Abstract
Purpose
To evaluate benefits of sentinel lymph node (SLN) biopsy for staging accuracy in prostate cancer. Extended pelvic lymph node dissection (ePLND) is a preferred staging tool; however, it may underestimate the incidence of nodal involvement.
Methods
Eighty patients with estimated risk of lymphadenopathy above 5 % based on Briganti nomogram had Tc-99m-labeled nanocolloid injected into the prostate. Planar lymphoscintigraphy and single-photon emission computed tomography/CT were performed to localize SLNs. Radioguided SLN dissection was followed by backup ePLND comprising external iliac, obturator and internal iliac regions. All SLNs were serially sectioned every 150 μm and examined using hematoxylin and eosin; immunohistochemical staining was applied every 300 μm.
Results
A total of 335 SLNs were detected, and 17 % were located outside ePLND template. Nodal metastases were diagnosed in 32 patients (40 %). Without radioguided SLN localization, solitary metastases posteriorly to the branches of the internal ilaic vessels, in pararectal and common iliac regions would not have been removed in five of 32 patients (16 %). Using standard histology protocol, we would have diagnosed metastases in 23 patients with median size of 2.8 mm. Serial sectioning of SLN and immunohistochemistry led to the detection of metastases in additional nine patients (28 %) with median size of 0.2 mm.
Conclusion
ePLND comprised 83 % of SLNs, at least one SLN laid outside its template in 28 % of patients. ePLND and SLN dissection combined with nodal serial sectioning and immunohistochemistry increased the detection rate of nodal metastases by 68 % in comparison with ePLND alone and standard histology protocol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lymph node metastasis is an unfavorable prognostic factor in prostate cancer [1]. Pelvic lymph node dissection (PLND) is still considered to be a staging procedure of choice as imaging studies have shown low sensitivity for the detection of micrometastatic disease [2]. Several retrospective studies have even pointed to possible therapeutic benefits of PLND, especially with minimal nodal involvement [3].
In last 30 years, widespread prostate-specific antigen (PSA) testing led to the shift in clinical stages of prostate cancer with fewer nodal metastases being diagnosed. At the same time, PLND was less frequently indicated and its extent diminished, which resulted in a decreasing number of removed lymph nodes and low probability of accurate staging [4]. The actual rate of nodal metastases may be underestimated if anatomically adequate PLND is not performed. At the present time, extended PLND (ePLND) comprising external iliac, obturator and internal iliac regions is recommended template [2].
In certain solid tumors, lymphadenectomy has been replaced by sentinel lymph node (SLN) dissection, which provides individualized localization of primary draining nodes, even beyond accepted borders of lymphadenectomy. Previous studies have also proven that the meticulous histologic examination of SLN improves the detection of nodal metastases [5].
SLN dissection has been used for lymphatic mapping studies in prostate cancer, but its clinical role still needs to be defined. Nowadays it mostly complements PLND. The aim of the present study was to evaluate staging benefits of a more thorough histologic examination of SLNs and radionavigated dissection beyond the borders of ePLND.
Materials and methods
Patients
A total of 80 patients (median age 63.5 years; range 51–72) with biopsy-confirmed localized or locally advanced prostate cancer, whose estimated risk of lymphadenopathy was above 5 % based on Briganti nomogram [6], were included in this study from October 2010 to March 2013. The study was approved by the Committee for Ethics, and informed consent was obtained from all patients. Patients’ data were prospectively collected. The exclusion criteria included previous pelvic surgery, radiotherapy and androgen ablation. Macroscopic lymphadenopathy was excluded by computer tomography (CT) or magnetic resonance imaging.
Preoperative imaging
On the day of surgery, 100 MBq in 0.8 ml of Tc-99m-labeled nanocolloid (Nanocoll®, GE Healthcare, Milan, Italy) was injected into the prostate with a Chiba needle 21G under ultrasound guidance. Radiocolloid was applied in two fractions per lobe, in the peripheral and central zones of the upper and lower quadrants. We aimed to achieve homogeneous tracer distribution.
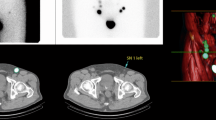
Planar lymphoscintigraphy (Fig. 1a) and single-photon emission computer tomography (SPECT) fused with CT images (Fig. 1b) were performed after approximately 2 h to localize SLNs on Picker Prism 2000 XP gamma camera. Two to six static scintigraphic images were recorded for 5 min. SPECT images were acquired from 72 projections during 15 min with subsequent reconstruction of data and software correction of attenuation. For better anatomic localization of SLNs, SPECT images were fused with CT scan with Siemens software.
