Abstract
Purpose
Design short hairpin RNA (shRNA) interference sequence to silence glutathione S-transferase P1 (GSTP1) gene of androgen-independent prostate cancer cell line DU145, and then to explore its effect on sensitivity to chemotherapeutics.
Methods
Target sequence was picked up to form the shRNA. DU145 cell was divided into five groups according to the shRNA added for transfection: shRNA255, shRNA554, shRNA593, negative-shRNA and blank group. Fluorescence microscope was used to pick up the shRNA with the highest transfection ratio. Western blotting and RT-PCR were taken to pick up the shRNA with the best gene silencing result. 3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide assay and terminal de-oxynucleotidyl transferase-mediated dUTP nick end-labeling assay were used to detect survival ratio and apoptosis ratio of DU145 administered of fluorouracil (5-FU) or paclitaxel (PA) at different concentrations before and after shRNA transfection.
Results
Three different shRNA oligonucleotides (shRNA255; shRNA554; shRNA593) targeting the coding sequence of GSTP1 mRNA and one negative control shRNA were constructed. The transfection ratio of shRNA554 (76.2 ± 0.68 %) was higher than that of shRNA255 (63.3 ± 1.04 %) (P < 0.01) or shRNA593 (72.7 ± 0.33 %) (P < 0.01). After transfection of shRNA554, the mRNA and protein of level were the lowest, P < 0.01. The survival ratio of DU145 administered with 5-FU of different concentrations (30, 60, 120, 240 μg/ml) declined after transfection (P < 0.01). Besides, the apoptosis ratio increased after transfection (P < 0.01). Similarly the survival ratio of DU145 administered with PA of different concentrations (0.2, 2, 10, 20 μg/ml) declined (P < 0.01) and the apoptosis ratio increased (P < 0.01) after transfection.
Conclusions
The gene GSTP1 silence via shRNA transfection to androgen-independent prostate cancer cell line DU145 enhances the sensitivity to chemotherapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is now recognized as one of the most common medical problems facing the male population in the Western countries. In Europe, the incidence rate of PCa was 214 cases per 1,000 men in 2004 [1]. There were an estimated 417,000 new cases of PCa in Europe in 2012 [2]. Furthermore, PCa is currently the second most common cause of cancer death in male population [3]. Androgen withdrawal is effective at the beginning because PCa is androgen dependent, but with progression PCa may become androgen-independent and resistant to hormone therapy [4]. Chemotherapy is often given to patients of hormone-refractory prostate cancer (HRPC) when secondary hormonal therapy or radiotherapy stops working [5]. Gene Glutathione S-transferase P1 (GSTP1) codes for the protein glutathione S-transferases-π (GST-π), which is an acidic protein contains two subunits, each subunit is composed of 209 amino acids and overexpressed in androgen-independent prostate cancers [6] and closely related to the tumorigenesis and tumor progression [7]. Research of the gastrointestinal malignant tumor indicated that GSTP1 participates in the drug resistance, and inhibition of the GSTP1 expression could depress the drug resistance and induce apoptosis [8]. Li et al. [9] reported that GSTP1 was upregulated in the stromal compartment of hormone-independent prostate cancer, which may contribute to chemoresistance of advanced prostate cancer. Thus, the knockdown of any key gene that can regulate androgen independence might provide a tool for the HRPC patients [6]. RNA interference (RNAi), developed by Fire et al. [10] in 1998, is a gene silencing method which could inhibit the target gene. In our experiment, the specific short hairpin RNA (shRNA) interference sequence was designed to silence GSTP1 and cloned into plasmid as carrier for transfection to the androgen-independent cell line DU145, then to investigate the effect of gene silence on the chemosensitivity of DU145.
Materials and methods
Establishment and screening of shRNA for transfection
GSTP1 shRNA (shRNA255(#A02349), shRNA554(#A02347), shRNA593(#A02350) and negative-shRNA(#A02348)) was purchased from GenePharma (Shanghai, China). Silencer negative control with no significant homology to any known human genes was used as a negative control shRNA. The shRNA-annealed oligonucleotides were ligated into pGPU6/GFP/Neo(GeneSil Biotech, Wuhan, China) shRNA expression vectors between the BdmHI and HindIII sites by T4 DNA ligase (TaKaRa, Otsu, Japan). All constructs were verified by sequence analysis. The target sequences were shRNA255: 5′-GCC CTA CAC CGT GTC TAT TT-3′; shRNA554: 5′-GCA AAT ACA TCT CCC TCA TCT-3′; shRNA593: 5′-GCA AGG ATG ACT ATG TGA AGG-3′; negative-shRNA: 5′-GTT CTC CGA ACG TGT CAC GT-3′.
