Abstract
Purpose
To evaluate nuclear factor erythroid 2-related factor 2 (Nrf2) and nuclear factor-kappaB (NF-κB) mRNA expression in nondialysis chronic kidney disease (CKD) patients, comparing with data from hemodialysis (HD) patients and healthy individuals.
Methods
Twenty nondialysis CKD patients (62.0 ± 8.1 years old, 11 men, estimated glomerular filtration rate of 36.8 ± 13.6 mL/min/1.73 m2), twenty HD patients (55.0 ± 15.2 years old, 13 men, and dialysis vintage of 76.5 ± 46.3 months) and eleven healthy individuals (50.9 ± 8.0 years old, 6 men) were enrolled in the study. The peripheral blood mononuclear cells were isolated and processed for the evaluation of expression of NF-κB and Nrf2 by quantitative real-time polymerase chain reaction.
Results
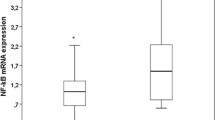
Nrf2 mRNA expression was significantly higher in nondialysis (1.12 ± 0.57) when compared to HD patients (0.58 ± 0.35, p = 0,006) but similar to healthy individuals (1.13 ± 0.64). Inversely, NF-κB mRNA expression was lower in nondialysis (1.21 ± 0.71) when compared to HD patients (2.08 ± 0.7, p < 0.0001) and similar to healthy individuals (1.04 ± 0.22). Nrf2 mRNA was positively correlated with NF-κB mRNA expression in nondialysis CKD patients (r = 0.52, p = 0.02) and healthy individuals (r = 0.77, p < 0.006). By contrast, Nrf2 mRNA was inversely correlated with NF-κB mRNA expression (r = −0.65, p = 0.003) in HD patients.
Conclusion
Nondialysis CKD patients may conserve regular homeostatic balance between Nrf2 and NF-κB expressions, being comparable to healthy individuals. However, HD patients seem to have Nrf2 downregulation and NF-κB upregulation. Thus, the association among Nrf2 and NF-κB expressions and nutritional status, kidney disease progression or immune deregulation deserve further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nuclear factor erythroid 2-related factor 2 (Nrf2) has been known as an important transcriptional activator for antioxidant genes [1]. Its transcriptional activity is controlled by the cytoplasmic protein inhibitor Kelch-like ECH-associated protein 1 (Keap1). When cells are exposed to electrophiles or reactive oxygen species (ROS), Nrf2 is released from Keap1 cytoplasmic capture, leading to its translocation to the nucleus where Nrf2 activates transcription of target genes that encode antioxidant and phase II detoxifying enzymes, as NADPH quinone oxidoreductase-1 and heme oxygenase-1 [2–4]. Nrf2 activation also produces a variety of anti-inflammatory effects through modulation of nuclear factor-kappaB (NF-κB), a transcription factor that regulates transcription of several genes encoding pro-inflammatory cytokines, chemokines, and leukocyte adhesion molecules [3, 5–7].
Chronic kidney disease (CKD) is associated with immune deregulation, systemic inflammation and oxidative stress, which promote cardiovascular disease. Recent studies addressed the role of impaired Nrf2 activity in the pathogenesis of these complications in nephrectomized animals [8, 9]. However, in CKD patients, the clinical relevance of Nrf2 has yet to be investigated. Zaza et al. [10] demonstrated that Nrf2 was upregulated in peritoneal dialysis. Inversely, Pedruzzi et al. [11] showed that Nrf2 gene expression is downregulated and possibly associated with oxidative stress and inflammation observed in hemodialysis (HD) patients.
Nrf2 is also related to important antifibrotic effects in the kidney and could attenuate the pathophysiological tissue remodeling observed in the course of CKD [12]. Thus, considering the potential beneficial effects of Nrf2 in CKD progression and the paucity of data about Nrf2 system in nondialysis CKD patients, this study aimed to investigate mRNA expression of Nfr2 and NF-κB in this population of patients, comparing the results with those obtained in HD patients and a group of healthy individuals.
Subjects and methods
This is a cross-sectional analysis of 20 nondialysis CKD patients, 20 HD patients and 11 healthy individuals. Nondialysis patients were treated at Hospital da Lagoa (Rio de Janeiro, Brazil), and HD patients were enrolled from Clínica Nefrológica (Niterói, Rio de Janeiro, Brazil). Inclusion criteria were age higher than 18 years and glomerular filtration rate <60 mL/min/1.73 m2 (nondialysis patients) and HD treatment for at least 6 months (HD patients). Patients with autoimmune/inflammatory diseases, known malignancies, active infections, AIDS, smokers and patients using shunts or central catheters as blood access for HD were excluded. The dialysis treatment consisted of 3.5–4.5 h/session three times a week with a blood flow greater than 300 ml/min, dialysate flow of 500 ml/min, and the use of bicarbonate buffer.
