Abstract
Objective
Pentoxifylline (PTF) has anti-inflammatory properties, which may be beneficial for diabetic nephropathy (DN). A meta-analysis was conducted to assess the additive effect of pentoxifylline and its safety among patients with type 2 DN under blockade of angiotensin system.
Data sources
Relevant studies were searched from PubMed, CBM, EMBASE, CENTRAL and Cochrane renal group specialized register.
Selection criteria
All RCTs that compared the benefits and harms of pentoxifylline and ACEI/ARB with ACEI/ARB alone for DN were included.
Data extraction and analysis
Pertinent data were extracted independently by two authors. Meta-analyses were performed when more than one study provided data on a comparable outcome. Standard mean differences (SMDs) for proteinuria and albuminuria, mean differences (MDs) for systolic blood pressure (SBP), diastolic blood pressure (DBP), HbA1c, serum creatinine (Scr), creatinine clearance (CrCl) and urine tumor necrosis factor-alpha (UTNF-α), 95 % confidence intervals (CIs) were calculated, and heterogeneity was assessed with the I 2 test. Adverse effects were assessed using descriptive techniques.
Results
Eight studies including 587 patients with a median duration of 5 months were identified. Compared with ACEI/ARB alone, the combination of PTF and ACEI/ARB significantly reduced proteinuria (SMD 0.76, 95 % CI 0.52–0.99), albuminuria (SMD 0.36, 95 % CI 0.12–0.59) and UTNF-α (MD 1.56 ng/g, 95 % CI 0.09–3.03). However, no statistically significant changes were observed for SBP, DBP, HbA1c, Scr and CrCl. The most frequent adverse effects in patients treated with PTF were gastrointestinal symptoms (28/298) and dizziness (7/298), but in most cases, these symptoms were mild, only six participants withdrew due to intractable nausea and vomiting.
Conclusions
Pentoxifylline can significantly provide additive antiproteinuric effect independent from the decrease in BP or improvement in glycemic control in DN patients under blockade of angiotensin system. Further large, multicenter, high-quality studies with long duration are necessary to prove whether it really has renoprotective effects in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy is now considered to be the major cause of end-stage renal disease (ESRD) [1]. Patients with diabetic nephropathy present much higher risk of cardiovascular events and mortality, compared with those of other causes of kidney disease [2, 3]. Albuminuria and proteinuria are the hallmarks of diabetic nephropathy, contributing to the progression of kidney disease and cardiovascular complications [4]. More urinary protein excretion causes greater renal damage, while a reduction in it by intensive therapy would have a renoprotective effect. Current therapy for diabetic nephropathy still primarily relies on the antiproteinuric, antihypertensive and nephroprotective effects of renin–angiotensin system (RAS) blockers [5, 6]. However, these standard therapies are insufficient to prevent progression to ESRD in a substantial number of patients with residual proteinuria (albuminuria) [7]. Dual blockade of the RAS might decrease proteinuria more effectively than single RAS blockade. However, this strategy is not recommended by the authoritative guidelines as no additional efficacy in terms of renal function has been demonstrated and the incidence and severity of adverse effects (such as hyperkalemia and AKI) are increased [5, 6, 8]. The ALTITUDE and VA NEPHRON‑D RCTs showed no beneficial effects of dual versus single RAS blockade on renal function, and both studies were stopped prematurely for safety reasons [9, 10]. Despite great advances in the knowledge of molecular and cell signaling pathways involved in kidney injury, few new drugs are coming into the market to treat diabetic nephropathy due to the lack of effects or serious safety concerns.
