Abstract
Purpose
Several epidemiologic studies were performed to clarify the protective effect of regular aspirin use on prostate cancer risk; however, the results remain controversial. Therefore, we conducted this meta-analysis to assess the association between regular aspirin use and risk of prostate cancer.
Methods
Electronic databases including PubMed, EMBASE and Cochrane Library were searched between January 1966 and April 2013 to identify eligible studies. Pooled relative ratios (RRs) and 95 % confidence intervals (CIs) were computed to assess the influence of aspirin use on prostate cancer risk. All statistical tests were two-sided.
Results
A total of 24 observational studies including 14 case–control studies and 10 cohort studies were eligible for this meta-analysis. Regular aspirin use was associated with reduction in overall and advanced prostate cancer risk (pooled RR 0.86, 95 % CI 0.81–0.92; pooled RR 0.83, 95 % CI 0.75–0.91, respectively). When we restricted our analyses to studies with long-time regular aspirin use (equal or more than 4 years), reverse association became stronger (pooled RR 0.82, 95 % CI 0.72–0.93; pooled RR 0.70, 95 % CI 0.55–0.90, respectively).
Conclusions
Our findings suggest that regular, especially long-time regular aspirin use may reduce the risk of overall and advanced prostate cancer. Considering the limitation of included studies, further well-designed large-scaled cohort studies and RCTs are required to draw more definitive conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most commonly diagnosed non-skin cancer and the second leading cause of cancer death in man [1]. Primary prevention of prostate cancer is, therefore, a significant public health issue. The mechanism of prostate carcinogenesis is still not fully understood. Inflammation was proved to have large beneficial effects in colorectal, esophageal and gastric cancer. Recent laboratory and animal studies indicated that inflammation may also influence prostate carcinogenesis through inhibiting the cyclooxygenase (COX) pathway, which is an inducible enzyme that facilitates inflammation by promoting production of prostaglandin [2].
Aspirin is one of the most common used nonsteroid anti-inflammatory drugs (NSAIDs), which was proved having protective effects in colorectal adenoma through inhibiting of COX-2 enzymes, restoring of normal apoptosis and reducing of angiogenesis [3]. Several epidemiologic studies were performed to illuminate the association with prostate cancer. However, the results remain controversial. No significant difference was reported in a meta-analysis [4] based on 15 relevant studies [pooled relative ratio (RR) 0.98, 95 % CI 0.95–1.01]. In 2010, Mahmud et al. [5] reported an updated result, a significant inverse association was found in patients who took aspirin regularly [pooled odds ratio (OR) 0.83, 95 % CI 0.77–0.89]. In the more recent meta-analysis [6], 10 % reduction in prostate cancer was reported among regular aspirin users. The protective effect of aspirin use against prostate cancer was suggestive, but not conclusive due to the large heterogeneity between included studies.
Recently, several large-scaled studies [7–9] were published and showed controversial associations between regular aspirin use and risk of prostate cancer. Besides, so far there were no meta-analyses evaluating the association of long-time regular aspirin use on the risk of overall and advanced prostate cancer. We, therefore, conducted this meta-analysis to update these associations.
Methods
Data source and search strategy
Electronic databases including PubMed, EMBASE and Cochrane library were searched between January 1966 and 21 April 2013 to identify eligible studies, using following key words: “aspirin or acetylsalicylic acid or nonsteroidal anti-inflammatory agent or NSAID or analgesics,” “prostate or prostatic” and “cancer or carcinoma or neoplasm or neoplasms or tumor”. Furthermore, the reference lists of every article retrieved and reviews were manually searched to identify additional eligible studies.
Criteria for inclusion and exclusion
Studies are eligible for inclusion if they meet the following criteria: (1) had to be case–control or cohort studies; (2) evaluated the association between aspirin use and the risk of prostate cancer separated from other NSAIDs; (3) had explicit description of aspirin exposure and (4) provided RRs or ORs and their 95 % CIs or sufficient information to calculate them. Review articles, case reports, letters to the editor and editor comments were excluded.
