Abstract
Purpose
To evaluate the efficacy and safety of the calcineurin inhibitors (CNIs) cyclosporine (CyA) and tacrolimus (TAC) in the induction and maintenance treatment of lupus nephritis (LN).
Methods
The Cochrane library, PubMed, Embase, and CENTRAL databases were searched and reviewed up to February 2015. Randomized controlled trials were analyzed using RevMan 5.2 software.
Results
Ten randomized controlled trials were selected and included in this study according to our inclusion and exclusion criteria, and six were included in the meta-analysis. The analysis results indicated that, in induction treatment, no statistically significant difference was observed in the rates of complete remission (CR), partial remission (PR), or response between the CNIs and intravenous cyclophosphamide (ivCYC). However, the rates of adverse events such as infection (RR 0.65, P = 0.04), leukocytopenia (RR 0.32, P = 0.04), and menstruation disorder (RR 0.37, P = 0.01) following the use of the CNIs were remarkably lower than those after ivCYC. No differences in the CR, PR, infection, or leukocytopenia rates were observed between the CNIs and mycophenolate mofetil (MMF). In the maintenance treatment period, the relapse rate between the CNIs and azathioprine (AZA) was similar (RR 0.44, P = 0.27), while the leukocytopenia rate was lower with the CNIs (RR 0.26, P = 0.0005).
Conclusion
The efficacy of the CNIs CyA and TAC in induction therapy for lupus nephritis is comparable to ivCYC and MMF, and they are much safer than ivCYC. CNI treatment during the maintenance period was also as effective as AZA treatment, with a much lower risk of adverse effects. The CNIs CyA and TAC should be recommended for both induction and maintenance therapy of LN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease that affects multiple organs [1]. Renal involvement, i.e., lupus nephritis (LN), in SLE occurs in up to 60 % of cases and is a major determinant of the outcome of the disease [2]. Currently, glucocorticoids in combination with cyclophosphamide are the main regimen of induction therapy for lupus nephritis [3]. This regimen is quite effective and may improve the long-term prognosis of patients. However, adverse effects such as bone marrow suppression, infection, and gonadal toxicity limit its use in clinical practice. New immunosuppressant, such as mycophenolate mofetil (MMF) and calcineurin inhibitors (CNIs), has been used to treat LN in recent years. CNIs are potent immunosuppressants that inhibit the nucleus factor of activated T cell (NFAT) family of transcription factors, leading to the reduced function of effector T-cells [4] Meanwhile, CNIs inhibit the transcription of the early activation genes of interleukin (IL)-2 and suppress T cell-induced activation of tumor necrosis factor-α, IL-1β, and IL-6 [5]. Although several studies indicated that CNIs, such as cyclosporine (CyA) and tacrolimus (TAC), are effective at treating LN [6, 7], no systematic review has been performed to clarify their efficacy in the induction and maintenance treatment of LN in comparison with ivCYC and MMF. Therefore, this study sought to evaluate the efficacy and safety of these CNIs in treating LN.
Search strategy
A literature search was performed in PubMed, the Cochrane Library, and the Embase databases. PubMed (1966–February 2015), Embase (1974–February 2015), the Central Register of Controlled Trials (1999–January 2015), and the Cochrane Renal Group (1999–January 2015) were searched for the identification of relevant randomized controlled trials (RCTs). The following search terms were used: lupus nephritis, lupus glomerulonephritis, systemic lupus erythematosus, tacrolimus, TAC, FK506, cyclosporine A, CsA, CyA, and calcineurin inhibitors. Relevant text words relating to eligible interventions were also searched. We also hand-searched the bibliographies of articles for additional references. The results were limited to human studies with no restrictions on language.
Inclusion criteria and risk of bias
Articles were selected and subsequently screened based on the patient/problem intervention comparison outcome (PICO) principle. The studies included were RCTs and quasi-RCTs, whether published or unpublished, that evaluated any of the following treatment options: alone or in combination for more than 6 months in induction and 9 months in the maintenance period with corticosteroids, cyclophosphamide, MMF, tacrolimus, azathioprine, or cyclosporine. Only trials enrolling patients with biopsy-proven lupus nephritis and clearly defined remission criteria, remission outcome data and safety data were included. All potential articles were retrieved for the full text and reviewed independently by at least two investigators to determine whether the inclusion criteria were met.