Pentoxifylline (PTF) is a methylxanthine phosphodiesterase inhibitor with significant hemorheological effects, clinically used to treat patients with occlusive peripheral arterial disorders for more than 40 years [11]. PTF also has anti-inflammatory, antifibrotic and antiproliferative actions in animal models of DN [12]. Inflammation is recognized as a key contributor in the pathogenesis and progression of DN [13]. Phosphodiesterase inactivates the intracellular second messengers cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), inactivates protein kinase A (PKA) and enhances leukotriene and TNF synthesis, leading to inflammation. Urinary TNF-α level was an independent predictor of urinary albumin excretion in individuals with type 2 diabetes [14]. As a non-selective phosphodiesterase inhibitor, PTF can reduce inflammatory factors including IL-1, IL-6 and TNF-α which play important roles in the pathogenesis and progression of diabetic nephropathy [15]. Clinical studies in patients with DN have also shown that pentoxifylline reduces inflammatory effects and attenuates proteinuria [14, 16].
To date, clinical trials evaluating pentoxifylline in patients with DN had small sample sizes or were single-center designs. Though McCormick et al. and Shan et al. conducted a meta-analysis, respectively, which assessed the effect of pentoxifylline on proteinuria in patients with DN, but the two analyses were limited to articles published before 2010. In addition, they did not specially detect the additive effects of PTF based on RAS blockers [17, 18]. Therefore, we conducted a meta-analysis of appropriate published RCTs to determine the efficacy and safety of pentoxifylline plus RAS blockers in diabetic nephropathy.
Methods
Search strategy and study selection
A computerized search of the PubMed, CBM, EMBASE, CENTRAL and Cochrane renal group specialized register was independently undertaken by two authors to identify potentially eligible RCTs. The search was not limited to English language or publication type. All searches were up to date as of December 2014. The following subject heading terms or key words were used in our search: (“diabetes mellitus or diabetic nephropathies or diabetic kidney disease or diabetic or diabetes”) and (“pentoxifylline or pentoxifylline or oxpentifylline or torental or trental or agapurin or bl-191”). The reference lists from included RCTs, relevant systematic reviews and narrative reviews identified by electronic databases were hand searched to identify other potentially eligible articles. Studies were included in the meta-analysis if the following criteria were met: Randomized controlled trials that compared the benefits and harms of oral pentoxifylline plus ACEI/ARB with ACEI/ARB alone for patients of DN defined as albuminuria with greater than 30 mg/d, or estimated glomerular filtration rate <60 mL/min/1.73 m2; reporting at least one of the following outcomes: proteinuria, albuminuria, serum creatinine level, creatinine clearance or estimate of glomerular filtration rate. Trials that included subjects with renal replacement therapy or kidney damage relating to diseases other than diabetes were excluded. There was no restriction on sample size or intervention duration. Two investigators independently searched and assessed all citations for potentially eligible studies. Titles and abstracts from the electronic search were reviewed. After the initial review of the abstracts, the relevant studies were identified and a detailed evaluation of the full text was done. Disagreements or uncertainties were adjudicated by consensus or by consulting a third reviewer. In the case of multiple reports of with the same or overlapping data published by the same authors, we combined the informative data and retained only the complete article to avoid duplication of information.
Data extraction and quality scoring
Data extraction was performed independently by two authors using standardized data extraction forms. The following information was extracted from the included studies: first author’s name and publication year, sample size, trial design, demographic data (DM type and duration, body mass index, mean age, gender and location), daily dosage of PTF and control therapy, length of follow-up, dropouts and adverse events. We were interested in the following outcomes, including information on baseline and final concentrations (or net changes) of proteinuria, albuminuria, Scr, r, SBP, DBP, HbA1c and UTNF-α. These values were captured as the mean change from baseline to follow-up (with mean ± SD). The mean changes were calculated by subtracting the final values from the baseline values. Additionally, the standard deviations of the mean changes [SD(C)] were calculated according to the following formula:
We assumed a pre–post study correlation R of 0.5 to get an estimate of the mean change in SD. This allowed for the calculation of the mean effect size between pre–post change for PTF and control. We also conducted a sensitivity analysis assuming 0.25 and 0.75 as correlation between baseline and final values. Results did not change, and thus, data using a correlation of 0.5 are presented in this analysis. The quality of each study was evaluated using validated Jadad 5-point scale [19].