The etiologies of renal disease of patients from both nondialysis and HD group were hypertensive nephrosclerosis (29 of 40), diabetic nephropathy (3 of 40), chronic glomerulonephritis (2 of 40), polycystic kidney disease (2 of 40), and other diseases or unknown cause (4 of 40).
The patients studied were compared to eleven individuals without any disease and not on any medication. These healthy individuals were mainly the staff members of the dialysis unit. The institutional review board of the Faculty of Medicine of the Fluminense Federal University approved all the procedures of the study, which were explained to patients and controls who gave their written informed consent.
Analytic procedures and sample processing
Blood samples were drawn from each subject in the morning, after overnight fasting and before the dialysis session (for HD patients) using syringe containing EDTA (1.0 mg/mL) as anticoagulant. Plasma was separated (15 min, 3000×g, 4 °C) and stored at −80 °C until analysis.
To obtain the peripheral blood mononuclear cells (PBMCs), blood samples with EDTA were diluted in PBS and cells were separated in 5 mL ficoll gradient (lymphocyte isolation solution, Axis-Shield, Oslo, Norway) by centrifugation at 800 g for 20 min. PBMCs were collected and washed twice with cold PBS and re-suspended and stored (−80 °C) with 1 mL of recovery cell culture freezing (Invitrogen) for RNA isolation.
Quantitative real-time PCR analysis
Nrf2 and NF-κB mRNA levels were assessed in PBMCs using quantitative real-time polymerase chain reaction (qRT-PCR). PBMCs were isolated from blood and RNA was extracted with SV Total RNA Isolation System (PROMEGA). The cDNA was synthesized with the high-capacity cDNA reverse transcription kit (Applied Biosystems). One µg of RNA was reversed transcribed using random primer and MultiScribe reverse transcriptase. Real-time PCR amplifications reactions were performed in duplicate in 20 µl of final volume via TaqMan Gene Expression Assays on ABI Prism 7500 Sequence Detection System (Applied Biosystems). PCR protocol was performed using TaqMan Primer Assays (Applied Biosystems) for Nrf2 (Hs00975961_g1), NF-κB (Hs00765730_m1) and the control gene, GAPDH (Hs02758991_g1): 50oC for 2 min, 95oC for 10 min and 40 two-step cycles: 95oC for 15 sec and 60oC for 1 min. The expression of Nrf2 and NF-κB mRNA was normalized against ABL1, and the expression level was calculated using the ΔΔCT (delta delta threshold cycle) method. The ratio Nrf2/NF-κB was also determined.
Biochemical and anthropometrics data
Blood urea nitrogen (BUN), creatinine and albumin were determined through Bioclin® kits by automatic biochemical analyzer (Bioclin BS-120 Chemistry Analyzer). Tumor necrosis factor-alpha (TNF-α) was measured by ELISA using R&D systems® duoset kits (DY 210, Minneapolis, MN, USA). Estimated glomerular filtration rate (eGFR) was obtained with CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation [13]. Dialysis dose (Kt/V) was calculated from values of BUN, pre- and post-dialysis, body weight, and dialysis duration using standard formula [14].
The body mass index (BMI) was calculated as the dry body weight (Kg), obtained after HD sessions, divided by the squared height (m) [15].
Statistical analysis
All data were analyzed for the normality distribution using the Shapiro–Wilk test. Data are expressed as mean ± standard deviation (SD) or median (interquartile range). ANOVA was used to examine the difference between continuous data. The Pearson or Spearman correlation coefficient was calculated to examine the relationship between variables. Statistical significance was accepted as p < 0.05. Statistical analyses were performed using the SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Demographic data of studied groups are presented in Table 1. Age and gender were not different among healthy individuals, HD and nondialysis CKD patients. However, BMI was higher for HD patients; but it was not related to others studied variables, except for age (r = 0.4, p = 0.008).
Biochemical characteristics of nondialysis and HD patients are summarized in Table 2. Forty percent of nondialysis CKD patients was in stage 3B (eGFR between 30 and 44 mL/min/1.73 m2), 30 % in stage 3A (eGFR between 45 and 59 mL/min/1.73 m2) and 30 % in stage 4 (eGFR between 15 and 29 mL/min/1.73 m2). As expected, BUN and creatinine were higher in HD patients. For HD patients, dialysis dose was adequate (>1.2).