Because of the variable quality of the articles included, no assessment of validity was made for qualifying studies. Moreover, a risk of bias table recommended by the Cochrane risk of bias tool [8] was used to assess the risk of bias of the included RCTs.
Data extraction and management
Two authors (Xiaoyan Zhang and Ling Ji) performed data extraction independently using standard data extraction forms, and Wei Qin was consulted when there was a discrepancy. For studies from which detailed data could not be extracted, the authors were contacted by e-mail. Basic information such as first author, year of publication, study design, inclusion criteria, study sample size, basic characteristics of the study subject, intervention regimen, drug dosage, follow-up time, outcome data, and adverse effects was recorded for each study included.
Outcome measures
The primary outcomes for the induction period were the complete remission (CR) rate, the partial remission (PR) rate, and the response rate, as defined by the sum of the complete and partial remission rates. The serum creatinine (sCr) level and 24-h urine protein level were used as efficacy indexes, whereas the rates of infection, leukocytopenia, hypertension, hyperglycemia, and menstruation disorders were used as safety indexes. Complete and partial remission criteria and rates were established within each article and are described in Table 1. All studies used proteinuria reduction as a criterion for remission and required a reduction in proteinuria to less than 0.5 g/day for complete remission. Some studies used even lower thresholds of less than 0.3 g/day, and several used serum creatinine levels, serum albumin levels, urine red blood cell numbers, and extra-renal lupus activity as additional criteria for remission. Partial remission criteria varied between articles, but all required a greater than 50 % reduction in proteinuria or less than 3.5 g/day (Table 2). When data were missing or incomplete, the investigators of the trials were contacted for clarification. The method provided by the Cochrane Handbook [8] was used to convert the mean and the 95 % confidence interval (CI) range to the mean and standard deviation.
Statistical analysis
Review Manager 5.2 software was used to analyze the data. Risk ratios (RR) and 95 % CIs were used to express the results of dichotomous outcomes. The mean difference (MD) was used for results with continuous scales, and the standardized mean difference (SMD) was used when different scales were used. Heterogeneity was analyzed using a Cochran Q test (n − 1 df), with P < 0.05 denoting statistical significance and I 2 measuring the proportion of variation in efficacy estimates due to heterogeneity beyond chance [9]. Random-effects analysis (I 2 > 50 %) and fixed-effects analysis (I 2 < 50 %) were used in meta-analysis according to the protocol. A Z test was used to analyze the overall effect, with P < 0.05 denoting statistical significance. Publication bias was estimated using funnel plots.
Result
Study selection
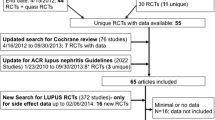
We identified 2765 articles in the first search. Of these, 2747 articles, including duplicate references, reviews, case reports, basic studies, non-controlled trials, systematic reviews, and meta-analyses, were excluded after examination of the title and abstract. Full texts of the remaining 18 articles were retrieved for further selection. An additional eight articles were excluded, including four non-RCT studies [10–13], one study of children [14], one historical controlled trial [15], one study without outcome assessment [16], and one early research study [17]. Eventually, 10 studies including eight induction period studies [18–25] and two maintenance period studies [26, 27] were included in this systematic review and meta-analysis. Among them was El-Sehemy’s [18] study, which compared CyA and ivCYC or AZA, and Li’s [25] study, which compared TAC and ivCYC or MMF. Therefore, separate comparisons were performed during the meta-analysis. Miyasaka’s [20] study was not included in the quantitative analysis because it compared TAC with a placebo. Although El-Sehemy’s [18] and Griffiths’ [22] studies both compared CyA and AZA, a quantitative analysis was not performed because there were few identical indices between the two studies. Zavada’s [16] study was not included in the quantitative analysis of the maintenance period because it only contained subjects from the induction period rather than complete remission subjects into the maintenance period. The article search strategy used in our review is described in Fig. 1.
