Abstract
Objectives
Controversial results were reported among several epidemiologic studies on the relationship between coffee consumption and urologic cancer risk. We, therefore, conducted this meta-analysis to clarify these associations.
Methods
Electronic databases including Pubmed, Embase and Cochrane library were searched between January 1966 and August 2013 for eligible studies. Pooled relative risk (RR) and its 95 % confidence interval (CI) were calculated. All P values are two tailed.
Results
Thirteen cohorts were eligible for inclusion. As to prostate cancer (PCa), significant reverse association was found among highest versus none/lowest analysis with acceptable heterogeneity (RR 0.86, 95 % CI 0.79–0.95; I 2 25 %, P value for heterogeneity: 0.221). A pooled RR which assessed advanced PCa was 0.73 (with 95 % CI 0.50–1.07), and a slight stronger reverse association was found in fatal PCa. However, a slight insignificant reverse association, basing on 8 studies with 9 outcomes, was found in dose–response analysis (RR 0.98, 95 % CI 0.93–1.03). For kidney and bladder cancer, insignificant associations were found in both highest versus none/lowest analyses and dose–response analyses.
Conclusions
Our findings suggest that coffee consumption may reduce the risk of PCa. No associations were found with both bladder and kidney cancer. Further well-designed large-scaled cohort studies are warranted to provide more definitive conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coffee, one of the most frequently consumed beverages worldwide, has demonstrated its protective effect against several chronic diseases, including type two diabetes mellitus, cardiovascular diseases, stroke and Parkinson’s disease. Considering antioxidant and anti-inflammatory nature of its beneficial components, many scholars had great interest in the relationship between coffee consumption and risk of cancer. As for urologic cancer, the associations of coffee consumption with kidney cancer [1–3], prostate cancer (PCa) [1, 4–6] and bladder cancer [7–9] risk were reported in several cohort studies, all of which had not detect any statistically significant associations. In 2010 [10], a meta-analysis, which assessed the association between coffee consumption and risk of total PCa, reported a contradictory result based on eight case–control studies and four cohort studies. Statistically significant association was found in case–control studies (relative risk (RR) 1.21, 95 % confidence interval (CI) 1.03–1.43), but not in cohorts (RR 1.06, 95 % CI 0.83–1.35). In relation to bladder cancer, a dose–response meta-analysis published in 2012 [11], which was based on 23 case–control studies and five cohort studies, did not report a clear conclusive evidence due to inconsistencies between case–control and cohort studies. Half of the included studies in both meta-analyses are case–control studies, which are with nature of recall bias and selection bias, so it is hard to obtain persuasive results.
Recently, several large-scale prospective cohort studies [12–14] were published and showed an inverse association between coffee consumption and risk of PCa. Therefore, updated dose–response meta-analysis was needed to quantitatively assess this association. Besides, the relations between coffee consumption and bladder (or kidney) cancer were also clarified in present meta-analysis.
Methods
Data source and search strategy
The present meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [15]. Electronic databases including Pubmed, Embase and Cochrane library were searched between January 1966 and August 2013 to identify eligible studies, using following key words: “coffee or caffeine or beverages or diet or dietary or drink” and “bladder or prostate or kidney or testicular or urinary tract or urologic” and “cancer or carcinoma or neoplasm or tumor or mass”. Furthermore, the reference lists of every article retrieved and reviews were manually searched to identify additional eligible studies.
Criteria for inclusion and exclusion
Studies are eligible for inclusion if they meet the following criteria: (1) had to be cohort study; (2) the exposure of interest was coffee consumption; (3) reported RRs or hazard ratios (HRs) and their corresponding 95 % CIs, or sufficient data to calculate them; (4) reported three or more quantitative categories of coffee consumption; (5) provided person-year of every categories or data to calculate them. Studies will be excluded if they achieve the following requirements: (1) the study was case–control study, review article, case report, letter to the editor or editor comment; (2) no categories of coffee consumption were reported; (3) no available data to calculate person-year. When multiple reports based on the same study are published, only the most recent or complete report can be used. When more than one estimate is available, we will choose the one adjusted for most variables.
Data extraction and outcomes of interest
Eligibility evaluation and data abstraction were carried out independently by two reviewers (Tian-bao Huang and Zhui-feng Guo), which according to the meta-analysis of observational studies in epidemiology, guidelines and discrepancies were adjudicated by discussion with a third reviewer (Jun-hua Zheng) [16]. The following data which were extracted from each study included first author; year of publication; country; follow-up period; number of subjects and cases; age; cancer sites; category amounts of coffee consumption; person-years of every category; RR or HR; and its 95 % CI for every category of coffee intake and adjusted factors.