In nondialysis patients, Nrf2 mRNA expression was markedly higher and, in contrast, NF-κB mRNA expression was lower when compared to HD patients. Interestingly, mRNA expressions of Nrf2 and NF-κB were similar between nondialysis patients and healthy individuals (Fig. 1). Moreover, it was not observed difference in Nrf2/NF-κB ratio between nondialysis patients and healthy individuals (Table 3). Nrf2 expression was positively correlated with NF-κB in nondialysis CKD patients (r = 0.52, p = 0.02) and healthy individuals (r = 0.77, p = 0.006). However, in HD patients this association was inverse (r = −0.65, p = 0.003) (Fig. 2).
NF-κB expression was positively associated with creatinine (r = 0.52, p = 0.001, n = 40) and BUN levels (r = 0.4, p = 0.02, n = 40). In contrast, Nrf2 expression was correlated negatively with creatinine levels (r = -0.37, p = 0.02, n = 40). TNF-α plasma levels was negatively correlated with Nrf2 expression (r = −0.4, p = 0.008, n = 40) and positively with NF-κB expression (r = 0.4, p = 0.01, n = 40). Although patients have presented different BMI values, it was not associated with NF-κB and Nrf2 expression.
Discussion
This study demonstrated that Nrf2 and NF-κB gene expressions in nondialysis patients were comparable to healthy subjects. Nondialysis patients presented high Nrf2 and low NF-κB mRNA expression when compared to HD patients. Moreover, Nrf2 was positively correlated with NF-κB expression in nondialysis patients, which is at odds with data from HD patients.
The interaction between Nrf2 and NF-κB is very complex and not completely understood [1]. Under physiological conditions, oxidative stress triggers upregulation of the endogenous antioxidant and cytoprotective proteins and enzymes via Nrf2 activation [3, 4, 8]. In turn, Nrf2 activation produces a wide variety of anti-inflammatory effects, including lowered NF-κB pathway [1]. However, recent experimental evidence indicates that NF-κB may directly repress Nrf2 signaling at the transcriptional level [16]. This is based on the observation that the active subunits of NF-κB (P65 and P53) could interfere with the dissociation of Nrf2 from its repressor molecule, Keap 1, in the cell cytoplasm and with binding of Nrf2 to the antioxidant response elements of the target genes in the cell nucleus [8, 9, 17–19].
The upregulation of NF-κB and its active subunit P65 by indoxyl sulfate, a uremic toxin, exemplifies the exacerbation of inflammatory status in renal disease [20]. In addition, the impaired Nrf2 activity in HD patients can also be explained by the prominent systemic inflammation caused by intermittent blood exposure to extracorporeal circuit and its consequences (membrane biocompatibility, dialysate backflow and endotoxemia) [21]. Hence, both the state of uremia and several dialysis related pro-inflammatory stimuli might be crucial determinants of NF-κB and Nrf2 gene expression profile. In this regard, it is reasonable to compare healthy individuals with CKD patients at early stages, not on dialysis.
At a molecular level, inflammation is induced through activation of pattern recognition receptors, exogenous pathogen-associated molecular patterns (PAMPs) or endogenous damage-associated molecular patterns (DAMPs) [22, 23]. In HD patients, the uremia per se, dialysis procedure and CKD complications are associated with immunity disturbances [24]. In this sense, it has been observed that the contact of PBMCs with short bacterial-derived DNA fragments [25], a PAMP, and with apoptotic cell-free DNA [26], a DAMP, are associated with inflammation in HD patients. Recently, Elefhteraidis et al. [23] also observed an association between inflammation and the mitochondrial protein cytochrome c, a marker of released mitochondrial DAMPs. Thus, this chronic pro-inflammatory state could explain the increased NF-κB and decreased Nrf2 in PBMCs of HD patients and reinforce the immune deregulation that characterizes the HD population [24].
Two studies evaluated Nrf2 gene expression in CKD patients undergoing dialysis. Pedruzzi et al. [11] found reduced Nrf2 gene expression in HD patients compared to healthy individuals. On the other hand, Zaza et al. [10] observed upregulation of Nrf2 gene expression in PBMCs and its nuclear extracts in 15 peritoneal dialysis (PD) patients when compared to healthy subjects. For PD patients, intraperitoneal and systemic inflammation reflect different processes and consequences [27] and these independent findings could explain the discrepant results reported for HD and PD patients. For nondialysis CKD patients, data about Nrf2 expression were absent until now. Thus, studies on Nrf2 regulation and its effects in CKD patients are still needed.