Studies with scores of 4 or higher are considered to be ones of high quality, and Jadad score not more than two indicates the low quality.
Data analysis and synthesis
According to the guideline in the Cochrane reviewers’ handbook, all analyses were performed with RevMan5.0 software. Due to different scales used in studies or the wide difference of the mean, standardized mean differences (SMDs) with 95 % CIs were calculated for proteinuria and albuminuria. For Scr, CrCl, SBP, DBP, HbA1c and UTNF-α, mean differences (MDs) with 95 % CIs were counted. Between-study heterogeneity was assessed using the Chi-square test. Studies with an I 2 statistic of 25–50 % were considered to have low heterogeneity, an I 2 statistic of 50–75 % were considered to have moderate heterogeneity, and an I 2 statistic of >75 % were considered to have a high heterogeneity [20]. Fixed-effect analysis was used when I 2 ≤ 50 %. Otherwise, the random-effect model was employed. Statistical significance was set at a two-tailed level of 0.05 for hypothesis testing. Adverse effects were assessed using descriptive techniques. Funnel plots and subgroup analyses could not be conducted because of the few included studies.
Results
Characteristics of included studies
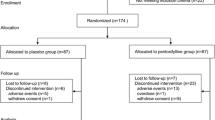
As outlined in Fig. 1, the search strategy generated 358 studies. After applying inclusion and exclusion criteria, eight randomized controlled trials with a total of 587 patients were included in this meta-analysis [21–28]. The characteristics and details of the included studies are summarized in Tables 1, 2 and 3. Studies generally were of small sample size, and the median number of participants was 58. Only two trials had 100 or more than 100 participants. All patients had been diagnosed as type 2 DN. The baseline characteristics of the patients were balanced between the PTF and control group. In five trials, pentoxifylline dose was 1200 mg/d; in one trial, dose was 600 mg/d; and in two trials, dose was 400 mg/d. The duration of therapy ranged from 21 days to 2 years, with a median of 5 months. The primary outcome of proteinuria was reported in five studies, and the other three trails reported results of albuminuria. Jadad scores were low, with a median score of 3 (range 2–4). The investigated populations of each study were too small to turn out significant results in these variables, but when they were assessed together in a meta-analysis, sufficient numbers of patients were available for a more reliable analysis.
Quantitative data synthesis
-
(1)
Proteinuria, albuminuria and UTNF-α
The pooled SMDs for proteinuria and albuminuria from the fixed-effect model are shown in Fig. 2. Five trials reported on the primary outcome of proteinuria. The meta-analysis suggested that proteinuria levels were decreased significantly in the PTF plus ACEI/ARB group compared with that of the control group (SMD0.76; 95 % CI 0.52–0.99, p < 0.001). The test for heterogeneity was low (I 2 = 24 %). The effect of PTF on albuminuria was assessed in three studies with 280 DN patients. Compared with ACEI/ARB alone, PTF plus ACEI/ARB could lead to a greater reduction in albuminuria (SMD0.36; 95 % CI 0.12–0.59, p = 0.004; I 2 = 0 %). Two studies reported UTNF-α (Fig. 3). The combination of PTF and ACEI/ARB could significantly reduce urine TNF-α level (WMD 1.56 ng/g; 95 % CI 0.09–3.03, p = 0.04; I 2 = 23 %).