Trial characteristics and qualities
Table 3 shows the characteristics of the studies that were included in the meta-analysis. With the exception of two studies, all patients had biopsy-proven proliferative lupus nephritis [18, 22]. Comparators for induction therapy included cyclophosphamide, MMF, tacrolimus, and cyclosporine. The efficacy and safety of the CNIs and ivCYC were compared in five studies [18, 19, 21, 23, 25], totaling 188 and 194 patients, respectively, and the efficacy and safety of the CNIs and MMF were compared in two studies [24, 25] with a total of 56 patients. Only the CNIs and AZA were compared in maintenance therapy, which included two studies [26, 27] and 139 total patients.
A risk of bias assessment of the RCTs included in our analysis was performed using a risk of bias table recommended by the Cochrane risk of bias tool (Table 4). A lack of allocation concealment resulted in a high risk of bias in Zavada’s study, and selective reporting in El-Sehemy’s and Griffiths’ studies also increased the risk of bias.
Comparison of the CNIs and ivCYC in induction therapy
The comparison of the efficacy of the CNIs and ivCYC in induction therapy of LN patients included four RCTs. No significant difference was observed in the CR rate (RR 1.33, 95 % CI 0.93–1.90, P = 0.12), PR rate (RR 0.91, 95 % CI 0.60–1.36, P = 0.64), or response rate (RR 1.12, 95 % CI 0.94–1.33, P = 0.20). Moreover, there were no significant differences in the proteinuria or serum creatinine levels between CNI and ivCYC treated patients (Fig. 2). Five RCTs were used to compare the adverse effects of the CNIs and ivCYC during the induction therapy of LN patients. There were lower rates of infection (RR 0.65, 95 % CI 0.43–0.98, P = 0.04), leukocytopenia (RR 0.32, 95 % CI 0.11–0.93, P = 0.04), and menstrual disorder (RR 0.37, 95 % CI 0.17–0.80, P = 0.01) in patients treated with the CNIs than in patients treated with ivCYC (Fig. 3). However, no differences were found in the incidence of other adverse effects, such as liver dysfunction, hyperglycemia, and the transient increase of serum creatinine (Table 5).
Comparison of the CNIs and MMF in induction therapy
Two RCTs were used to compare the efficacies of the CNIs and MMF in the induction therapy of LN patients. No significant difference was observed in the CR rate (RR 0.57, 95 % CI 0.12–2.75, P = 0.48), PR rate (RR 1.33, 95 % CI 0.58–3.08, P = 0.50), or response rate (RR 0.94, 95 % CI 0.68–1.30, P = 0.71), and no difference was found in the incidence of infection or leukocytopenia (Fig. 4; Table 5).
Comparison of the CNIs and AZA in maintenance therapy
Comparison of the efficacies of the CNIs and AZA in the maintenance therapy of LN patients included two RCTs. No significant difference was observed in the relapse rates (RR 0.44, 95 % CI 0.10–1.89, P = 0.27), but a slight decrease in the infection incidence (RR 0.56, 95 % CI 0.28–1.10, P = 0.09) and a lower apparent leukocytopenia incidence (RR 0.26, 95 % CI 0.12–0.55, P = 0.0005) were observed in the CNI groups (Fig. 5; Table 5), indicating a lower incidence of adverse effects and a better tolerance for the CNIs in maintenance therapy.
Publication bias
The funnel plots of the five RCTs comparing the CNIs and ivCYC in induction therapy are shown in Fig. 6. The symmetric distribution suggests that there was no publication bias in these studies. No funnel plot analysis could be performed in the CNIs versus MMF or the CNIs versus AZA comparisons because only two RCTs were included.
Discussion
SLE is a classic autoimmune disease with a range of multi-system disorders; the kidney is the most vulnerable target of SLE. Lupus nephritis is one of the most severe complications of SLE. Many guidelines recommend intravenous CYC [28–30] as the first choice of induction treatment. Although the CYC regimen achieved a relatively high remission rate, 15 % of LN patients were unresponsive, and up to 50 % of patients developed end-stage renal disease (ESRD) during treatment [31]. Moreover, CYC could cause severe adverse effects such as liver toxicity, infection, malignancy, and infertility [3]. Thus, new immunosuppressants, such as MMF and the CNIs CyA and TAC, have been used recently to improve the clinical management of LN patients. The most effective immunosuppressive therapy, however, is controversial. A recently published systematic review [32] reported that there was insufficient evidence to determine which immunosuppressive agent provides the best induction treatment. To clarify the effect of the CNIs CyA and TAC on the induction and maintenance treatment of LN with the most reliable and credible results, we performed a systematic review and meta-analysis that included all of the high-quality RCTs ever published.