Nrf2 antioxidant system may have a protective role against CKD progression due to its modulation of transforming growth factor β1 (TGFβ1), a profibrogenic cytokine [12]. TGFβ is related to renal disease by regulating the expression of epithelial-to-mesenchymal transition-related genes and by elevating extracellular matrix protein genes [28]. Regarding NF-κB, its higher activity in glomerulus could explain the increased susceptibility to injury and development of glomerulosclerosis [29]. In fact, the present study observed a direct correlation among NF-κB and BUN and creatinine levels when all of CKD patients were analyzed. Moreover, there was an inverse correlation between Nrf2 and creatinine levels. As serum levels of creatinine could be affected by muscle mass (mainly in HD patients), the association between Nrf2 and renal disease progression or nutritional status deserves further investigation.
Although phase III clinical trial of bardoxolone methyl, a potent synthetic activator of Nrf2, in type 2 diabetes and stage 4 CKD patients has failed due to safety issues [30] probably caused by modulation in endothelin pathway [31], experimental studies reinforce the beneficial effects of Nrf2 on renal disorders through modulation of fibrogenesis [12]; attenuation of hyperglycemia-induced renal damage by ROS neutralization and TGFβ1 regulation [32]; and attenuation of oxidative stress and improvement of renal lesion and endothelial function [8, 33].
Dietary compounds targeting Nrf2 activation also presented beneficial results to attenuate renal damage and preserve renal function [34]. Sulforaphane, a compound found in vegetables, improves nephropathy in streptozotocin-induced diabetes animals [35]. Epigallocatechin-3-gallate, a polyphenol compound found in green tea, attenuates renal lesions in animals with systemic lupus erythematosus [36], in the mouse model of rapidly progressive glomerulonephritis [37], and in animals with cisplatin-induced acute kidney injury [38]. Curcumin, a natural Nrf2 inducer, isolated from turmeric, protects against chromium-induced nephrotoxicity in experimental animals [39]. Hence, it is conceivable to suggest that in the future, Nrf2 modulation may become a valuable point for therapeutic intervention in order to reduce progression and clinical complications in CKD patients undergoing conservative treatment [12, 40].
The present study has some limitations. Firstly, the results were generated in a cross-sectional design from a small but well selected patient population. Thus, the inferences about causality between genes expressions and kidney disease progression could not be established. Secondly, Nrf2 gene expression was not measured specifically in cell nucleus, where Nrf2 acts through activation of target genes [2–4]. Thirdly, gene expression of Keap 1, antioxidant enzymes and cytokines were not performed. Fourthly, Western blot should be performed to identify these specific proteins.
As conclusion, the results from this study revealed for the first time the Nrf2/NF-κB profile gene expression in nondialysis CKD patients, which is comparable to healthy individuals. In HD patients, the high NF-κB expression and, conversely, the reduced Nrf2 gene expression by PMBCs could be related with the prominent systemic inflammation or immune deregulation. Moreover, the inverse association between Nrf2 and creatinine pointed that kidney disease progression or nutritional status could be associated with Nrf2 regulation. Nevertheless, further studies are necessary to better delineate the biological and biochemical mechanisms involved in the regulation and effects of Nrf2 and NF-κB gene expression.
References
Pall ML, Levine S (2015) Nrf2, a master regulation of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Acta Physiologica Sinica 67:1–18
Itoh K, Wakabayaschi N, Katoh Y et al (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13:76–86
Kim J, Cha YN, Surh YJ (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutation Res 690:12–23
Wu KC, Cui JY, Klaassen CD (2012) Effect of grades Nrf2 activation on phase-I and –II drug metabolizing enzymes and transporters in mouse liver. PLoS ONE 7(7):e39006
Kobayashi M, Yamamoto M (2006) Nrf2-Keap 1 regulation of cellular defense mechanism against electrophilles and reactive oxygen species. Adv Enzyme Regul 46(113):140
Antunes F, Han D (2009) Redox regulation of NF-kappaB: from basic to clinical research. Antiox redox signal 11:2055–2056
Banning A, Brigelius-Flohe R (2005) NF-kappaB, Nrf2, and HO-1 interplay in redox regulated VCAM-1 expression. Antioxid Redox Signal 7:889–899
Aminzadeh MA, Nicholas SB, Norris KC, Vaziri ND (2013) Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol Dial Transplant 28:2038–2045
Kim HJ, Sato T, Rodriguez-Iturbe B, Vaziri ND (2011) Role of intrarenal angiotensin system activation, oxidative stress, inflammation, and impaired nuclear factor-erythroid-2-related factor 2 activity in the progression of focal glomerulosclerosis. J Pharmacol Exp Ther 337:583–590