Date extraction
Eligibility evaluation and data abstraction were carried out independently by 2 investigators (Tian-bao Huang, Yang Yan) according to the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [10], and discrepancies were adjudicated by consensus. For each study, the following data were extracted: first author; year of publication; country; study design; type of controls; sample size; definition of aspirin exposure; RRs or ORs; and their 95 % CIs. Estimates of the association between aspirin use and the risk of advanced prostate cancer were also extracted. When more than one estimate was available, we chose the “most adjusted or multi-adjusted” estimate.
Statistical analysis
Due to the low incidence of prostate cancer, the RR mathematically approximates the OR in case–control studies. To simplify, only pooled RR and its 95 % CI were used as effect of interest to assess the association between aspirin use and the risk of prostate cancer. When data of different duration of use or different intake levels were available, we chose the one with longest duration or highest intake. Regular aspirin use refers to “more than one table per day for at least 4 days per week in a certain period”. Long-time regular aspirin use refers to “period of regular aspirin use is more than 4 years”. Besides, advanced prostate cancer is defined as “tumor stage ≥2c or Gleason score ≥ 7”. The statistical heterogeneity among studies was evaluated using the Cochrane’s Q and I 2 statistics. As for Q statistic, heterogeneity was considered exist for P < 0.1. When P > 0.1 and I 2 < 50 %, the included studies were identified as with acceptable heterogeneity, and fixed-effects model was used. Otherwise, the random-effects model was used.
To detect the source of heterogeneity, subgroup analyses based on study design (case–control vs. cohort study, population-based vs. hospital-based case–control study), geographic location (America vs. Europe vs. others) and number of adjusted confounders (equal or more than 5 vs. less than 5) were carried out. It is known that age, race and family history are proved as three major risk factors for prostate cancer [1]. Therefore, we limited the analysis to studies which had adjusted for at least two major factors to eliminate their impact.
Finally, the potential publication bias was evaluated graphically with funnel plots of log risk ratio against the standard error of the included studies. If the funnel plot is asymmetrical, rank correlation method proposed by Begg et al. and linear regression approach suggested by Egger et al. will be used to evaluate the potential publication bias. If the P value is less than 0.05, sensitive analyses will be conducted to explore whether the final effect was strongly influenced by individual studies. All statistical analyses were performed using STATA Statistical Software version 11.0 (Stata Corp., College Station, Texas, USA). All P values are two-tailed.
Results
Study characteristics
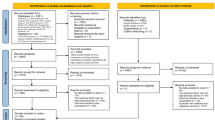
The detailed steps of our literature search are displayed in Fig. 1. Briefly, one study, which assessed the association between aspirin use and cancer mortality, was excluded [11]. Besides, two another studies [12, 13] were also excluded because two updated reports from the same study populations were published. Finally, a total of 24 observational studies were eligible and included in this meta-analysis.
For simplicity, four nested case–control studies including 14,231 cases and 40,698 controls [14–17] were classified as case–control studies. As a result, 13 case–control studies and nine cohort studies, which assessed the association between aspirin use and overall prostate cancer risk, were included. Among these studies, more than half of the case–control studies were population-based [9, 16–22], whereas the remaining five were hospital-based [14, 15, 23–25]. As to geographic location, 12 studies were carried out in the USA [7, 8, 21–23, 25–31], 3 studies were in Canada [15, 17, 20], 2 studies were in the UK [14, 16] and one each were in New Zealand [18], France [19], Italy [24], Finland [9] and the Netherlands [32]. When it comes to confounding factors, most studies adjusted for age [8, 9, 14, 16, 18, 20–32], race [7, 8, 21–23, 28, 30, 31] and family history of prostate cancer [8, 16, 24, 25, 31] (Table 1).
