorical variables with the Chi-square test and by Mann–Whitney test or independent T test for the remaining variables, as appropriate. The normality of the distribution was assessed by the Shapiro–Wilk test, and logarithmic conversion was performed for non-normally distributed variables.
Pearson and point biserial correlation coefficient was used to determine correlations between FMD and all the other investigated variables. Backward stepwise multivariate regression analysis, including all univariate associates (with p < 0.05), was used to select the most informative model (based on Akaike information criterion (AIC)) to assess the determinants for FMD.
Time-to-event analysis of death was performed using Kaplan–Meier and Cox analyses. The Kaplan–Meier curves were compared using the log-rank test. Cox analysis was performed initially only with the two groups of patients (group 1: low ADMA–low uric acid, low ADMA–high uric acid, and high ADMA–low uric acid; group 2: high ADMA–high uric acid levels), subsequently adjusting for several groups of covariates. In model 1, we adjusted for conventional cardiovascular risk factors: age, gender, smoking status, diabetes, systolic blood pressure, HDL, and total cholesterol. In model 2, we adjusted for renal-specific cardiovascular risk factors: eGFR, proteinuria, and hsCRP. In model 3, we adjusted for all the variables used in the previous two models. To avoid the problem of overfitting due to the low number of incident outcomes, we performed bootstrapping validation, in order to determine the confidence intervals for estimating B in the Cox proportional hazard regression.
The diagnostic accuracy of the combination between ADMA and uric acid levels was tested by the C statistic difference, continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI) using methods accounting for censoring [26, 27] evaluated from the Cox proportional hazards models including traditional and renal-specific cardiovascular risk factors (age, gender, smoking status, diabetes, systolic blood pressure, HDL, total cholesterol, eGFR, proteinuria, and hsCRP) with and without continuous ADMA and uric acid levels. We used the Hosmer and Lemeshow test to evaluate the calibration of the models. Additionally, we calculated the Bayesian information criterion (BIC) and the AIC for each of the two Cox models; there is no statistical test to compare different BIC or AIC estimations, and a lower value indicates a better-fitted model.
Results
Baseline characteristics
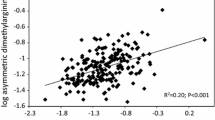
Two hundred and fifty-nine patients (mean age 51.5 ± 12 years, 22.4 % diabetics) were included in the current study. Baseline demographic, clinical, biological, and vascular characteristics of the entire population are presented in Tables 1 and 2. The mean values of serum ADMA and uric acid were 3.1 μmol/L and 6.9 mg/dL, respectively. Serum uric acid levels were correlated closely with serum ADMA levels (r = 0.73, p < 0.0001). Patients were divided into two groups according to median values of ADMA and uric acid (group 1: low ADMA–low uric acid, low ADMA–high uric acid, and high ADMA–low uric acid; group 2: high ADMA–high uric acid) levels. Patients in group 2 had a higher prevalence of diabetes and hypertension (Table 1), worse inflammatory status (as assessed by hsCRP), and lower renal function (Table 2). There was also a significant difference between the two groups regarding calcium, phosphate, and PTH (as markers of CKD—mineral and bone disorder) and FMD (as a marker of vascular abnormalities) (Table 2). As shown in Table 3, log FMD value was positively associated with log eGFR, but negatively associated with log ADMA and log uric acid levels. When performing a stepwise multiple linear analysis for assessing the independent determinants for FMD, all three variables remained in the final model (Table 3, R 2 of the model = 0.877). Interestingly, the final model included also the interaction term between ADMA and uric acid levels, showing a more important and negative association between uric acid and FMD values with increasing ADMA levels.
Survival and prognostic analysis
During the follow-up (mean 34.8, median 38 months), there were recorded 24 (9.3 %) all-cause deaths, 90 (34.7 %), CVE (fatal and non-fatal), and 95 (36.7 %) composite outcome (all-cause deaths and CVE). As shown in Table 4 and Fig. 1, patients from group 2 had a 5.06-, 4.75-, and 4.13-fold increase in the risk of all-cause mortality, CVE, and the composite outcome occurrence, respectively. In the Cox survival analysis, this association remained significant for CVE and the composite outcome after adjustment for traditional (model 1) and renal-specific risk factors (model 2), and even in the fully adjusted model (model 3). However, when analyzing the all-cause mortality outcome, the inclusion in the multivariable Cox survival analysis of the renal-specific risk factors (model 2 and model 3) made this association nonsignificant (Table 4).