Zaza G, Granata S, Masola V et al (2013) Downregulation of nuclear-encoded genes of oxidative metabolism in dialyzed chronic kidney disease patients. PLoS ONE 8(10):e77847
Pedruzzi LM, Cardozo LFMF, Daleprane JB et al (2015) Systemic inflammation and oxidative stress in hemodialysis patients are associated with down-regulation of Nrf2. J Nephrol 28:495–501
Ryoo IG, Ha H, Kwak MK (2014) Inhibitory role of the KEAP1-NRF2 pathway in TGFβ1-stimulated renal epithelial transition to fibroblastic cells: a modulatory effect on SMAD signaling. PLoS ONE 9(4):e93265
Levey A, Stevens L, Schmid C et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Daugirdas JT (1993) Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol 4:1205–1213
World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. Technical report series. 894:1–253
Buelna-Chontal M, Zazueta C (2013) Redox activation of Nrf2 and NF-KB: a double end sword? Cell Signal 25:2548–2557
Bolati D, Shimizu H, Yisireyili M, Nishijima F, Niwa T (2013) Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-kappaB. BMC Nephrol 14:56
Faraonio R, Vergara P, Di Marzo D et al (2006) p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem 281:39776–39784
Yu M, Li H, Liu Q et al (2011) Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal 23:883–892
Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T (2010) Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-KappaB activation. Am J Nephrol 31:435–451
Meuwese CL, Stenvinkel P, Dekker FW, Carrero JJ (2011) Monitoring of inflammation in patients on dialysis: forewarned is forearmed. Nat Rev Nephrol 7:166–176
Denk S, Perl M, Huber-Lang M (2012) Damage- and pathogen-associated molecular patterns and alarmins: keys to sepsis? Eur Surg Res 48:171–179
Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I (2014) Damage-associated molecular patterns derived from mitochondria may contribute to the hemodialysis-associated inflammation. Int Urol Nephrol 46:107–112
Eleftheriadis T, Antoniadi G, Liakpoulos V, Kartsios C, Stefanidis I (2007) Disturbances of acquired immunity in hemodialysis patients. Semin Dial 20(5):440–451
Bossola M, Sanguinetti M, Scribane D et al (2009) Circulating bacterial-derived DNA fragments and markers of inflammation in chronic hemodialysis patients. Clin J Am Soc Nephrol 4(2):379–385
Atamaniuk J, Kopecky C, Skoupy S, Saemann MD, Weichhart T (2012) Apoptotic cell-free DNA promotes inflammation in hemodialysis patients. Nephrol Dial Transplant 27(3):902–905
Lambie M, Chess J, Donovan KL et al (2013) Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J Am Soc Nephrol 24:2071–2080
Biernacka A, Dobaczewski M, Frangogiannis NG (2011) TGF-beta signaling in fibrosis. Growth Factors 29:196–202
Wiggins JE (2012) Aging and glomerulus. J Gerontol A Biol Sci Med Sci 67:1358–1364
De Zeeuw D, Akizawa T, Audhya P et al (2013) Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369:2492–2503
Chin MP, Reisman SA, Bakris GL et al (2014) Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am J Nephrol 39:499–508
Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD (2010) The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59:850–860
Aminzadeh MA, Reisman SA, Vaziri ND et al (2013) The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores endothelial function impaired by reduced Nrf2 activity in chronic kidney disease. Redox Biol 1:527–531
Cardozo LF, Pedruzzi LM, Stenvinkel P et al (2013) Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 95:1525–1533
Zheng H, Whitman SA, Wu W et al (2011) Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 60:3055–3066
Tsai PY, Ka SM, Chang JM et al (2011) Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Rad Biol Med 51:744–754
Peng A, Ye T, Rakheja D et al (2011) The green tea polyphenol (-)-epigallocatechin-3-gallate ameliorates experimental immune-mediated glomerulonephritis. Kidney Int 80:601–611
Sahin K, Tuzcu M, Gencoglu H et al (2010) Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci 87:240–245
Molina-Jijon E, Tapia E, Zazueta C et al (2011) Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radical Biol Med 51:1543–1557
Pedruzzi LM, Stockler-Pinto MB, Leite M Jr, Mafra D (2012) Nrf2-keap1 system versus NF-kappaB: the good and the evil in chronic kidney disease? Biochimie 94:2461–2466
Acknowledgments
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Medicine Faculty of Federal University Fluminense and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individuals participants included in the study.
Statement of animal studies
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Leal, V.O., Saldanha, J.F., Stockler-Pinto, M.B. et al. NRF2 and NF-κB mRNA expression in chronic kidney disease: a focus on nondialysis patients. Int Urol Nephrol 47, 1985–1991 (2015). https://doi.org/10.1007/s11255-015-1135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1135-5