For advanced prostate cancer, 12 studies including 9,783 cases were used for analysis, which included seven case–control studies including 5,846 cases and 29,053 controls [9, 18, 21, 22, 24, 25, 33] and five cohort studies including 3,937 cases among 272,736 subjects [7, 8, 28, 31, 34]. The detailed characteristics of the studies included are summarized in Table 2.
Overall prostate cancer
For the 22 studies included, nine of them showed protective effects of aspirin use, while the remaining 13 studies did not detect any association of aspirin use on the risk of overall prostate cancer. The pooled estimates data revealed a significant association between regular/any aspirin use and the risk of prostate cancer (pooled RR 0.90, 95 % CI 0.86–0.95) (Fig. 2). Reverse associations were stronger, when we limited our analyses to studies that assessed regular aspirin use versus non-use (pooled RR 0.87, 95 % CI 0.81–0.92). And there were little evidence of heterogeneity (I 2 = 30.6 %, P value for heterogeneity = 0.174). The heterogeneity could be subside after stratification by design (case–control study: I 2 = 0.0 %, P value for heterogeneity = 0.843; cohort study: I 2 = 0.0 %, P value for heterogeneity = 0.427, respectively). Besides, estimated pooled data, which assessed daily aspirin use, showed a deeper reverse association with remarkable heterogeneity (pooled RR 0.82, 95 % CI 0.72–0.93; I 2 = 53.6 %, P value for heterogeneity = 0.091) (Supplementary Fig. 1). In addition, a significant reverse association was detected in the association between long-time aspirin use and overall prostate cancer (pooled RR 0.82, 95 % CI 0.72–0.93), which, however, with some evidence of heterogeneity (I 2 = 53.6 %, P value for heterogeneity = 0.091).
Forest plot and meta-analysis of the association between any aspirin use and prostate cancer risk. Any aspirin use was associated with a reduction in prostate cancer risk (pooled RR 0.90, 95 % CI 0.85–0.95). Subgroup analysis based on study design obtained a consistent result in cohorts with few heterogeneity. However, some evidence of heterogeneity were detected in case–control studies (I 2 = 68.2 %, P for heterogeneity = 0.000)
A series of subgroup analyses were carried out to detect the source of heterogeneity (Table 3). The heterogeneity for 13 case–control studies was large (I 2 = 68.2 %, P value for heterogeneity = 0.000) and did not subside after stratification by type of control subjects, while no evidence of heterogeneity among nine cohort studies existed (I 2 = 0.0 %, P value for heterogeneity = 0.703), indicating that the results of the cohort studies (pooled RR 0.90, 95 % CI 0.86–0.94) were homogeneous. The pooled estimated data in subgroup analyses revealed that geographic location, race and family history of prostate cancer may confuse the assessed association and influence the effect of aspirin use on risk of prostate cancer. It was obvious that the heterogeneity would become weaker when the studies were adjusted for more confounding factors (for equal or more than five:I 2 = 4.2 %, P value for heterogeneity = 0.398; for less than five:I 2 = 61.7 %, P value for heterogeneity = 0.001). No publication bias was found through Egger’s test (P = 0.169) or Begg’s test (P = 0.955) (Fig. 3).
Advanced prostate cancer
A stronger reverse association was detected in the association between aspirin use and the risk of advanced prostate cancer (pooled RR 0.86, 95 % CI 0.78–0.95) (Fig. 4). However, there were some evidence of heterogeneity (I 2 = 41 %, P value for heterogeneity = 0.068). When we restricted our analyses to long-time regular usage of aspirin, a 30 % reduction in advanced prostate cancer risk was found with little evidence of heterogeneity (I 2 = 0.0 %, P value for heterogeneity = 0.969). In subgroup analysis, a moderate protective rate was obtained in cohort studies with little heterogeneity among studies (I 2 = 0 %, P value for heterogeneity = 0.743), which confirmed the protective effect of aspirin use on advanced prostate cancer (Table 4).