Finally, we also determined whether using both ADMA and uric acid levels could improve the risk prediction of all-cause mortality, CVE, or the composite outcome, beyond clinical and biological parameters. Therefore, we tested the potential incremental prognostic value of adding ADMA and uric acid to the fully adjusted prediction model, based on both traditional (age, gender, smoking status, diabetes, systolic blood pressure, HDL, and total cholesterol) and renal-specific risk factors (eGFR, proteinuria, and hsCRP), using three measurements of performance: calibration, discrimination, and reclassification. All models showed good calibration (p > 0.05 for the Hosmer–Lemeshow—Table 5). The models that included ADMA and uric acid levels had the lowest AIC and BIC scores, showing better global goodness of fit than the baseline models for both outcomes (Table 5). Nevertheless, the addition of serum ADMA and uric acid did not increase the discrimination abilities for none of the outcomes (Table 5). Similarly, it did not improve the IDI or continuous NRI for all-cause mortality, although it did show some improved reclassification abilities over the baseline model for CVE or the composite outcome (NRI 17.1 and 16.3 %, respectively) (Table 5).
Discussion
We tested the hypothesis that the combination of elevated serum uric acid and ADMA levels carries a worse cardiovascular risk in subjects with CKD. Several findings were obtained. First, we showed that both uric acid and ADMA are correlated with endothelial dysfunction. Second, we demonstrated that higher uric acid and ADMA levels were associated with a higher risk of all-cause mortality and/or CVE, but this association remained independent of the renal-specific risk factors only for the CVE and composite outcome. Third, the addition of log ADMA and log uric acid into two baseline models for predicting cardiovascular death or CVE did not increase the risk prediction abilities of the models for any of the three outcomes.
Previous studies have shown that both uric acid [1, 18] and ADMA [12, 13] are independent risk factors for increased CVE in CKD and the general population. Both ADMA and uric acid are thought to be involved in important pathophysiologic processes such as inflammation, oxidative stress, and endothelial dysfunction [28, 29]. Serum uric acid levels are also correlated with ADMA levels, and a recent study found serum uric acid to be an independent predictor of ADMA [13, 28, 29]. Given these findings, we hypothesized that uric acid and ADMA in combination may play a synergistic, predictive role in the occurrence of new CVE.
Our study clearly shows that the patients with high levels in both biomarkers are at an increased risk of CVE, independently of traditional and renal-specific risk factors. However, we demonstrate for the first time that the addition of both biomarker into baseline prediction models for cardiovascular death or fatal and non-fatal CVE does not improve the prediction abilities of those models. While speculative, there are several potential explanations. First, uric acid or ADMA may not be as specific as eGFR with respect to cardiovascular risk prediction, and it is also possible that the relationship of uric acid or ADMA with CVE may simply reflect their association with eGFR levels. Indeed, a multivariate analysis by the Framingham heart study failed to demonstrate a relationship between uric acid and future cardiovascular disease [30]. However, this explanation may not be satisfactory since there are numerous studies showing that uric acid is an independent risk factor for CVE [18, 31, 32] and because mechanistically uric acid has been shown to block endothelial function in both cell culture and animal models [7, 9, 33–37].
Second, it is also possible that uric acid and ADMA lie in the same causal pathway. Indeed, the levels of uric acid and ADMA were found to be correlated. The possibility that uric acid may be upstream of ADMA is suggested by a recent study in which the rs734553 polymorphism in the urate transporter, SLC2A9, the strongest genetic marker of uric acid levels discovered so far, interacts with ADMA in determining the risk of CKD progression in CKD patients [17]. Indeed, in a double-blind study allopurinol decreased ADMA levels in chronic heart failure patients [29]. In contrast, endothelial dysfunction may also drive up uric acid levels, as noted by studies showing that mice lacking endothelial nitric oxide synthase develop hyperuricemia and features of metabolic syndrome [38, 39].
