Abstract
Background and objective
End-stage renal failure (ESRF) patients under palliative care could live for months or even years after deciding not to start dialysis. They could experience significant symptom burden with recurrent fluid overload due to poor renal reserve. This could imply repeated hospital admissions for parenteral diuretics, which may destabilize their community support and limit their precious time spent with family. Diuretic therapy remains the cornerstone of managing fluid overload, but when per-oral administration become ineffective, parenteral diuretics may cause extra discomfort with potential infective complications. Metolazone, since its introduction in 1970s, has been proven effective in managing refractory heart failure, but whether its potential effect could be applied in ESRF patients not receiving dialysis is awaited to be proven.
Method
In our case series, we recruited elderly renal failure patients under palliative care with refractory fluid overload resistant to oral furosemide (120–160 mg daily dose), which was successfully managed after addition of low-dose metolazone (2.5–5 mg) for short duration (2–5 days). Reasoning behind not to initiate intravenous diuretics was discussed.
Results
All patients show good tolerance to combined diuretics without significant blood pressure fluctuation or electrolytes disturbance. Peripheral and pulmonary edema was clinically improved. Body weight reduction of 2.0–5.0 kg was achieved.
Conclusion
Our case series support the use of above regimen as a potential alternative in ESRF patients under palliative care, without bearing the parenteral route of treatment burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the advances in dialysis therapy and transplant medicine, many elderly patients with comorbidities are deemed inappropriate to receive renal replacement treatment. Failure to initiate dialysis is fundamentally different from the withdrawal of dialysis in which imminent death is expected. Patients can live for months or even years after deciding not to start dialysis [1]. End-staged renal failure (ESRF) patients experienced significant symptom burden comparable to terminal malignancy [2]. Common symptoms included weakness, lack of energy, poor appetite, edema, dyspnea and pruritis [1]. Besides, symptom distress severity tends to increase in the month prior to death [3].
Patients with ESRF are less able to compensate for their poor renal reserve and develop signs and symptoms of fluid overload with weight gain, peripheral edema and pulmonary edema. Previous studies demonstrated that refractory edema could affect up to 58–73 % of ESRF patients under palliative care [2–4]. Initially, patients may respond well to fluid restriction and loop diuretics. However, as the ESRF gets worse, fluid balance may become so precarious, complicated by increasing diuretic resistance, that the patient has repeated hospital admissions for parenteral diuretics. This may destabilize community support and limited their time spent with family members.
Metolazone, the potent thiazide-like diuretic [7-chloro-2-methyl-3-(2-methylphenyl)-4-oxo-2,3-dihydro-1H-quinazoline-6-sulfonamide], if often used as a drug of last resort on top of a loop diuretic [5]. Use of metolazone in refractory heart failure had been reported in up to 23 % in specialized heart failure clinics [6].
The application of metolazone in chronic renal failure patients dated back to year 1972 [7]. Fourteen patients with non-edematous stable ESRF were given high-dose metolazone 20–150 mg, which reported a significant increase in urine flow and sodium excretion. However, no previous studies have reported the efficacy and safety of combination treatment of metolazone and furosemide in ESRF patients under palliative care. Here, we reported a case series of three patients with ESRF resistant to high-dose oral furosemide and examined the clinical utility of combination diuretic in managing refractory fluid overload in a hospice setting. All recruited patients were previously seen by nephrologist and decided not to receive dialysis. All patients provided verbal consent for this case series. Patients recruited underwent treatment in a regional palliative care unit in Hong Kong and received close monitoring of blood pressure, fluid balance and electrolytes during treatment.