The effect of the CNIs on the induction and maintenance treatment of LN was analyzed in this study. Eight RCTs were included in the systematic review and meta-analysis of the CNIs in induction therapy, and of them, five compared the CNIs with ivCYC (two TAC and three CyA). A total of 202 LN III-V patients were included, with a 6 to 12 month follow-up period. Although some studies suggested that the effect of TAC or CyA was superior to ivCYC, our systematic analysis and meta-analysis indicated that the CNIs TAC and CyA are comparable to ivCYC in terms of their CR, PR and response rates. The advantage of the CNIs is a significantly lower incidence of the adverse effects (infection, leukocytopenia, and menstrual disorder) that limited the use of ivCYC in many patients. A quantitative analysis about hypertension was not performed because only few articles can be merged. However, Griffiths’s and Moroni’s studies showed that the incidence of hypertension in CyA group was higher than azathioprine group. This question should be addressed further after more data could be achieved in RCTs. Two RCTs that compared the efficacy of the CNIs and MMF in LN induction treatment were also included in this study. A total of 56 patients with LN III-V were enrolled in studies with 6 to 24 month follow-up periods. TAC was used in both trials. Moreover, a systematic review and meta-analysis reported no difference in therapeutic effectiveness (CR, PR rate) or adverse effects between TAC and MMF. Similar to induction therapy, the effect of the CNIs on LN maintenance therapy was comparable to that of AZA, but the leukocytopenia rate was much lower. However, only two RCTs were included in the meta-analysis, and this result needs further confirmation.
In the recently published KDIGO (Kidney Disease Improving Global Outcomes) guidelines for lupus nephritis treatment, ivCYC, and MMF were recommended for proliferative and membranous lupus nephritis. CNIs, by contrast, were suggested only as an alternative membranous lupus nephritis treatment. In the ACR guidelines, CNIs were also suggested as a treatment option for refractory LN [28]. Webster reported that tacrolimus could be applied in patients with lupus nephritis during pregnancy [33]. Based on the results of the current study, we recommend the CNIs (CyA and TAC) as an alternative induction LN III-V treatment when patients either refuse or are intolerant to ivCYC/MMF treatment.
Given that no “head-to-head” comparison of TAC and CyA in LN treatment has been performed, we could not determine which one was more effective. However, in this study, we found that, considering the CR, PR and response rates, neither TAC nor CyA is superior to ivCYC in LN induction treatment, but the renal toxicity of TAC is much lower than that of CyA, suggesting that TAC is safer, especially in patients with impaired renal function.
Previously, several systematic reviews [34–37] comparing CNIs and ivCYC in LN induction therapy have been published. For maintenance therapy, Henderson LK et al. reported that MMF was more effective than azathioprine at preventing relapse [38]. A recent study [37] also compared CNIs and CYC (oral or iv) in four RCTs, one case control study and one cohort study. The results suggested that CNIs are superior to CYC in the response rate and adverse effect incidence. However, the inclusion of non-RCTs violated the principle of meta-analysis and compromised the reliability of the results.
The current study is the only systematic review to analyze the effect and safety of the CNIs CyA and TAC in the induction and maintenance therapy of LN in high-quality RCTs. No case–control or cohort studies were included to prevent bias and to guarantee the reliability of the results. Moreover, this study is the only systematic review comparing these CNIs and MMF in LN treatment; therefore, it provides very useful information.
Our study had several limitations. First, most of the RCTs included were small sample size, single-center studies, whereas large sample size, multi-center studies are needed to avoid inclusion bias. Second, only six RCTs [19, 21–23, 26, 27] indicated the exact randomization method, whereas three RCTs [23, 26, 27] concealed the random assignment protocol, potentially compromising the reliability of these studies. Third, heterogeneities in pathological subtypes and drug dosages, and a combined regimen in different RCTs may have also obscured the meta-analysis results. Therefore, long-term, large-sample, multi-center RCTs are needed to confirm the efficacy of the examined CNIs in LN treatment.