Statistical analysis
Pooled RRs and their 95 % CIs were computed to assess the associations between coffee consumption and risk of urologic cancer. If RRs are not available, crude RRs and their 95 % CIs will be calculated from exposure distributions. In study reported by Ellison et al. [6], coffee consumption was indicated in milliliters, and we assumed 250 ml as approximately equivalent to 1 cup. Reference refer to never drink [6, 7, 13, 14, 17–20], <1cup/day [21], <2 cups/day [1, 2] or 1 to 3 cups/day [12]. One study which was published in 2000 [22] used 4–5 cups/day in man group and 3–4 cups/day in women group as reference. The advanced PCa refers to Gleason Score (GS) equal or more than seven. The statistical heterogeneity among studies was evaluated by using the Cochrane’s Q and I 2 statistics. As for Q statistic, heterogeneity was considered exist for P < 0.05. When P > 0.05 and I 2 < 50 %, the including studies were considered with acceptable heterogeneity, and fixed-effects model was used. Otherwise, the random-effects model was used.
For PCa, subgroup analyses were performed based on geographic location (Europe vs America vs Asia), published data (before 2010 vs after 2010), and the following factors were adjusted or not: smoking, body mass index (BMI), family history of PCa, diabetes, physical activity and the number of adjusted factors (equal or more than 10 vs <10). However, as to bladder cancer, we only carried out two subgroup analyses which were based on geographic location and sex. Moreover, sensitivity analysis was also conducted to explore whether the final effects were strongly influenced by individual studies.
For the dose–response meta-analyses, methods which were proposed by Greenlan and Orsini were used to estimate study-specific slopes. If data are available, the median or mean coffee consumption for each category will be assigned to each corresponding RR. If it is unavailable, we will assign the midpoint of the upper and lower boundaries in each category as the average consumption. When the lowest category was unrestricted, we set the lower boundary to zero. If the upper boundary for the highest category was open-ended, we assessed that the boundary will have the same amplitude as the preceding category. Moreover, if total person-years are not available, it will be approximated from follow-up duration and number of subjects [23], and if total number of cases and person-years are available, we will estimate the distribution of person-years using method reported by Aune et al. [24]. All the meta-analyses were done by using Stata 11 software (Stata Corp LP, College Station, TX, USA).
Results
Study characteristics
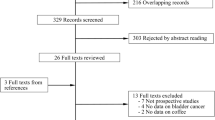
The detailed steps of our literature search were displayed in Fig. 1. Briefly, two papers, which evaluated the association between coffee consumption and aggressiveness or prognosis of PCa, were excluded. Nine papers, seven of them did not have quantitative coffee intake data and the remaining two did not have enough information to calculate person-years, were also excluded. After that, 13 cohort studies were eligible for inclusion in this meta-analysis. The characteristics of the included studies were showed in Table 1. The 13 cohort studies included five studies including 969 bladder cancer cases which occurred in 201,272 participants [1, 2, 7, 17, 22], eight studies including 32,735 PCa cases which involved in 447,458 participants [1, 2, 6, 12–14, 18, 20] and four studies including 366 kidney cancer among 310,625 participants [1, 2, 19, 21]. When it came to geographic location, five studies were conducted in Europe [1, 2, 12, 18, 22], five studies were in America [6, 7, 13, 20, 21] and the other three studies were conducted in Asia [14, 17, 19].
Highest versus none/lowest analysis
In overall analysis, highest coffee consumption had an inverse association with risk of PCa (RR 0.86, 95 % CI 0.79–0.95; Fig. 2c), but not bladder cancer and kidney cancer (RR 1.08, 95 % CI 0.71–1.66; RR 0.99, 95 % CI 0.52–1.89, respectively, Fig. 2a). However, there was some evidence of heterogeneity. We, therefore, conducted subgroup analyses among single cancer, respectively. For bladder cancer, coffee consumption might be a risk factor of bladder cancer in men population without reaching statistically significant difference (RR 1.34, 95 % CI 0.99–1.8, P value = 0.056; Fig. 2a), while in female population, insignificant reverse association was detected with bladder cancer risk (RR 0.55, 95 % CI 0.25–1.18, P = 0.123).