-
(2)
Kidney function, blood pressure and HbA1c
Six trials reported serum creatinine levels. Pooled analysis showed no significant effect of pentoxifylline on change in serum creatinine levels (WDM, −0.04 mg/dL; 95 % CI −0.15 to 0.06; p = 0.42). The test for heterogeneity was high (I 2 = 52 %). Four trials reported creatinine clearance, and PTF plus ACEI/ARB treatment did not significantly change the creatinine clearance level compared with that of control group (WDM, −0.65 mL/min; 95 % CI −4.21 to 2.91; p = 0.72; I 2 = 0 %). Seven trials reported blood pressure. There were no significant differences in either systolic (WDM, 0.3 mmHg; 95 % CI −2.3 to 2.9; p = 0.82; I 2 = 81 %) or diastolic blood pressure (WDM 0.8 mmHg; 95 % CI −0.06 to 1.66; p = 0.72; I 2 = 48 %) between two groups. Six trials reported HbA1c. No significant change was observed in the PTF group compared with that of the control group (WDM 0.09 %; 95 % CI −0.04 to 0.21; p = 0.16; I 2 = 0 %). These detailed results are shown in Fig. 3.
Adverse effects
The most frequent adverse effects in patients treated with PTF were gastrointestinal symptoms (28/298) and dizziness (7/298), but in most cases, these symptoms were mild, only six participants withdrew due to intractable nausea and vomiting. According to Roozbeh et al. [24], four participants were excluded: One person from each group was excluded due to uncontrolled hypertension, one from control group due to hyperkalemia as a result of captopril use and one in PTF group due to nausea and vomiting as a result of PTF use. In one trail, six patients from PTX group were excluded because of chest pain and dyspnea in one, retinal hemorrhage in another and intractable nausea and vomiting in four patients [27]. In another study, five patients initiated dialysis during the study (three in the control group and two in the PTF group); one patient in each group died; one participant in PTF group withdrew because of intolerant gastrointestinal symptom [28].
Discussion
This meta-analysis included a total of eight studies with 587 patients focusing on the efficacy and safety of oral pentoxifylline plus ACEI/ARB for diabetic nephropathy. Compared with ACEI/ARB alone, the addition of PTF causes a significant reduction in urinary protein excretion and this effect seems to be associated with a reduction in urinary TNF-α excretion, yet independent from blood pressure and glycemic control. In this review, pooled analysis did not show significant effect of pentoxifylline plus ACEI/ARB on change in serum creatinine or creatinine clearance levels. This lack of benefit for them may be related to the shorter observation time, with median duration of 5 months in the included studies. Several clinical trials with longer follow-up indeed found that PTF could reduce the rate of progression of renal disease, and the between-group difference in the reduction of eGFR reached statistical significance only after a year of administration [28–30]. Recently, the PREDIAN trial found that treatment with PTF for 24 months led to a significant mean difference of 4.3 mL/min per 1.73 m2 in the reduction of eGFR among patients with type 2 diabetes who had stages 3–4 CKD and were receiving the maximum recommended dosage of ACEIs or ARBs. Moreover, the proportion of patients with a rate of eGFR decline greater than the median (0.16 mL/min/1.73 m2 per month) was significantly lower in the PTF group (33.3 %) than in the control group (68.2 %, p < 0.001) [28]. Regarding the antiproteinuric effects of PTF, the onset time in different trails is inconsistent, from the earliest 21 days to the latest 6 months after administering the drug [24, 26, 28]. The definitive onset time and effect degree of PTF on diabetic nephropathy should be answered by large-scale and multicenter studies. The adverse effects were consistent with the known safety profile of PTF obtained from a wide clinical experience for more than 40 years in patients with peripheral vascular disease, and transient digestive symptoms, dizziness and headache were the most common adverse reactions in patients of diabetic nephropathy.