Transfection of DU145 with shRNA plasmids
DU145 were divided into five groups, which were DU145 + shRNA255, DU145 + shRNA554, DU145 + shRNA593, DU145 + negative-shRNA and blank group (DU145 + reagents for transfection, without shRNA). DU145 cultured in flask with RPMI1640 (Gibco-BRL, Grand Island, NY, USA) for 6 days in 5 % CO2 at 37 °C. The medium was then discarded, and the cells washed twice with phosphate-buffered saline (PBS) to remove unattached cells. Trypsin was added at 2 ml per flask, and after 2 min at 37 °C, cells were seeded (5 × 104 cells/well) on 6-well plates and cultured in RPMI1640 without antibiotics to achieve greater than 70 % confluence on the day of transfection. For transfection, 4 μg of each shRNA expression construct was used per well of a 6-well plate. Lipofectamine 2000 (Invitrogen Inc. CA, USA) was used as the transfection reagent at a ratio of 1 μg shRNA:1.5 μL Lipofectamine, according to the manufacturer’s instructions. After 20 min of incubation, the complexes were used per well of DU145 and culture medium. All the designed shRNA including shRNA255, shRNA554, shRNA593 and negative-shRNA were transfected, and the transfection efficiency was compared to pick up the shRNA with the highest transfection efficiency for the downstream experiment. All the cells were counted by light microscope, and the fluorescent cells were counted by fluorescence microscope, transfection rate = fluorescent cell number/cell number × 100 %.
RNA isolation and reverse transcription-PCR analysis
Total RNA was isolated from cultured cells 96 h after transfection using the Trizol reagent (#15596-026, Invitrogen Inc. CA, USA). RNA was converted to cDNA using oligo (dT) primers and cDNA synthesis kit (#FSK-100, TOYOBO Co., Ltd. Osaka, Japan) according to the manufacturer’s instructions. Primers designed for GSTP1 and the control gene GAPDH were synthesized by Sangon (Shanghai, China). GSTP1 primer sequence: F: 5′-TCT CCC TCA TCT ACA CCA AC-3′, R: 5′-TTG TAG TCA GCG AAG GAG AT-3′, 151 bp. GAPDH : F: 5′-ACC ACA GTC CAT GCC ATC AC-3′, R: 5′-TCC ACC ACC CTG TTG CTG TA -3′, 450 bp. The primers were purified using polyacrylamide gel electrophoresis (PAGE). After centrifugalization for 5 min and then 95 °C pre-degeneration for 5 min, the reaction mixture was subjected to polymerase chain reaction in a thermal cycler (Biometra, UNO-II, Göttingen, Germany). The amplification products were then subjected to electrophoresis and dyeing in ethidium bromide (EB) reflecting the amount.
Western blotting
DU145 cells were washed twice with PBS and lysed in lysis buffer (#SJ-200501, RIPA, ProMab. Biotechnologies, Inc., CA, USA.). The extracts equally used for each blot were separated by polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The following antibodies were used for immunodetection: mouse GSTP1 antibody (#sc-66000, 1:500, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and mouse GAPDH antibody (#Mab-2005079, 1:8,000, ProMab. Biotechnologies, Inc., CA, USA.).
MTT and TUNEL
DU145 cells were divided into four groups according to the concentration of chemotherapeutics administered with before and after shRNA transfection. The concentration of 5-FU was 30, 60, 120, 240 μg/ml and of PA was 0.2, 2, 10, 20 μg/ml. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to detect the survival ratio of DU145. Terminal de-oxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay was used to detect the apoptosis ratio of DU145.
Statistical analysis
Statistical analyses were performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Student’s t test was used to test the measurement data, and Chi-square test was used to test the enumeration data. Results are reported as mean ± SD. Statistically significant results were accepted at p value of 0.05.