Meta-analysis of the association between coffee consumption and risk of bladder cancer and PCa, respectively. a Forest plot of coffee consumption and risk of bladder cancer stratified by sex. b Coffee consumption and risk of bladder cancer, dose–response analysis, per 2 cups/day. c Forest plot of coffee consumption and risk of PCa in highest Vs none/lowest analysis. d Coffee consumption and risk of PCa, dose–response analysis, per 2 cups/day. No statistically significant difference was showed in the association between highest coffee consumption and risk of bladder cancer, while a significant reverse association was detected with PCa. The summary RR of each increment of 2 cups of coffee consumption dairy by using random-effects models was 1.03 (95 % CI 0.80–1.32) for bladder cancer, 0.98 (0.93–1.03) for PCa. There was no evidence of a nonlinear association between coffee consumption and both cancer, P-nonlinearity = 0.827 for bladder cancer, P-nonlinearity = 0.361 for PCa
As for PCa, series of subgroup analyses were carried out to detect the source of heterogeneity and relevant confounding factors, which were presented in Table 2. In the subgroup analysis by geographic location, a statistically significant inverse association was observed in studies carried out in Europe (RR 0.82; 95 % CI 0.72–0.93; I 2 0 %, P value for heterogeneity: 0.702), but not in America and Asia (RR 0.92; 95 % CI 0.79–1.07; RR 0.63, 95 % CI 0.39–1.01, respectively). Pooled RR from five studies published after 2010 [12–14, 18, 20] showed a moderate result that compared with none/lowest coffee consumption, and highest coffee intake may reduce the PCa risk (RR 0.87, 95 % CI 0.81–0.95; I 2 16.2 %, P value for heterogeneity: 0.309); however, no association was detected among studies published before 2010 (RR 0.84, 95 % CI 0.49–1.44). Finally, a pooled RR which assessed highest coffee consumption and risk of advanced PCa was 0.73 (with 95 % CI 0.50–1.07) and a slightly stronger reverse association was found in fatal PCa (RR 0.70,95 % CI 0.46–1.05), but both of which turned out to be insignificant.
Dose–response meta-analysis
In dose–response meta-analysis, we found that no associations were detected between coffee consumption (2 cups/day increased) and urologic cancer risk (bladder cancer: RR 1.03, 95 % CI 0.8–1.3; kidney cancer: RR 0.95, 95 % CI 0.56–1.59; PCa: RR 0.98, 95 % CI 0.93–1.03, respectively, Fig. 2). As to fatal PCa and advanced PCa, still no associations were found (RR 0.95, 95 % CI 0.77–1.16; RR 0.98, 95 % CI 0.86–1.12; Fig. 3).
Meta-analysis of the association between coffee consumption and risk of fatal, advanced and localized PCa. a Forest plot of coffee consumption and risk of fatal, advanced and localized cancer in highest versus none/lowest analysis. b Coffee consumption and fatal PCa, dose–response analysis, per 2 cups/day. c Coffee consumption and advanced PCa, dose–response analysis, per 2 cups/day. d Coffee consumption and localized PCa, dose–response analysis, per 2 cups/day. In highest versus none/lowest analysis, the estimated RR were 0.70 (95 % CI 0.46–1.05) for fatal prostate/kidney cancer, 0.73 (95 % CI 0.50–1.07) for advanced PCa, 0.89 (95 % CI 0.83–0.96) for localized PCa. There were no evidence of a nonlinear association between coffee consumption and fatal prostate/kidney cancer (RR for two cups/day increased = 0.95, 95 % CI 0.77–1.17, P-nonlinearity = 0.621), advanced PCa (RR for two cups/day increased = 0.98, 95 % CI 0.86–1.12, P-nonlinearity = 0.737) and localized PCa (RR for 2 cups/day increased = 0.98, 95 % CI 0.92–1.04, P-nonlinearity = 0.465)
Discussion
There were two systematic reviews summarizing the evidence about coffee consumption and risk of urologic cancer. Zhou et al. [11] reported an inconsistent outcome between case–control and cohort studies. Data from case–control studies suggested that coffee was a risk factor for bladder cancer (RR for 4 cups/day vs. non-drinkers: 1.29, 95 % CI 1.12–1.48), but no significant association was obtained from cohort studies (RR 0.77, 95 % CI 0.77–1.56). Park et al. [10] performed a meta-analysis in 2010 and showed that coffee consumption might have harmful effect on PCa. Data from cohort studies did not reach statistically significant differences, but the RR was >1, with a wide CI. No meta-analysis was conducted to evaluate the dose–response relationship between coffee consumption and PCa risk, especially fatal and advanced PCa. So we performed this meta-analysis to renew these data. Considering recall bias and select bias of case–control studies, we only took cohort studies into consideration.