The combined therapy of PTF and ACEI/ARB may offer greater antiproteinuric and renoprotective actions than ACEI/ARB alone. In addition to its rheologic effect with reduction in blood viscosity and a subsequent decrease in glomerular hydraulic pressure, preclinical researches indicate that PTF treatment can also lead to improvements in markers of inflammation, oxidative stress, cell proliferation and fibrosis [12, 31]. Although exact mechanisms of the renoprotective effect of PTF for diabetic nephropathy are not clearly understood, the most likely explanation may involve its ability to inhibit the production of proinflammatory cytokines, such as monocyte chemoattractant protein 1(MCP-1), IL-1, IL-6 and TNF-α [15, 32]. Diabetic nephropathy is a primary inflammatory state, and existing pieces of evidence suggest that proinflammatory cytokines may have a pathogenic role in increasing glomerular permeability to serum protein [33]. Among them, especially TNF-α has been shown to be cytotoxic to glomerular mesangial and epithelial cells, which causes significant glomerular injury [34]. PTF administration has been shown to inhibit the production of TNF-α in animal models and humans by inhibiting the transcription and translation of TNF-alpha gene [35]. In the PREDIAN trial and other clinical studies, the change in urinary TNF-α levels correlated directly with change in UAE and inversely with change in eGFR. There was no significant correlation between the serum and urinary concentrations of TNF-α, which suggests PTF might modulate intrarenal TNF production [12, 23, 28].
Strengths of our meta-analysis include composing of RCTs and selection of a homogenous type 2 diabetes population. The I 2 statistics for proteinuria and albuminuria display an acceptable risk of between-study heterogeneity, and this combined with narrow confidence intervals suggests that our findings are valid. Our study also has some potential limitations. Firstly, there was a marked absence of blinding or placebo use reported in the included studies, which often favors the treatment group. Secondly, there was a notable absence of data on clinically hard outcomes such as ESRD incidence, cardiovascular events and mortality at long-term follow-up. The included studies focused mainly on albuminuria, proteinuria, Scr, CrCl and blood pressure, which acted as surrogate endpoints. Thirdly, funnel plots for publication bias could not be made because of the limited numbers of studies for each outcome. Last but not least, due to the limited number of studies of PTF for the specific outcomes, subgroup analyses could not be conducted to compare dose, treatment duration, type of RAS blockers and baseline proteinuria level for each outcome.
Conclusions
Combining an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker and pentoxifylline could lead to a greater reduction in proteinuria and albuminuria in patients of DN independent of the decrease in BP or improvement in glycemic control. Further large, multicenter, high-quality studies with long duration are necessary to prove whether it can cause a reduction in hard endpoints, such as death or the need for dialysis.
References
Gregg EW, Li Y, Wang J et al (2014) Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 370(16):1514–1523
Haffner SM, Lehto S, Rönnemaa T et al (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339(4):229–234
Afkarian M, Sachs MC, Kestenbaum B et al (2013) Kidney disease and increased mortality risk in type 2 diabetes. J Am Socm Nephrol 24(2):302–308
Parving HH (1996) Initiation and progression of diabetic nephropathy. N Engl J Med 335(22):1682–1683
Kdigo BP (2012) Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2:337–414
American Diabetes Association (2014) Standards of medical care in diabetes 2014. Diab Care 37(Suppl 1):S14–S80
Perkins BA, Ficociello LH, Silva KH et al (2003) Regression of microalbuminuria in type 1 diabetes. N Engl J Med 348(23):2285–2293
National Kidney Foundation (2012) KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 60(5):850–886
Parving HH, Brenner BM, Investigators ALTITUDE et al (2012) Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367(23):2204–2213
Fried LF, Emanuele N, Investigators VANEPHRON-D et al (2013) Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369(20):1892–1903