The study has some limitations. Cause and effect cannot be inferred, although the longitudinal nature does demonstrate that both uric acid and ADMA are risk factors for CVE in subjects with CKD. Indeed, there are now some studies showing that lowering uric acid can reduce CVE in subjects with CKD [40, 41]. Second, the measurements were taken at only one time point. Third, the patients were recruited from one center and the results cannot be generalized. Finally, our sample size and number of outcomes were relatively small, but we performed additional statistical adjustments to overcome this limitation. Nevertheless, this is the only study to our knowledge that combines ADMA and uric acid to investigate their interaction in a longitudinal study evaluating endothelial function and all-cause mortality and/or CVE in subjects with CKD.
In conclusion, both uric acid and elevated ADMA are associated with cardiovascular death and/or fatal and non-fatal CVE in subjects with CKD. Combining the two measures does not increase predictability for none of the outcomes. Nevertheless, these studies continue to emphasize the importance of endothelial function and of two key markers, ADMA and uric acid, in influencing cardiovascular risk in subjects with CKD.
References
Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ (2013) The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 99:759–766
Oda M, Satta Y, Takenaka O, Takahata N (2002) Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol 19:640–653
Ames BN, Cathcart R, Schwiers E, Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 78:6858–6862
Kanbay M, Afsar B, Covic A (2011) Uric acid as a cardiometabolic risk factor: to be or not to be. Contrib Nephrol 171:62–67
Kanbay M, Ikizek M, Solak Y, Selcoki Y, Uysal S, Armutcu F, Eryonucu B, Covic A, Johnson RJ (2011) Uric Acid and pentraxin-3 levels are independently associated with coronary artery disease risk in patients with stage 2 and 3 kidney disease. Am J Nephrol 33:325–331
Schwartz IF, Grupper A, Chernichovski T, Grupper A, Hillel O, Engel A, Schwartz D (2011) Hyperuricemia attenuates aortic nitric oxide generation, through inhibition of arginine transport, in rats. J Vasc Res 48:252–260
Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, Patel J (2008) Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol 295:C1183–C1190
Sautin YY, Nakagawa T, Zharikov S, Johnson RJ (2007) Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 293:C584–C596
Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN (2008) Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids 27:967–978
Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ (2006) A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 290:F625–F631
Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, Duranay M, Covic A, Johnson RJ (2011) A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol 6:1887–1894
Ando R, Ueda S, Yamagishi S, Miyazaki H, Kaida Y, Kaifu K, Yokoro M, Nakayama Y, Obara N, Fukami K, Takeuchi M, Okuda S (2013) Involvement of advanced glycation end product-induced asymmetric dimethylarginine generation in endothelial dysfunction. Diabetes Vascu Disease Res 10:436–441
Tenderenda-Banasiuk E, Wasilewska A, Taranta-Janusz K, Korzeniecka-Kozerska A (2013) Asymmetric and symmetric dimethylarginine in adolescents with hyperuricemia. Dis Markers 35:407–412
Boger RH, Maas R, Schulze F, Schwedhelm E (2005) Elevated levels of asymmetric dimethylarginine (ADMA) as a marker of cardiovascular disease and mortality. Clin Chem Lab Med/FESCC 43:1124–1129
Sen N, Ozlu MF, Akgul EO, Kanat S, Cayci T, Turak O, Yaman H, Sokmen E, Ozcan F, Maden O, Demir AD, Covic A, Kanbay M (2011) Elevated plasma asymmetric dimethylarginine level in acute myocardial infarction patients as a predictor of poor prognosis and angiographic impaired reperfusion. Atherosclerosis 219:304–310
Kuwahata S, Hamasaki S, Ishida S, Kataoka T, Yoshikawa A, Orihara K, Ogawa M, Oketani N, Saihara K, Okui H, Shinsato T, Kubozono T, Ichiki H, Fujita S, Takumi T, Yoshino S, Nakazaki M, Miyata M, Tei C (2010) Effect of uric acid on coronary microvascular endothelial function in women: association with eGFR and ADMA. J Atheroscler Thromb 17:259–269
Testa A, Mallamaci F, Leonardis D, Spoto B, Pisano A, Sanguedolce MC, Tripepi G, Zoccali C, Investigators MS (2015) Synergism between asymmetric dimethylarginine (ADMA) and a genetic marker of uric acid in CKD progression. Nutr Metab Cardiovasc Dis 25:167–172
Kanbay M, Yilmaz MI, Sonmez A, Solak Y, Saglam M, Cakir E, Unal HU, Arslan E, Verim S, Madero M, Caglar K, Oguz Y, McFann K, Johnson RJ (2012) Serum uric acid independently predicts cardiovascular events in advanced nephropathy. Am J Nephrol 36:324–331