For PCa, several biological mechanisms have been proposed which may explain the inverse association between coffee consumption and risk of PCa, especially the association with fatal or advanced PCa. One possible mechanism was that coffee inhibited intestinal glucose absorption and improved glucose handling [25]. Several cross-sectional studies found that coffee intake has been associated with lower glucose levels and circulating C-peptide [26], which is a marker of insulin secretion. Growing evidence showed that higher insulin levels might have harmful effect on PCa progression through the insulin and insulin-like growth factor 1 receptors. Inflammation was hypothesized to play a role in the development of PCa, and several recent studies showed that there is a positive association between coffee drinking and lower inflammation markers. Recently published data [27] indicated that dietary total antioxidant content had a weak inverse association with PCa (RR = 0.91 for total PCa, RR = 0.82 for advanced PCa) and this association mainly due to coffee. Besides, coffee had influence on the levels of circulating sex hormones by affecting the levels of sex-hormone-binding globulin [28, 29].
In the present meta-analysis, a stronger association was detected in fatal or advanced cancer without reaching statistically significant difference, which might result from small number of included studies with few cases. Further well-designed large-scaled studies are needed to strengthen those findings. In subgroup analyses, we found that there was a distinct difference in finding between studies published before and after 2010. Studies published after 2010 may have better design with adjusting for more confounding factors, including BMI, tea consumption and physical activity and so on. After ruling out the influence of these factors, a moderate reverse association still existed, which confirmed our findings.
For bladder cancer, a slightly increased risk was found among heavy coffee drinkers. Beside, opposite associations were found between male and female. However, the reason is still unclear. Regarding the differences in findings by the geographic location where the studies carried out, several reasons could be considered, such as potential risks of bladder cancer in each population, national differences of coffee intake, type of coffee beans and the brewing method and so on [10].
No association was found between coffee drinking and kidney cancer risk based on four relevant studies. However, recently published animal experiments [30] showed that insulin-mediated oxidative stress might result in genomic damage and antioxidants might exert protective effects in clear cell renal cell carcinoma. Active substances contained in coffee may result in lower production of insulin mentioned before. Maybe there existed several other mechanisms, which neutralized the protection effect.
Several limitations should be taken into account in the present meta-analysis. Firstly, cohort studies were less susceptible to bias due to their prospective design, and risk estimates which adjusted for the most factors were extracted. These data might reduce the influence of potential confounders to a large extent. However, there were still several potential known or unknown confounders failed to be adjusted. If data were not available, crude RRs were calculated [1, 2]. These factors might influence conclusion drawn from the meta-analysis compiled from these studies. Secondly, seven studies only reported frequencies of coffee consumption which were hard to convert to cups per day. Two studies did not provide person-years or enough data to calculate them. And several studies only reported <3 categories. All these studies were excluded in this meta-analysis, which may result in a certain degree of bias. Thirdly, misclassification of coffee consumption also should be taken into account. Most of included studies used the number of cups to assess coffee consumption. However, the size of cups may be different. Bracken et al. [31] pointed out that serving sizes and brewing methods for coffee can vary substantially within and between countries. Finally, only published studies were included in present meta-analysis, which might result in publication bias.
In conclusion, our findings suggest that coffee consumption may reduce the risk of PCa. No associations were found with both bladder and kidney cancer. Considering the limitation of included studies, further well-designed large-scaled cohort studies are warranted to provide more definitive conclusions.