Results (summarized in Table 1)
Patient A
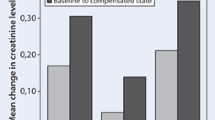
A 78-year-old man with stage V chronic kidney disease (CKD) due to diabetic nephropathy was admitted to hospice for progressive fluid overload. His eGFR was 4.2 ml/min. His body weight increased from baseline 58–62.3 kg upon admission and experienced shortness of breath requiring 4L of oxygen supplement. He had 3+ pitting edema up to mid-shins bilaterally. Chest X-ray confirmed pulmonary edema. His usual oral furosemide 60 mg twice daily was titrated to 80 mg twice daily, but his dyspnea and fluid overload failed to response. He had difficult intravenous access due to generalized edema. Metolazone 5 mg daily was initiated and continued for 5 days in total. He responded well with significant diuresis, and his weight decreased to 58.2 kg, and successfully weaned off oxygen supplement. There was no significant blood pressure fluctuation, and his electrolytes balance remained satisfactory. Serum creatinine was 1,215 µmol/L (13.74 mg/dL) comparing with 1,128 µmol/L (12.76 mg/dL) pre-treatment. His diuretic regimen was transitioned back to furosemide 60 mg twice daily. With careful fluid restriction and medication adjustment, he did not have symptomatic fluid overload during the remainder of his home hospice care over the next 3 months.
Patient B
An 86-year-old man with stage V CKD (eGRF 5.8 ml/min) due to diabetic nephropathy, congestive heart failure and old stroke was admitted for progressive worsening ankle edema and orthopnea. During this time, he experienced a 3-kg weight gain despite a regular oral diuretic regimen of furosemide 60 mg twice per day. He had multiple hospital admission in recent 2 months due to uncontrolled fluid overload, requiring intravenous diuretics complicated by drip-site infection and multiple skin bruises. His oral furosemide dosage was continued at 60 mg twice per day, with an addition of metolazone 2.5 mg daily for 3-day course. Patient achieved good diuresis with body weight reduced from 74.5 to 69.5 kg. His oxygen saturation improved from 92 to 97 % on room air, with an associated increased tolerance in physiotherapy. There was a mild laboratory increase in his serum creatinine from 848 to 941 µmol/l (9.59–10.64 mg/dl), but his electrolyte and blood pressure remained stable during combined treatment. His condition improved during stay and was successfully discharged home after 14 days of inpatient care.
Patient C
A 74-year-old man with stage IV CKD (eGFR 20.8 ml/min) due to hypertensive nephropathy and atrophic right kidney was admitted for refractory generalized edema despite maintenance oral furosemide 80 mg twice per day. He had 3+ pitting edema up to knee upon admission, but there was no associated pulmonary edema. Upon discussion, he expressed his wish of comfort care by minimizing inhospital stay and declined the placement of intravenous cannula as far as possible. Oral metolazone 5 mg daily was prescribed for 2 days, in addition to his usual furosemide regimen. He responded well to treatment, with pitting ankle edema subsided to minimal and achieved a weight reduction of 2 kg. His blood pressure, serum creatinine and electrolytes all remained stable. He was satisfied with treatment and successfully discharged on day 3.
Discussion
With the expected growth in non-dialytic ESRF patients, the Renal Physicians Association recently updated their practice guideline affirming the rights of patients to refuse dialysis initiation [8]. The guideline mentions the importance of balancing the burden of dialysis versus the potential benefits, especially in the very elderly group of patients with multiple comorbid diseases. Most nephrologists currently extrapolate fluid and electrolyte management from earlier-stage CKD. In China Hong Kong SAR, several palliative care centers are starting to develop specialized renal palliative care program to address concurrent renal care and palliative care needs of patients [9, 10].
While refractory fluid overload remains a prevalent symptom affecting up to 70 % of renal palliative care patients [2], its management should remain distinct with special emphasis placed on comfort measures in symptom management. Parenteral route of drug administration had been reported in up to 90 % in certain disease population under palliative care [11]. Intravenous administration of diuretics, as used in most acute care unit, requires catheter insertion and may cause palliative care patients an elevated hardship of discomfort, complications and cost [12]. Combination of low-dose oral metolazone with loop diuretics, as our case series support, may be a potential alternative in the management of refractory renal failure in hospice setting.