Forest plot and meta-analysis of the association between any aspirin use and advanced prostate cancer risk. Any aspirin use was associated with a reduction in advanced prostate cancer (pooled RR 0.86, 95 % CI 0.77–0.95). Subgroup analysis based on study design obtained a consistent result in cohorts with few heterogeneity. However, some evidence of heterogeneity were detected in case–control studies (I2 = 61.0 %,P for heterogeneity = 0.018)
Discussion
In this meta-analysis of 24 observational studies, we found that regular aspirin use was associated with reduction in overall and advanced prostate cancer risk (pooled RR 0.86, 95 % CI 0.81–0.92; pooled RR 0.83, 95 % CI 0.75–0.91, respectively). When it comes to long-time regular aspirin use, reverse association became stronger (pooled RR 0.82, 95 % CI 0.72–0.93; pooled RR 0.70, 95 % CI 0.55–0.90, respectively). Previously, there were three systematic reviews [4–6] summarizing the evidence about the association of aspirin use and prostate cancer risk. Of these, a significant inverse association was computed in the meta-analysis conducted by Mahmud et al. [5]. In addition, an updated systematic review, which performed by Bosetti et al., suggested that prostate cancer risk is reduced by 10 % in regular aspirin users, with similar risk reductions reported in both case–control and cohort studies. Recently, several well-designed studies which adjusted more confounding factors were published and reported controversial results. A cohort of 51,529 health professionals aged 40–75 years old was conducted by Dhillon et al. [8] to evaluate the association between long-term aspirin use and the incidence of total, high-grade, regionally advanced and lethal prostate cancer. Any use more than 10 years had no influence with overall prostate cancer risk (pooled RR 0.99; 95 % CI 0.87–1.12). But significantly reverse association was observed in high-grade and lethal prostate cancer which associated with higher doses of aspirin (≥6 adult-strength tablets per week). Another large-scaled study [9] carried out in Finland at population level, which suggested a decreased overall prostate cancer risk (OR 0.90, 95 % CI 0.84–0.96) in a dose-dependent fashion.
Several mechanisms were proposed to interpret the protective of aspirin and other NSAIDs on cancers, which included induction of apoptosis via COX-independent pathways, inhibition of cellular proliferation and angiogenesis by up-regulating of tumor suppressor genes [35]. In relation to prostate cancer, inhibition of the COX enzymes involved in prostaglandin synthesis also played a role in the prevention of prostate cancer. Gupta et al. [36] compared levels of COX-2 mRNA in pair-matched benign and cancer tissue obtained from the same prostate cancer patients and found that COX-2 is over expressed in prostate cancers. Consistent results were obtained from some others studies [37, 38]. However, the influence of prostatitis on prostate cancer risk remains unfathomed.
Several limitations should be taken into account in the present meta-analysis. Firstly, half of included studies were case–control studies, which were susceptible to recall bias and select bias. These kinds of bias might be reduced to a large extent in cohort studies. However, there were still several potential known or unknown confounders, which may influence conclusion drawn from the meta-analysis compiled from these studies. Secondly, our literature search was restricted to the studies published in PubMed, EMBASE and Cochrane Library. It is well known that negative studies were less likely to be published in indexed journals, which may bias our results, though there was no evidence of publication bias basing on either Egger’s test or Begg’s test. Thirdly, studies included were different in terms of populations, dose and duration of aspirin use, selection of control group and confounders adjusted. Subsequently, subgroup analyses were performed to reduce the considerable heterogeneity. Moderate results were demonstrated when our analyses got rid of the influence of the factors mentioned before. The results also became acceptable with little heterogeneity when we restricted our analyses to regular aspirin use and long-time regular aspirin use. But subgroup analyses and sensitivity analyses were far from removing all the heterogeneity. Finally, it was impossible to clarify the dose–response association because of lack of data. So it is hard to quantitatively assess the aspirin use on prostate cancer risk.
However, subgroup analyses based on several known confounding factors such as age, race and family history of prostate cancer were performed. And moderate results with little heterogeneity were obtained. From this meta-analysis, 10 % reduction in prostate cancer risk and 14–15 % in advanced prostate cancer risk were observed associated with any use of aspirin in overall and cohort studies.