Conclusion
In conclusion, our systematic review and meta-analysis of recent RCTs indicated that the CNIs CyA and TAC were equally as effective as ivCYC/MMF and AZA in the induction and maintenance treatment of LN patients. Moreover, the CNIs were safer than ivCYC, with lower rates of leukocytopenia, infection, and menstruation disorder. Therefore, the CNIs could be recommended to LN patients as an alternative method of induction and maintenance treatment, especially for those who refuse or are intolerant to ivCYC or MMF.
Abbreviations
- CNIs:
-
Calcineurin inhibitors
- CyA:
-
Cyclosporine
- TAC or FK506:
-
Tacrolimus
- MMF:
-
Mycophenolate
- AZA:
-
Azathioprine
- SLE:
-
Systemic lupus erythematosus
- LN:
-
Lupus nephritis
- ivCYC:
-
Intravenous cyclophosphamide
- CR:
-
Complete remission
- PR:
-
Partial remission
- NFAT:
-
Nucleus factor of activated T cell
- RCTs:
-
Randomized controlled trials
- PICO:
-
The patient/problem intervention comparison outcome principle
References
Namendys-Silva SA et al (2009) Prognostic factors in patients with systemic lupus erythematosus admitted to the intensive care unit. Lupus 18(14):1252–1258
Hogan J, Appel GB (2013) Update on the treatment of lupus nephritis. Curr Opin Nephrol Hypertens 22(2):224–230
Petri M (2004) Cyclophosphamide: new approaches for systemic lupus erythematosus. Lupus 13(5):366–371
Ferraccioli GF, Tomietto P, De Santis M (2005) Rationale for T cell inhibition by cyclosporin A in major autoimmune diseases. Ann NY Acad Sci 1051:658–665
Tanaka H et al (2012) Treatment of young patients with lupus nephritis using calcineurin inhibitors. World J Nephrol 1(6):177–183
Moroni G, Doria A, Ponticelli C (2009) Cyclosporine (CsA) in lupus nephritis: assessing the evidence. Nephrol Dial Transplant 24(1):15–20
Yap DY et al (2014) Long-term data on tacrolimus treatment in lupus nephritis. Rheumatology (Oxford) 53(12):2232–2237
Higgins JP, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration
Higgins JPT et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Yin PD, Yang XY (1994) A clinical study on low dose cyclosporin A in the treatment of lupus nephritis. Zhonghua Nei Ke Za Zhi 33(10):684–686
Petrovic R et al (2002) Therapy of lupus nephritis with standard therapeutic protocols and cyclosporine. Srp Arh Celok Lek 130(Suppl 3):13–18
Wang S et al (2012) Tacrolimus versus cyclophosphamide as treatment for diffuse proliferative or membranous lupus nephritis: a non-randomized prospective cohort study. Lupus 21(9):1025–1035
Ikeuchi H et al (2014) Efficacy and safety of multi-target therapy using a combination of tacrolimus, mycophenolate mofetil and a steroid in patients with active lupus nephritis. Mod Rheumatol 24(4):618–625
Tanaka H et al (2012) Long-term tacrolimus-based immunosuppressive treatment for young patients with lupus nephritis: a prospective study in daily clinical practice. Nephron Clin Pract 121(3–4):c165–c173
Szeto CC et al (2008) Tacrolimus for the treatment of systemic lupus erythematosus with pure class V nephritis. Rheumatology (Oxf) 47(11):1678–1681
Zavada J et al (2014) Extended follow-up of the CYCLOFA-LUNE trial comparing two sequential induction and maintenance treatment regimens for proliferative lupus nephritis based either on cyclophosphamide or on cyclosporine A. Lupus 23(1):69–74
Dammacco F et al (2000) Cyclosporine-A plus steroids versus steroids alone in the 12-month treatment of systemic lupus erythematosus. Int J Clin Lab Res 30(2):67–73
El-Sehemy MS et al (2006) Comparative clinical prospective therapeutic study between cyclophosphamide, cyclosporine and azathioprine in the treatment of lupus nephritis. Egypt J Immunol 13(1):39–52
Austin HA III et al (2009) Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol 20(4):901–911