References
Jacobsen BK, Bjelke E, Kvale G, Heuch I (1986) Coffee drinking, mortality, and cancer incidence: results from a Norwegian prospective study. J Natl Cancer Inst 76:823–831
Stensvold I, Jacobsen BK (1994) Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control 5:401–408
Ozasa K, Nakao M, Watanabe Y et al (2005) Association of serum phytoestrogen concentration and dietary habits in a sample set of the JACC Study. J Epidemiol 15(Suppl 2):S196–S202
Nomura AM, Kolonel LN, Hankin JH, Yoshizawa CN (1991) Dietary factors in cancer of the lower urinary tract. Int J Cancer 10(48):199–205
Severson RK, Nomura AM, Grove JS, Stemmermann GN (1989) A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res 1(49):1857–1860
Ellison LF (2000) Tea and other beverage consumption and prostate cancer risk: a Canadian retrospective cohort study. Eur J Cancer Prev 9:125–130
Mills PK, Beeson WL, Phillips RL, Fraser GE (1991) Bladder cancer in a low risk population: results from the Adventist Health Study. Am J Epidemiol 1(133):230–239
Michaud DS, Spiegelman D, Clinton SK et al (1999) Fluid intake and the risk of bladder cancer in men. N Engl J Med 6(340):1390–1397
Nagano J, Kono S, Preston DL et al (2000) Bladder-cancer incidence in relation to vegetable and fruit consumption: a prospective study of atomic-bomb survivors. Int J Cancer 1(86):132–138
Park CH, Myung SK, Kim TY, Seo HG, Jeon YJ, Kim Y (2010) Coffee consumption and risk of prostate cancer: a meta-analysis of epidemiological studies. BJU Int. 106:762–769
Zhou Y, Tian C, Jia C (2012) A dose-response meta-analysis of coffee consumption and bladder cancer. Prev Med 55:14–22
Discacciati A, Orsini N, Andersson SO et al (2013) Coffee consumption and risk of localized, advanced and fatal prostate cancer: a population-based prospective study. Ann Oncol 24:1912–1918
Bosire C, Stampfer MJ, Subar AF, Wilson KM, Park Y, Sinha R (2013) Coffee consumption and the risk of overall and fatal prostate cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control 24:1527–1534
Li Q, Kakizaki M, Sugawara Y et al (2013) Coffee consumption and the risk of prostate cancer: the Ohsaki Cohort Study. Br J Cancer 11(108):2381–2389
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 8:336–341
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 19(283):2008–2012
Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane S (2009) Coffee, green tea, and caffeine consumption and subsequent risk of bladder cancer in relation to smoking status: a prospective study in Japan. Cancer Sci 100:294–914
Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS (2012) Coffee consumption and prostate cancer risk: further evidence for inverse relationship. Nutr J 11:42
Washio M, Mori M, Sakauchi F et al (2005) Risk factors for kidney cancer in a Japanese population: findings from the JACC Study. J Epidemiol. 15(Suppl 2):S203–S211
Wilson KM, Kasperzyk JL, Rider JR et al (2011) Coffee consumption and prostate cancer risk and progression in the health professionals follow-up study. J Natl Cancer Inst 8(103):876–884
Lee JE, Giovannucci E, Smith-Warner SA, Spiegelman D, Willett WC, Curhan GC (2006) Total fluid intake and use of individual beverages and risk of renal cell cancer in two large cohorts. Cancer Epidemiol Biomarkers Prev. 15:1204–1211
Zeegers MP, Dorant E, Goldbohm RA, van den Brandt PA (2001) Are coffee, tea, and total fluid consumption associated with bladder cancer risk? Results from the Netherlands cohort study. Cancer Causes Control 12:231–238
Moskal A, Norat T, Ferrari P, Riboli E (2007) Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. Int J Cancer 1(120):664–671
Aune D, Greenwood DC, Chan DS et al (2012) Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol 23:843–852
Bhathena SJ, Velasquez MT (2002) Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr 76:1191–1201
Wu T, Willett WC, Hankinson SE, Giovannucci E (2005) Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in US women. Diabetes Care 28:1390–1396
Russnes KM, Wilson KM, Epstein MM, et al. Total antioxidant intake in relation to prostate cancer incidence in the health professionals follow up study. Int J Cancer. 2013
Platz EA, Giovannucci E (2004) The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J Steroid Biochem Mol Biol 92:237–253
Kotsopoulos J, Eliassen AH, Missmer SA, Hankinson SE, Tworoger SS (2009) Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer 15(115):2765–2774
Safarinejad MR (2011) Insulin-like growth factor binding protein-3 (IGFBP-3) gene variants are associated with renal cell carcinoma. BJU Int. 108:762–770
Bracken MB, Triche E, Grosso L, Hellenbrand K, Belanger K, Leaderer BP (2002) Heterogeneity in assessing self-reports of caffeine exposure: implications for studies of health effects. Epidemiology 13:165–171
Acknowledgments
Tian-bao Huang was responsible for the initial plan, data collection, statistical analysis and for conducting the study. Tian-bao Huang, Yang Yan and Zhui-feng Guo contributed to data collection, data extraction, data interpretation and manuscript drafting. Huan Liu, Xiao-long Zhang, Jiang Geng and Xu-dong Yao contributed to data interpretation and study design. Jun-hua Zheng was the guarantor for this paper and has full responsibility for this study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tian-bao Huang and Zhui-feng Guo have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, Tb., Guo, Zf., Zhang, Xl. et al. Coffee consumption and urologic cancer risk: a meta-analysis of cohort studies. Int Urol Nephrol 46, 1481–1493 (2014). https://doi.org/10.1007/s11255-014-0699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0699-9