Smoking has not been established as risk factors for prostate cancer, but they are important risk factors for other human cancers and potentially major avoidable factors. Recently, a published large prospective study among Japanese found that smoking was inversely associated with prostate cancer risk among total subjects, but tended to increase the risk of advanced prostate cancer [39]. To evaluate the effect of smoking on prostate cancer risk, subgroup analysis based on smoking was performed. However, no significant heterogeneity between subgroups (P = 0.244) was obtained.
In conclusion, the results of this meta-analysis of 24 observational studies provide quantitative evidence that aspirin may reduce the risk of overall and advanced prostate cancer, especially long-time regular aspirin use. Further well-designed large-scaled cohort studies are needed to provide more definitive conclusions.
References
Brawley OW (2012) Prostate cancer epidemiology in the United States. World J Urol 30:195–200
Sabichi AL, Lippman SM (2004) COX-2 inhibitors and other nonsteroidal anti-inflammatory drugs in genitourinary cancer. Semin Oncol 31:36–44
Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo AM (2004) Cyclooxygenases in cancer: progress and perspective. Cancer Lett 215:1–20
Bosetti C, Gallus S, La Vecchia C (2006) Aspirin and cancer risk: an updated quantitative review to 2005. Cancer Causes Control 17:871–888
Mahmud SM, Franco EL, Aprikian AG (2010) Use of nonsteroidal anti-inflammatory drugs and prostate cancer risk: a meta-analysis. Int J Cancer 127:1680–1691
Bosetti C, Rosato V, Gallus S, La Vecchia C (2012) Aspirin and urologic cancer risk: an update. Nat Rev Urol. 9:102–110
Shebl FM, Sakoda LC, Black A et al (2012) Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. Br J Cancer 107:207–214
Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL (2011) Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988–2006. Int J Cancer 128:2444–2452
Veitonmaki T, Tammela TL, Auvinen A, Murtola TJ (2013) Use of aspirin, but not other non-steroidal anti-inflammatory drugs is associated with decreased prostate cancer risk at the population level. Eur J Cancer 49:938–945
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283:2008–2012
Ratnasinghe LD, Graubard BI, Kahle L, Tangrea JA, Taylor PR, Hawk E (2004) Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res 24:3177–3184
Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW Jr (1993) Aspirin use and risk of fatal cancer. Cancer Res 53:1322–1327
Leitzmann MF, Stampfer MJ, Ma J et al (2002) Aspirin use in relation to risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 11:1108–1111
Garcia Rodriguez LA, Gonzalez-Perez A (2004) Inverse association between nonsteroidal anti-inflammatory drugs and prostate cancer. Cancer Epidemiol Biomarkers Prev 13:649–653
Dasgupta K, Di Cesar D, Ghosn J, Rajan R, Mahmud S, Rahme E (2006) Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J 12:130–135
Murad AS, Down L, Davey Smith G et al (2011) Associations of aspirin, nonsteroidal anti-inflammatory drug and paracetamol use with PSA-detected prostate cancer: findings from a large, population-based, case–control study (the ProtecT study). Int J Cancer 128:1442–1448
Mahmud SM, Franco EL, Turner D et al (2011) Use of non-steroidal anti-inflammatory drugs and prostate cancer risk: a population-based nested case–control study. PLoS One 6:e16412
Norrish AE, Jackson RT, McRae CU (1998) Non-steroidal anti-inflammatory drugs and prostate cancer progression. Int J Cancer 77:511–515
Irani J, Ravery V, Pariente JL et al (2002) Effect of nonsteroidal anti-inflammatory agents and finasteride on prostate cancer risk. J Urol 168:1985–1988
Perron L, Bairati I, Moore L, Meyer F (2003) Dosage, duration and timing of nonsteroidal antiinflammatory drug use and risk of prostate cancer. Int J Cancer 106:409–415