Metolazone only exists in an oral formulation for clinical use. It is excreted mainly in the urine with 70–95 % being unchanged [13]. Metolazone acts in the nephron on the intraluminal site of the tubule. It is believed that the main effect of metolazone is in the cortical diluting segment of the distal tubule by inhibition of the reabsorption of sodium and chloride ions. Presumably as a consequence of the drug action in the proximal tubule, metolazone, in contrast to other thiazide diuretics, is able to produce a diuresis despite a low glomerular filtration rate, which makes it a promising option for our ESRF patients [14]. Combination of metolazone with loop diuretic results in various point of action on the nephron. The multisegmental blockade diminishes the physiological renal counter-effects observed when using metolazone or furosemide as monotherapy, resulting in a synergistic diuretic effect [15].
A review of the literature demonstrated relatively few studies regarding combination treatment of metolazone with furosemide in renal palliative care management. Its combined usage had demonstrated success in refractory heart failure patients [5, 16]. Rosenberg et al. [5] reported a significant reduction in weight and NYHA functional class improvement in 42 episodes of combined treatment in advanced heart failure patients, with clinical important hypokalemia (<2.5 mM) or hyponatremia (<125 mM) observed in 10 % of cases with a relatively normal baseline renal function. Dargie et al. [7] demonstrated that high-dose metolazone use in chronic renal failure, in daily doses ranging from 20 to 150 mg, achieved a 51 % increase in mean urine flow rate and a 113 % increase in mean sodium excretion, with no reported side effects in their study on 14 subjects.
In our three reported cases of ESRF patients refractory to high-dose furosemide mono-therapy, combination of low-dose metolazone (2.5–5 mg) in short duration (2–5 days) with loop diuretics demonstrated a beneficial effect. Per-oral (PO) route of drug administration affords a more comfortable and accessible means than the intravenous (IV) counterpart and appears to have a synergistic diuretic effect in patients failing mono-furosemide therapy. PO route of drug administration could minimize the treatment burden of IV catheter, shorten inpatient length of stay and avert the requirement of repeated acute hospital admissions for IV diuresis. Although subcutaneous furosemide had demonstrated success in the management of refractory heart failure in palliative care and hospice setting [17, 18], its application in ESRF patients had not been reported. Besides, subcutaneous site reaction had been reported in up to 23 % of furosemide infusion subjects, and antibiotics treatment was indicated in the severe reaction group [17]. Another potential problem with furosemide subcutaneous infusion would be the large volume requirement. Since the concentration of the injection furosemide is 10 mg/ml, many ESRF patients would require high doses of loop diuretics that would require large volumes for subcutaneous injection. This may require the frequent change of syringe driver or the employment of multiple injection sites [18].
Our three reported patients all showed good tolerance to low-dose metolazone in combination with high-dose furosemide, with no significant blood pressure fluctuation (as defined by systolic BP drop >15 mmHg). Plasma sodium and potassium level remained stable during treatment. Outcome of treatment was good, with improvement in oxygen concentration in patient A and B, who suffered pulmonary edema. Peripheral edema was improved in all three patients. Combined treatment led to a reduction of body weight ranging from 2 to 5 kg. The three recruited patients underwent treatment in a palliative care setting, and the main method of monitoring fluid balance was by physical examination and body weight measures. Indwelling urinary catheters were not inserted taking into account of patients’ discomfort and associated risk of urinary tract infections [19]. Estimated glomerular filtration rate (eGFR) presented in our table was calculated by the Cockcroft and Gault formula. Although the formula estimation of GFR was known to carry small imprecision in elderly, formulaic estimates of clearance as presented can provide better information than serum creatinine alone [20].
We are encouraged by the initial results of this case series of combined oral diuretic treatment in the management of refractory fluid overload in renal failure elderly patients opted not to receive dialysis. Palliative care needs of elderly patients could be complex and often require a multidisciplinary approach in management [21]. Future larger-scale trials are warranted to evaluate its effectiveness on symptom management and patient safety, with the potential extended use in outpatient and home hospice settings.