SLNs (arrows) localized with planar scintigraphy (a) and single-photon emission computed tomography (SPECT) imaging (b), 2 h after injection of Tc-99m-labeled nanocolloid, the prostate with applied radiocolloid is shielded (asterisk). SPECT and CT images were fused to facilitate anatomic localization of SLNs (external iliac region in this case)
On visual inspection, the focal tracer accumulations that were found in presumed localization of draining lymphatic pathways were considered SLNs. Analysis was done by nuclear medicine physician.
Surgical procedure
After 3–6 h, patients underwent open retropubic radical prostatectomy and lymph node dissection. All lymphatic drainage regions of the prostate were extraperitoneally accessed and explored with the handheld gamma probe Europrobe 3 (Eurorad, Eckbolsheim, France) or Di Surpro (Delong Instruments, Brno, Czech Republic). A signal intensity of twice or more the background signal was defined as indicative of SLN.
Backup ePLND comprising the external iliac, obturator and internal iliac regions was performed to evaluate the sensitivity of SLN biopsy. The common iliac, presacral or pararectal lymph nodes were removed only if SLN occurred in that region and if technically justified. To approach common iliac and presacral SLNs, umbilical vessels were transected and peritoneum was pushed cranially. All removed fibro-fatty tissue was examined ex vivo to exclude previously missed SLNs. Every patient received low molecular weight heparin as prophylaxis against venous thromboembolic disease.
Histologic examination
The specimens from individual pelvic regions were sent separately. The lymph nodes were palpated and fixed with formalin. Smaller nodes were halved only longitudinally, and nodes thicker than 4 mm were sectioned to 2 mm slices and embedded in paraffin.
Standard examination consisted of three 4 μm sections stained with hematoxylin and eosin (H&E). In addition, we conducted a special histologic evaluation of SLNs comprising further serial sectioning every 150 μm with H&E staining and immunohistochemical staining using wide-spectrum anti-cytokeratin antibody (CK AE1/AE3, Biogenex) every 300 μm.
Statistics
Standard description of predictive power of SLN procedure was adopted—sensitivity and negative predictive value; calculation was based on true/false negative histologic finding in SLNs. Discrete variables were presented as rates or percentages, and continuous variables were characterized with median, range or interquartile range (IQR). When comparing the patient characteristics ML, chi-square test and Mann–Whitney U test were used for discrete and continuous variables, respectively. p value of significance was set at p < 0.05. Analysis was performed with the use of SPSS 21 software (IBM Corporation, 2012).
Results
Patients characteristics are summarized in Table 1. Median PSA was 12.7 ng/ml (range 2.8–80.0). Most of the patients were in high risk (59 %) or intermediate risk (40 %) subgroups.
Preoperative imaging of SLNs
Lymphoscintigraphy and SPECT/CT were performed in 78 patients; in two cases, only intraoperative localization was possible due to technical problems. Preoperative imaging of SLN was successful in 85 %, on both pelvic sides in 53 %. In total, 195 SLNs were delineated preoperatively with median of two (IQR 1–4).
Intraoperative detection of SLNs
SLNs were successfully localized with a gamma probe during the operation in 98 %, in 84 % on both pelvic sides. Anatomic distribution of 335 SLNs is shown in Fig. 2a: external iliac (n = 90; 27 %), obturator (n = 95; 28 %), internal iliac (n = 100; 30 %) including pararectal (n = 6; 2 %), common iliac (n = 24; 7 %), presacral (n = 19; 6 %), paraaortic (n = 5; 1 %) and inguinal (n = 2; 1 %). We removed 309 SLNs in total with median of four (IQR 3–5, range 0–8) (Fig. 2b). In 17 patients, 26 SLNs were not removed due to the limitation of extraperitoneal access and possible morbidity of the procedure.
Detection of lymph node metastases
The median number of 17 lymph nodes was removed (range 8–43; IQR 14–20), and nodal metastases were diagnosed in 32 of 80 patients (40 %). Positive lymph nodes were found in internal iliac (n = 23; 43 %) including pararectal (n = 2; 4 %), external iliac (n = 18; 33 %), obturator (n = 12; 22 %) and common iliac region (n = 1; 2 %).