Liu X, Plummer SJ, Nock NL, Casey G, Witte JS (2006) Nonsteroidal antiinflammatory drugs and decreased risk of advanced prostate cancer: modification by lymphotoxin alpha. Am J Epidemiol 164:984–989
Salinas CA, Kwon EM, FitzGerald LM et al (2010) Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol 172:578–590
Neugut AI, Rosenberg DJ, Ahsan H et al (1998) Association between coronary heart disease and cancers of the breast, prostate, and colon. Cancer Epidemiol Biomarkers Prev 7:869–873
Bosetti C, Talamini R, Negri E, Franceschi S, Montella M, La Vecchia C (2006) Aspirin and the risk of prostate cancer. Eur J Cancer Prev 15:43–45
Menezes RJ, Swede H, Niles R, Moysich KB (2006) Regular use of aspirin and prostate cancer risk (United States). Cancer Causes Control 17:251–256
Paganini-Hill A, Chao A, Ross RK, Henderson BE (1989) Aspirin use and chronic diseases: a cohort study of the elderly. BMJ 299:1247–1250
Schreinemachers DM, Everson RB (1994) Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 5:138–146
Habel LA, Zhao W, Stanford JL (2002) Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Causes Control 13:427–434
Platz EA, Rohrmann S, Pearson JD et al (2005) Nonsteroidal anti-inflammatory drugs and risk of prostate cancer in the Baltimore Longitudinal Study of Aging. Cancer Epidemiol Biomark Prev 14:390–396
Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE (2007) A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst 99:608–615
Brasky TM, Velicer CM, Kristal AR, Peters U, Potter JD, White E (2010) Nonsteroidal anti-inflammatory drugs and prostate cancer risk in the vitamins and lifestyle (VITAL) cohort. Cancer Epidemiol Biomark Prev 19:3185–3188
Siemes C, Visser LE, Coebergh JW, Hofman A, Uitterlinden AG, Stricker BH (2008) Protective effect of NSAIDs on cancer and influence of COX-2 C(-765G) genotype. Curr Cancer Drug Targets 8:753–764
Mahmud SM, Tanguay S, Begin LR, Franco EL, Aprikian AG (2006) Non-steroidal anti-inflammatory drug use and prostate cancer in a high-risk population. Eur J Cancer Prev 15:158–164
Jacobs EJ, Rodriguez C, Mondul AM et al (2005) A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst 97:975–980
Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G (2009) Aspirin, salicylates, and cancer. Lancet 373:1301–1309
Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H (2000) Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate 42:73–78
Kirschenbaum A, Klausner AP, Lee R et al (2000) Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology 56:671–676
Nithipatikom K, Isbell MA, Lindholm PF, Kajdacsy-Balla A, Kaul S, Campell WB (2002) Requirement of cyclooxygenase-2 expression and prostaglandins for human prostate cancer cell invasion. Clin Exp Metastasis 19:593–601
Sawada N, Inoue M, Iwasaki M et al (2014) Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: The Japan Public Health Center-based prospective study. Int J Cancer 134:971–978
Acknowledgments
Tian-bao Huang was responsible for the initial plan, data collection and statistical analysis and for conducting the study. Tian-bao Huang, Yang Yan, Zhui-feng Guo contributed to data collection, data extraction, data interpretation and manuscript drafting. Huan Liu, Xiao-long Zhang, Jiang Geng and Xu-dong Yao contributed to data interpretation and study design. Jun-hua Zheng was the guarantor for this paper and has full responsibility for this study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tian-bao Huang and Yang Yan have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11255_2014_703_MOESM1_ESM.tif
Forest plot and meta-analysis of the association between daily aspirin use and overall/advanced prostate cancer risk (TIFF 1590 kb).
Rights and permissions
About this article
Cite this article
Huang, Tb., Yan, Y., Guo, Zf. et al. Aspirin use and the risk of prostate cancer: a meta-analysis of 24 epidemiologic studies. Int Urol Nephrol 46, 1715–1728 (2014). https://doi.org/10.1007/s11255-014-0703-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0703-4