Results
Screening of the established shRNA
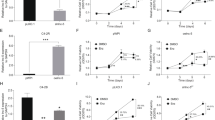
We constructed four shRNA expressing plasmids targeting the GSTP1 gene (shRNA255, shRNA554, shRNA593 and negative-shRNA). The transfection efficiency was found higher in shRNA554 (76.2 ± 0.68 %) than in shRNA225 (63.3 ± 1.04 %) (P < 0.01) or shRNA593 (72.7 ± 0.33 %) (P < 0.01). The transfection ratio of negative-shRNA was 80.1 ± 0.82 % (Fig. 1a–d). After transfection of shRNA554, the RT-PCR shows that the integral optical density (IOD) of GSTP1 mRNA was 43.24 ± 4.3, which was found lower than shRNA255 (128.31 ± 2.5) (P < 0.01) and shRNA593 (85.62 ± 6.3) (P < 0.01). The difference of GAPDH was insignificant (P > 0.05) (Fig. 2). Similarly, after transfection of shRNA554, the Western blotting shows that the IOD of GSTP1 protein was 65.38 ± 9.3, which was found lower than shRNA255 (163.92 ± 12.4) (P < 0.01) and shRNA593 (114.25 ± 16.7) (P < 0.01), while the difference of GAPDH was insignificant (P > 0.05) (Fig. 3). The shRNA554 was chosen for the downstream experiments.
Images of DU145 transfected with different shRNAs under fluoroscope. Count the total cell number under light microscope and the number of fluorescent cell under fluoroscope. The transfection ratio = fluorescent cell number/total cell number × 100 %. a shRNA255 transfection ratio 63.3 ± 1.04 %; b shRNA554 transfection ratio 76.2 ± 0.68 %; c shRNA593 transfection ratio 72.7 ± 0.33 %; d negative-shRNA transfection ratio 80.1 ± 0.82 %
Detection of GSTP1 mRNA by RT-PCR assay of DU145 transfected by different shRNAs. GSTP1 (lane 1–5) 151 bp 1 shRNA255: 128.31 ± 2.5; 2 shRNA554: 43.24 ± 4.3; 3 shRNA593: 85.62 ± 6.3; 4 negative-shRNA: 275.91 ± 9.7; 5 blank group: 304.73 ± 11.7. GAPDH (lane 6–10) 450 bp: 6 shRNA255: 642.18 ± 2.4; 7 shRNA554: 636.42 ± 3.5; 8 shRNA593: 638.59 ± 3.3; 9 negative-shRNA: 644.36 ± 2.9; 10 blank group: 630.07 ± 4.1. The best inhibitory effect of shRNA554 on GSTP1 of DU145 was confirmed in RT-PCR
Inhibitory effects of different shRNAs on GSTP1 expression. GSTP1 (23KD) (lanes 1–5) 1 shRNA255: 163.92 ± 12.4; 2 shRNA554: 65.38 ± 9.3; 3 shRNA593: 114.25 ± 16.7; 4 negative-shRNA: 245.03 ± 6.3; 5 blank group: 267.16 ± 4.3 GAPDH (37kD) (lanes 1–5) 1 shRNA255: 478.51 ± 4.2; 2 shRNA554: 466.19 ± 3.6; 3 shRNA593: 469.34 ± 6.7; 4 Negative-shRNA: 470.22 ± 5.9; 5 blank group: 465.76 ± 6.1. The best inhibitory effect of shRNA554 on GSTP1 expression of DU145 was confirmed in Western blotting
In TUNEL assay, apoptotic cell was identified by buffy granules in cell nucleus. The images exhibit the apoptosis ratio of DU145 administered with 5-FU of different concentrations in TUNEL assay before shRNA554 transfection. a 30 μg/ml, apoptosis ratio 5.5 ± 0.88 %. b 60 μg/ml, apoptosis ratio 10.2 ± 1.64 %. c 120 μg/ml, apoptosis ratio 15.2 ± 2.39 %. d 240 μg/ml, apoptosis ratio 25.1 ± 2.59 %
After shRNA transfection, the apoptosis ratio of DU145 administered with 5-FU of different concentrations in TUNEL assay. a 30 μg/ml, apoptosis ratio 10.8 ± 0.62 %. b 60 μg/ml, apoptosis ratio 15.7 ± 1.32 %. c 120 μg/ml, apoptosis ratio 20.4 ± 1.89 %. d 240 μg/ml, apoptosis ratio 34.9 ± 2.54 %. The apoptosis ratio increased compared with before transfection
Effect of shRNA transfection on the chemosensitivity of DU145
The survival rate of DU145, administered with 5-FU or PA before and after shRNA554 transfection, was measured by MTT. After transfection, the survival rate decreased significantly compared with before transfection administered with 5-FU (P < 0.01). With the increase in the concentration of 5-FU, the DU145 survival rate decreased (P < 0.01) (Table 1; Fig. 6). The results were similar of DU145 administered with PA (Table 2; Fig. 6). In TUNEL, apoptotic cell was identified if cell nucleus presented with buffy granules. Apoptosis index (AI) = apoptotic cell number/total cell number × 100 %. With the increase in the concentration of 5-FU, the AI increased (P < 0.01) (Fig. 4a–d). After transfection, the AI of DU145 increased administered with 5-FU (P < 0.01) (Table 3; Fig. 5a–d). The results of PA were similar (Table 4).