Miyasaka N, Kawai S, Hashimoto H (2009) Efficacy and safety of tacrolimus for lupus nephritis: a placebo-controlled double-blind multicenter study. Mod Rheumatol 19(6):606–615
Zavada J et al (2010) Cyclosporine A or intravenous cyclophosphamide for lupus nephritis: the Cyclofa-Lune study. Lupus 19(11):1281–1289
Griffiths B et al (2010) The BILAG multi-centre open randomized controlled trial comparing ciclosporin vs azathioprine in patients with severe SLE. Rheumatology (Oxf) 49(4):723–732
Chen W et al (2011) Short-term outcomes of induction therapy with tacrolimus versus cyclophosphamide for active lupus nephritis: a multicenter randomized clinical trial. Am J Kidney Dis 57(2):235–244
Yap DY et al (2012) Pilot 24 month study to compare mycophenolate mofetil and tacrolimus in the treatment of membranous lupus nephritis with nephrotic syndrome. Nephrology (Carlton) 17(4):352–357
Li X et al (2012) Mycophenolate mofetil or tacrolimus compared with intravenous cyclophosphamide in the induction treatment for active lupus nephritis. Nephrol Dial Transplant 27(4):1467–1472
Moroni G et al (2006) A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol 1(5):925–932
Chen W et al (2012) Outcomes of maintenance therapy with tacrolimus versus azathioprine for active lupus nephritis: a multicenter randomized clinical trial. Lupus 21(9):944–952
Anders H-J, Appel GB (2012) Lupus nephritis: implications of the new ACR lupus nephritis guidelines. Nat Rev Nephrol 8(9):500–501
Hahn BH et al (2012) American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res 64(6):797–808
Podracka L, Matousovic K (2013) Practice guideline and trends for immunosuppressive treatment of glomerulonephritides according to KDIGO (Clinical Practice Guideline for Glomerulonephritis). Vnitr Lek 59(2):113–118
Waldman M, Appel GB (2006) Update on the treatment of lupus nephritis. Kidney Int 70(8):1403–1412
Tian SY et al (2014) Immunosuppressive therapies for the induction treatment of proliferative lupus nephritis: a systematic review and network metaanalysis. J Rheumatol 41(10):1998–2007
Webster P et al (2014) Tacrolimus is an effective treatment for lupus nephritis in pregnancy. Lupus 23(11):1192–1196
Lee YH et al (2011) Efficacy and safety of tacrolimus therapy for lupus nephritis: a systematic review of clinical trials. Lupus 20(6):636–640
Deng J et al (2012) A meta-analysis of randomized controlled trials comparing tacrolimus with intravenous cyclophosphamide in the induction treatment for lupus nephritis. Tohoku J Exp Med 227(4):281–288
Zhou DJ, Wu XC (2011) Meta-analysis of calcineurin inhibitor in the treatment of lupus nephritis. Zhonghua Er Ke Za Zhi 49(4):287–293
Yang M et al (2014) Calcineurin inhibitors may be a reasonable alternative to cyclophosphamide in the induction treatment of active lupus nephritis: a systematic review and meta-analysis. Exp Ther Med 7(6):1663–1670
Masson P et al (2013) Induction and maintenance treatment of proliferative lupus nephritis: a meta-analysis of randomized controlled trials. Am J Kidney Dis 61(1):74–87
Authors contribution
Wei Qin. planned the study, analyzed data, and assisted in article preparation. Xiaoyan Zhang and Ling Ji searched the literature, selected articles, extracted data, analyzed data, and composed of the article. Lichuan Yang and Xiaohong Tang assisted in the data analysis. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any competing interests.
Additional information
Xiaoyan Zhang and Ling Ji have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Zhang, X., Ji, L., Yang, L. et al. The effect of calcineurin inhibitors in the induction and maintenance treatment of lupus nephritis: a systematic review and meta-analysis. Int Urol Nephrol 48, 731–743 (2016). https://doi.org/10.1007/s11255-015-1201-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1201-z