Schwedhelm E, Wallaschofski H, Atzler D, Dorr M, Nauck M, Volker U, Kroemer HK, Volzke H, Boger RH, Friedrich N (2014) Incidence of all-cause and cardiovascular mortality predicted by symmetric dimethylarginine in the population-based study of health in pomerania. PLoS ONE 9:e96875
K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis (2002) 39:S1–S266
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130(6):461–470
Solak Y, Yilmaz MI, Saglam M, Demirbas S, Verim S, Unal HU, Gaipov A, Oguz Y, Kayrak M, Caglar K, Vural A, Turk S, Covic A, Kanbay M (2013). Mean corpuscular volume is associated with endothelial dysfunction and predicts composite cardiovascular events in patients with chronic kidney disease. Nephrology (Carlton)
Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C (2006) The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 47:42–50
Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE (1992) Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340:1111–1115
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R (2002) International brachial artery reactivity task F. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39:257–265
Pencina MJ, D’Agostino RB (2004) Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 23:2109–2123
Pencina MJ, D’Agostino RB Sr, Steyerberg EW (2011) Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30:11–21
Fujimi-Hayashida A, Ueda S, Yamagishi S, Kaida Y, Ando R, Nakayama Y, Fukami K, Okuda S (2011) Association of asymmetric dimethylarginine with severity of kidney injury and decline in kidney function in IgA nephropathy. Am J Nephrol 33:1–6
von Haehling S, Bode-Boger SM, Martens-Lobenhoffer J, Rauchhaus M, Schefold JC, Genth-Zotz S, Karhausen T, Cicoira M, Anker SD, Doehner W (2010) Elevated levels of asymmetric dimethylarginine in chronic heart failure: a pathophysiologic link between oxygen radical load and impaired vasodilator capacity and the therapeutic effect of allopurinol. Clin Pharmacol Ther 88:506–512
Culleton BF, Larson MG, Kannel WB, Levy D (1999) Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 131:7–13
Kanbay A, Inonu H, Solak Y, Erden A, Uslu E, Yuksel SA, Ozturk MA, McFann K, Johnson RJ, Kanbay M (2014) Uric acid as a potential mediator of cardiovascular morbidity in obstructive sleep apnea syndrome. Eur J Int Med 25:471–476
Turak O, Afsar B, Ozcan F, Canpolat U, Grbovic E, Mendi MA, Oksuz F, Siriopol D, Covic A, Caliskan M, McFann K, Johnson RJ, Kanbay M (2014) Relationship between elevated morning blood pressure surge, uric acid, and cardiovascular outcomes in hypertensive patients. J Clin Hypertens 16:530–535
Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, Garcia-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ (2012) Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol 121:e71–e78
Sanchez-Lozada LG, Tapia E, Lopez-Molina R, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M (2007) Effects of acute and chronic l-arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol 292:F1238–F1244
Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ (2005) Hyperuricemia induces endothelial dysfunction. Kidney Int 67:1739–1742
Schwartz IF, Grupper A, Chernichovski T, Hillel O, Engel A, Schwartz D (2011) Hyperuricemia attenuates aortic nitric oxide generation, through inhibition of arginine transport, in rats. J Vasc Res 48:252–260
Kang DH, Park SK, Lee IK, Johnson RJ (2005) Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 16:3553–3562
Cook S, Hugli O, Egli M, Vollenweider P, Burcelin R, Nicod P, Thorens B, Scherrer U (2003) Clustering of cardiovascular risk factors mimicking the human metabolic syndrome X in eNOS null mice. Swiss Med Wkly 133:360–363
Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U (2001) Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104:342–345
Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, Arroyo D, Luno J (2010) Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 5:1388–1393
Goicoechea M, Garcia de Vinuesa S, Verdalles U, Verde E, Macias N, Santos A, Perez de Jose A, Cedeno S, Linares T, Luno J (2015) Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis 65:543–549
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest between authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Kanbay, M., Afsar, B., Siriopol, D. et al. Relevance of uric acid and asymmetric dimethylarginine for modeling cardiovascular risk prediction in chronic kidney disease patients. Int Urol Nephrol 48, 1129–1136 (2016). https://doi.org/10.1007/s11255-016-1271-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1271-6