Sensitivity of the SLN procedure was 91 % (95 % CI 84–97), and metastases were found in SLNs in 29 of 32 patients with stage pN1. False negativity was 9 % (95 % CI 3–16) and the negative predictive value 94 % (95 % CI 84–99). In three false negative cases, the solitary metastases were found in non-SLNs inside ePLND template.
The differences between pN0 and pN1 subgroups of the patients were significant in preoperative stage cT (p = 0.010), pathologic stage pT (p = 0.001) and pathologic Gleason score (GS) (p = 0.006) (Table 1). PSA did not differ in the subgroups (p = 0.898).
Templates of PLND
A total of 309/335 SLNs were removed, and 56 of them (16.7 %) in 22 patients (28 %) were located outside the ePLND template. Without the radioguided SLN dissection, metastases would not have been localized and removed in five patients (16 %). In two of them, a metastasis was found in pararectal region. In the other cases, a solitary metastasis was detected in common iliac region and posteriorly to vesical and umbilical branches of the internal iliac artery.
Table 2 depicts anatomic distribution of SLNs and impact of various PLND templates on probability of accurate staging. ePLND comprised 83 % of SLNs in our study, and it would have correctly staged 88 % and removed metastases in 84 % of the patients. ePLND combined with SLN biopsy had the highest staging accuracy and probability of metastatic nodes removal.
Nodal serial sectioning and immunohistochemistry
In total, 54 positive lymph nodes were diagnosed in 32 patients with median of 1 (range 1–6). Macrometastases, micrometastases and isolated tumor cells (ITC) occurred in 16 (50 %), 12 (37.5 %) and 4 (12.5 %) cases, respectively. In addition, non-SLNs were positive in 44 %. Using the standard pathological method for handling of nodes, we would have diagnosed metastases in 23 patients with median size of 2.8 mm (range 1.1–10.0; IQR 1.9–4.8). Serial sectioning of SLN and immunohistochemistry led to the detection of metastases in additional nine patients (28 %) with median size of 0.2 mm (range 0.1–1.1; IQR 0.1–0.5).
Radioguided SLN dissection beyond the borders of ePLND and serial sectioning with immunohistochemistry led to diagnosis of nodal disease in additional 13 patients (41 %) in comparison with ePLND and standard histology technique (Table 3). The detection rate of nodal metastases increased by 68 %.
Complications
Early ePLND-related complications (30-days) occurred in 9 (11 %) patients. In one patient, we encountered one major complication (Clavien grade 3b; 1 %), which required surgical intervention for bleeding from the external iliac vein postoperatively. Eight patients (10 %) experienced minor complications (Clavien grade 1–2), the most frequent complications were percutaneous drainage of symptomatic lymphocele (9 %), pulmonary embolism (3 %) and deep venous thrombosis (1 %).
Discussion
ePLND is currently the preferred staging tool in prostate cancer [2]. The SLN concept has replaced lymphadenectomy in several solid tumors, and it has also been validated in both open and laparoscopic surgery in prostate cancer [7, 8]; however, in this particular malignancy, further studies are needed to assess the prognostic and therapeutic implications of the procedure.
We evaluated individual lymphatic drainage in 80 patients by SLN biopsy and combined it with meticulous histologic examination of SLNs. In addition, data on positive LNs localization were collected. The study design was used to evaluate staging benefit of SLN biopsy with nodal serial sectioning and immunohistochemistry in comparison with ePLND with standard histology.
We can conclude that the incidence of lymph node metastases may be underestimated if an appropriate anatomic template is not pursued and only standard histologic evaluation is applied [5, 9]. The removal of obturator nodes alone is an inadequate procedure as only 34 % of the patients would have been staged correctly and all metastases would have been taken out in no more than 16 % (Table 2). The ePLND template would have correctly staged 88 % and removed metastases in 84 % of our patients which is in compliance with the results of the studies with SLN biopsy published previously [9, 10]. If PLND with curative intent is performed, meticulous dissection of the internal iliac region is of utmost importance. In our study, internal iliac region was the exclusive site of nodal involvement in 34 % of the patients and in 63 % in combination with other regions, which corresponds to the findings of the previous studies [9–11].