De Sanctis MT, Cesarone MR, Belcaro G et al (2002) Treatment of intermittent claudication with pentoxifylline: a 12-month, randomized trial–walking distance and microcirculation. Angiology 53(Suppl 1):S7–S12
Abdel-Salam OM, Baiuomy AR, El-Shenawy SM et al (2003) The anti-inflammatory effects of the phosphodiesterase inhibitor pentoxifylline in the rat. Pharmacol Res 47(4):331–340
Navarro-González JF, Mora-Fernández C, Muros de Fuentes M et al (2011) Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7(6):327–340
Navarro JF, Mora C, Maca M et al (2003) Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis 42(1):53–61
Ward A, Clissold SP (1987) Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs 34(1):50–97
Navarro JF, Mora C, Rivero A et al (1999) Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. Am J Kidney Dis 33(3):458–463
McCormick BB, Sydor A, Akbari A et al (2008) The effect of pentoxifylline on proteinuria in diabetic kidney disease: a meta-analysis. Am J Kidney Dis 52(3):454–463
Shan D, Wu HM, Yuan QY et al (2012) Pentoxifylline for diabetic kidney disease. Cochrane Database Syst Rev 15(2):CD006800
Jadad AR, Moore RA, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Harmankaya O, Seber S, Yilmaz M (2003) Combination of pentoxifylline with angiotensin converting enzyme inhibitors produces an additional reduction in microalbuminuria in hypertensive type 2 diabetic patients. Ren Fail 25(3):465–470
Navarro JF, Mora C, Muros M et al (2003) Effects of pentoxifylline administration on urinary N-acetyl-beta-glucosaminidase excretion in type 2 diabetic patients: a short-term, prospective, randomized study. Am J Kidney Dis 42(2):264–270
Navarro JF, Mora C, Muros M et al (2005) Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: a short-term, randomized, controlled trial. J Am Soc Nephrol 16(7):2119–2126
Roozbeh J, Banihashemi MA, Ghezlou M et al (2010) Captopril and combination therapy of captopril and pentoxifylline in reducing proteinuria in diabetic nephropathy. Ren Fail 32(2):172–178
Oliaei F, Hushmand S, Khafri S et al (2011) Efficacy of pentoxifylline for reduction of proteinuria in type II diabetic patients. Caspian J Intern Med 2(4):309–313
Jin-Lei LV, Liu-Qing LV, Yi SHAO et al (2012) Valsartan combined with pentoxifylline in treatment of diabetic retinopathy and diabetic Nephropathy. Rec Adv Ophthalmol 32(10):945–948
Ghorbani A, Omidvar B, Beladi-Mousavi SS et al (2012) The effect of pentoxifylline on reduction of proteinuria among patients with type 2 diabetes under blockade of angiotensin system: a double blind and randomized clinical trial. Nefrologia 32(6):790–796
Navarro-González JF, Mora-Fernández C, Muros de Fuentes M et al (2015) Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol 26(1):220–229
Perkins RM, Aboudara MC, Uy AL et al (2009) Effect of pentoxifylline on GFR decline in CKD: a pilot, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis 53(4):606–616
Goicoechea M, García de Vinuesa S, Quiroga B et al (2012) Effects of pentoxifylline on inflammatory parameters in chronic kidney disease patients: a randomized trial. J Nephrol 25(6):969–975
Dávila-Esqueda ME, Martínez-Morales F (2004) Pentoxifylline diminishes the oxidative damage to renal tissue induced by streptozotocin in the rat. Exp Diabesity Res 5(4):245–251
Chen YM, Lin SL, Chiang WC et al (2006) Pentoxifylline ameliorates proteinuria through suppression of renal monocyte chemoattractant protein-1 in patients with proteinuric primary glomerular diseases. Kidney Int 69:1410–1415
Dalla Vestra M, Mussap M, Gallina P et al (2005) Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol 16(Suppl 1):S78–S82
Ortiz A, González-Cuadrado R, Sharma M et al (1995) Tumor necrosis factor as a mediator of glomerular damage. J Nephrol 8:27–34
Doherty GM, Jensen JC, Alexander HR et al (1991) Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery 110(2):192–198
Acknowledgments
This analysis was supported by a grant from National Natural Science Foundation of China, the category of Funding belonged to Youth Science Fund Project, and the project number was 81200539.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, ML., Shen, Y., Sun, ZL. et al. Efficacy and safety of combining pentoxifylline with angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in diabetic nephropathy: a meta-analysis. Int Urol Nephrol 47, 815–822 (2015). https://doi.org/10.1007/s11255-015-0968-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-0968-2