References
O’Connor NR, Kumar P (2012) Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med 15(2):228–235
Saini T, Murtagh FE, Dupont PJ et al (2006) Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med 20:631–636
Murtagh FE, Addington-Hall J, Edmonds P et al (2010) Symptoms in the month before death for stage 5 chronic kidney disease patients managed without dialysis. J Pain Symptom Manage 40:342–352
Murtagh FE, Addington-Hall J, Edmonds P et al (2007) Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med 10:1266–1276
Rosenberg J, Gustafsson F, Galatius S, Hildebrandt PR (2005) Combination therapy with metolazone and loop diuretics in outpatients with refractory heart failure: an observational study and review of the literature. Cardiovasc Drugs Ther 19(4):301–306
Ramahi TM, Longo MD, Rohlfs K, Sheynberg N (2000) Effect of heart failure program on cardiovascular drug utilization and dosage in patients with chronic heart failure. Clin Cardiol 23:909–914
Dargie HJ, Allison ME, Kennedy AC, Gray MJ (1972) High dosage metolazone in chronic renal failure. BMJ 4(5834):196–198
Galla JH (2000) Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The Renal Physicians Association and the American Society of Nephrology. J Am Soc Nephrol 11(7):1340–1342
Yong DS, Kwok AO, Wong DM et al (2009) Symptom burden and quality of life in end-stage renal disease: a study of 179 patients on dialysis and palliative care. Palliat Med 23:111–119
Chan KY, Li CW, Wong H et al (2013) Use of sertraline for antihistamine-refractory uremic pruritus in renal palliative care patients. J Palliat Med 16(8):966–970
Cheng BHW, Sham MMK, Chan KY et al. (2013) Intensive palliative care for patients with hematological cancer dying in hospice: analysis of the level of medical care in the final week of life. Am J Hosp Palliat Care [Epub ahead of Print]. doi:10.1177/1049909113512412
Strasser F, Blum D, Bueche D (2010) Invasive palliative interventions: when are they worth it and when are they not? Cancer J 16(5):483–487
Hinsvark ON, Cohen AI (1970) The study of metolazone, a new diuretic, in human body fluids using thin layer separation, liquid chromatographic measurements and14C-counting techniques. Fed Proc 29:276 (Abstract)
Tilstone WJ, Dargie H, Dargie EN, Morgan HG, Kennedy AC (1974) Pharmacokinetics of metolazone in normal subjects and in patients with cardiac or renal failure. Clin Pharmacol Ther 16:322–329
Sigurd B, Olesen KH, Wennevold A (1975) The supra-additive natriuretic effect addition of bendroflumethiazide and bumetanide in congestive heart failure. Permutation trial tests in patients in long-term treatment with bumetanide. Am Heart J 89:163–170
Ng TM, Konopka E, Hyderi AF et al (2013) Comparison of bumetanide- and metolazone-based diuretic regimens to furosemide in acute heart failure. J Cardiovasc Pharmacol Ther 18(4):345–353
Zacharias H, Raw J, Nunn A, Parsons S, Johnson M (2011) Is there a role for subcutaneous furosemide in the community and hospice management of end-stage heart failure? Palliat Med 25(6):658–663
Farless LB, Steil N, Williams BR, Bailey FA (2013) Intermittent subcutaneous furosemide: parenteral diuretic rescue for hospice patients with congestive heart failure resistant to oral diuretic. Am J Hosp Palliat Care 30(8):791–792
Elpern EH, Killeen K, Ketchem A et al (2009) Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care 18(6):535–541
Lamb EJ, Webb MC, Simpson DE et al (2003) Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: is the modification of diet in renal disease formula an improvement? J Am Geriatr Soc 51(7):1012–1017
Cheng HWB, Li CW, Chan KY et al (2014) Bringing palliative care into geriatrics in a chinese culture society—results of a collaborative model between palliative medicine and geriatrics unit in Hong Kong. J Am Geriatr Soc 62:779–781. doi:10.1111/jgs.12760
Acknowledgments
The authors declared no potential conflicts of interest with respect to this research, authorship and publication of this article. We would like to thank all renal palliative care patients who participated in this case series.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, H.W.B., Sham, MK., Chan, KY. et al. Combination therapy with low-dose metolazone and furosemide: a “needleless” approach in managing refractory fluid overload in elderly renal failure patients under palliative care. Int Urol Nephrol 46, 1809–1813 (2014). https://doi.org/10.1007/s11255-014-0724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0724-z