In their mapping study, Mattei et al. located 37 % of SLNs outside ePLND, which is considerably more than 17 % in our study. Interestingly, in their study, 16 % and 12 % of SLNs were found in common iliac and paraaortic groups, respectively [12]. The question may arise if they represent true primary draining nodes of the prostate. Two teams have published their experience with fluorescent tracer indocyanine green (ICG) which enables to delineate not only SLNs but also afferent lymphatic channels and thereby distinguish between primary and secondary lymph nodes [13, 14]. Combining ICG with the radiocolloid technique, the authors detected more SLNs and they showed direct lymphatics to paraaortic region in 12 % of the patients [14]. The disadvantage is the need of near-infrared endoscopy to visualize the tracer.
Several studies have shown that 19–44 % of SLNs [9, 10, 12] and up to 13 % of metastases are located outside the ePLND template [9, 15]. The rate of presacral and common iliac metastases depends primarily on the stage of the primary tumor. In the pre-PSA era up to 50 % of the cases had nodal involvement in the presacral region [16], contemporary incidence is much lower in the range 4–12 % of the patients with stage pN1 [9, 15]. The teams from Bern and Leuven have proposed the extension of the dissection in common iliac and presacral region, respectively [9, 12]. Any expansion of the PLND template and accompanying morbidity increase must be outweighed by clear clinical benefit. In this sense, the patients with prostate cancer may benefit from SLN biopsy, which individually delineates the primary lymphatic spread and less frequent landing sites for lymph node metastases may be dissected only in case SLN is visualized in that region.
Our study confirmed that improved detection of nodal metastases by SLN biopsy has two origins. Firstly, SLNs beyond the borders of lymphadenectomy template are removed. Using the radioguided navigation, we found metastases in five patients (16 %) beyond the borders of ePLND. The metastases were located in pararectal, common iliac and internal iliac region. The latter is a part of ePLND, but we believe that without SLN localization, we would not have removed these solitary metastases located posteriorly to vesical and umbilical branches of internal iliac artery. This supports the need for dissection of medial and posterior aspect of the internal iliac vessels [3].
Secondly, a more thorough examination of SLNs by serial sectioning and immunohistochemistry is applied. We diagnosed micrometastases in further nine patients (28 %), which corresponds with published rates of 13–38 % [5, 17]. Together with radioguided dissection beyond the borders of ePLND, this method increased the number of the patients with stage pN1 in our study by 68 % in comparison with ePLND and standard histology (Table 3).
The incidence of stage pN1 (40 %) observed in this study was much higher than predicted by any preoperative nomogram. Even the updated Briganti nomogram which is based on ePLND showed little predictive accuracy, with median predicted risk of lymphadenopathy of 10 % and mean value of 20 % [18]. The reason is probably a higher detection of micrometastasis in SLN biopsy.
In four patients, only ITC, which are defined as tumor deposits below 0.2 mm, were found in SLNs. The benefit of diagnosing ITC is unclear and must be investigated as their presence may even represent passive transport of tumor cells after surgery or biopsy. In breast cancer, the results are contradictory, although several studies have shown that prognosis of the patients with ITC and micrometastases is not different [19]. In prostate cancer, Pagliarulo et al. used immunohistochemistry to reevaluate originally negative nodes. Compared to the original pN1 stage, newly detected micrometastases and ITC had a similar prognosis and were independent predictors of recurrence and death in a multivariable analysis [20].
Weakness of our study is the fact presacral and common iliac lymph nodes were not resected in every patient, only in case SLN occurred in that region. In addition, this study comprised patients with a higher risk of nodal involvement.
In the future, optimization of the technique and assessment of its main advantages in the current clinical practice will be necessary. SLN biopsy with meticulous histology in combination with ePLND in high risk patients may improve staging and locate metastases outside its borders. It may even replace ePLND in patients with lower risk of nodal involvement as the risk of non-SLN metastases is low in earlier stages of the disease. This could considerably decrease the operative time and risk of complications and have an impact on cost-benefit analysis of the procedure.
Conclusion
The incidence of nodal involvement in prostate cancer may be underestimated in the present surgical series. Due to radioguided localization of SLNs beyond the borders of ePLND and more thorough histologic examination, SLN biopsy gets close to the detection of a true incidence of nodal metastases. In comparison with ePLND with standard histologic evaluation, ePLND and SLN dissection combined with nodal serial sectioning and immunohistochemistry increased the detection rate of nodal metastases by 68 %. In our study, the median of removed SLNs was four per patient.
Patients may benefit from more accurate staging and tailored adjuvant treatment. Further studies are needed to define the role of SLN biopsy in the therapeutic management of prostate cancer.
Abbreviations
- CT:
-
Computed tomography
- ePLND:
-
Extended pelvic lymph node dissection
- ICG:
-
Indocyanine green
- IQR:
-
Interquartile range
- ITC:
-
Isolated tumor cells
- H&E:
-
Hematoxylin and eosin
- PSA:
-
Prostate-specific antigen
- SLN:
-
Sentinel lymph node
- SPECT:
-
Single-photon emission computed tomography
References
Cheng L, Zincke H, Blute ML et al (2001) Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer 91:66–73
Heidenreich A, Bellmunt J, Bolla M et al (2011) European Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localized disease. Eur Urol 59:61–71
Briganti A, Blute ML, Eastham JH et al (2009) Pelvic lymph node dissection in prostate cancer. Eur Urol 55:1251–1265
Abdollah F, Sun M, Thuret R et al (2010) Decreasing rate and extent of lymph node staging in patients undergoing radical prostatectomy may undermine the rate of diagnosis of lymph node metastases in prostate cancer. Eur Urol 58:882–892
Wawroschek F, Wagner T, Hamm M et al (2003) The influence of serial sections, immunohistochemistry and extension of pelvic lymph node dissection on the lymph node status in clinically localized prostate cancer. Eur Urol 43:132–137
Briganti A, Karakiewicz PI, Chun FK et al (2007) Percentage of positive biopsy cores can improve the ability to predict lymph node invasion in patients undergoing radical prostatectomy and extended pelvic lymph node dissection. Eur Urol 51:1573–1581
Holl G, Dorn R, Wengenmair H et al (2009) Validation of sentinel lymph node dissection in prostate cancer: experience in more than 2,000 patients. Eur J Nucl Med Mol Imaging 36:1377–1382
Jeschke S, Beri A, Grüll M et al (2008) Laparoscopic radioisotope-guided sentinel lymph node dissection in staging of prostate cancer. Eur Urol 58:126–133
Joniau S, Van den Bergh L, Lerut E et al (2013) Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol 63:450–458
Rousseau C, Rousseau T, Bridji B et al (2012) Laparoscopic sentinel lymph node (SLN) versus extensive pelvic dissection for clinically localized prostate carcinoma. Eur J Nucl Med Mol Imaging 39:291–299
Schumacher MC, Burkhard FC, Thalmann GN et al (2008) Good outcome for patients with few lymph node metastases after radical retropubic prostatectomy. Eur Urol 54:344–352
Mattei A, Fuechsel FG, Bhatta Dhar N et al (2008) The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol 53:118–125
Van der Poel HG, Buckle T, Brouwer OR et al (2011) Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur Urol 60:826–833
Jeschke S, Lusuardi L, Myatt A et al (2012) Visualisation of the lymph node pathway in real time by laparoscopic radioisotope and fluorescence-guided sentinel lymph node dissection in prostate cancer staging. Urology 80:1080–1087
Heidenreich A, Varga Z, Von Knobloch R (2002) Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol 167:1681–1686
Golimbu M, Morales P, Al-Askari S et al (1979) Extended pelvic lymphadenectomy for prostatic cancer. J Urol 121:617–620
Fukuda M, Egawa M, Imao T et al (2007) Detection of sentinel node micrometastasis by step section and immunohistochemistry in patients with prostate cancer. J Urol 177:1313–1317
Briganti A, Larcher A, Abdollah F et al (2012) Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol 61:480–487
De Boer M, van Deurzen CH, van Dijck JA et al (2009) Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med 361:653–663
Pagliarulo V, Hawes D, Brands FH et al (2006) Detection of occult lymph node metastases in locally advanced node-negative prostate cancer. J Clin Oncol 24:2735–2742
Acknowledgments
This study was funded by Institutional Resources for Supporting the Research Organization provided by the Czech Ministry of Health to Masaryk Memorial Cancer Institute.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Staník, M., Čapák, I., Macík, D. et al. Sentinel lymph node dissection combined with meticulous histology increases the detection rate of nodal metastases in prostate cancer. Int Urol Nephrol 46, 1543–1549 (2014). https://doi.org/10.1007/s11255-014-0704-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0704-3