Discussion
The current study was aimed at investigating the potential as therapy for HRPC of gene GSTP1 silencing in combination with chemotherapy. In this study, we present a strategy to silence the GSTP1 gene in DU145 using shRNA expression plasmids. We first constructed three plasmid-based expression shRNA, targeting the GSTP1 gene and transfected to DU145 to test efficacy of the vector by RT-PCR measurement of mRNA levels, and found that all constructs demonstrated successful silence of the gene. We further checked the protein level in DU145 by Western blotting and found that both results were in agreement. Repeated experiments indicated that shRNA554 was the most stable in its gene silencing efficacy and thus chosen for the further experiments of chemosensitivity altering in DU145. 5-FU and PA were chose as the chemotherapeutics administered for DU145. Previous reports have indicated that docetaxel remains as one of the standard treatments for HRPC [11]. 5-FU and PA were broad spectrum antitumor drugs and recently were chosen in many studies of combination with gene therapy or targeted chemotherapy strategy for HRPC [12, 13]. After transfection of shRNA554, the survival ratio of DU145 administered with 5-FU or PA declined and the apoptosis ratio increased. GSTP1 is found overexpressed in many malignant tumors, which is closely related to carcinogenesis and chemoresistance [7]. Mammalian glutathione S-transferases (GSTs) are now classified into the alpha, mu and pi classes [14]. GST-pi catalyses reduced glutathione combine with antitumor drugs promoting drug discharge, besides regulates the mitogen-activated protein kinase (MAPK) signal transduction pathway to affect the cell proliferation and apoptosis [15]. Overexpression of GST-pi leads to multidrug resistance, which suggests that GST-pi active in drug metabolism. The study of exploring treatment aimed at GST-pi in cellular experiment such as glutathione analogue TER199 was reported by O’Brien et al. [16]. Eilers et al. [17] found the biopsy washing DNA GSTP1 island hypermethylation of the promoter sequences as an early event in human prostate carcinogenesis. Lu et al. [18] reported an involvement of JNK and p38 activity in the inhibition effects on apoptosis of GSTP1. Beside GSTP1, other genes such as clusterin also affect the chemosensitivity of prostate cancers. Sowery et al. [19] has reported that knockdown of clusterin using the antisense oligonucleotide OGX-011 re-sensitizes docetaxel-refractory prostate cancer PC-3 cells to chemotherapy. Beside chemoresistance, GSTP1 has an important role in adapting prostate cancer for growth and metastasis involving an alteration in reactive oxygen species (ROS) signal [20].
The current study has its limitation. Cancer is not a single cell disease [9, 21]. Aberrant cancer cells and their interactive microenvironment are needed for prostate cancer to progress to androgen independence and distant metastasis. It is highly plausible that newly evolved prostate cancer cell clones dominate cancer metastasis after cell–cell and cell–matrix interaction with the host microenvironment, rather than the selection or expansion of a preexisting prostate cancer cell clone. Mice model of orthotopic androgen-independent prostate cancer or nude mice with human prostate cancer cell lines is needed to identify the chemosensitivity converting mechanism in androgen-independent prostate cancer. 5-FU is a cell-cycle-specific chemotherapeutic, and the S phase cell is sensitive to 5-FU [22]. Thus, the flow cytometry is needed to check the cell cycle change in DU145 after transfection. The exact mechanism of interaction between GSTP1 silence and chemotherapeutics effect augment should be identified in future.
Conclusions
In this study, we constructed three shRNA target sequences and demonstrated that silencing of GSTP1 by shRNA in androgen-independent human prostate cancer cell line DU145 enhances the chemosensitivity. The current study supports the concept of local therapy involving shRNA designed for GSTP1 injected to recurrent tumors in combination with systematic chemotherapy. The present findings provide preclinical proof of testing GSTP1 shRNA in second-line chemotherapy regimens for patients with androgen-independent prostate cancer.
References
Boyle P, Ferlay J (2005) Cancer incidence and mortality in Europe, 2004. Ann Oncol 16(3):481–488
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6):1374–1403
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA: Cancer J Clin 58(2):71–96
Feldman BJ, Feldman D (2001) The development of androgen-independent prostate cancer. Nat Rev Cancer 1(1):34–45
Heidenreich A, von Knobloch R, Hofmann R (2001) Current status of cytotoxic chemotherapy in hormone refractory prostate cancer. Eur Urol 39(2):121–130
Hokaiwado N, Takeshita F, Naiki-Ito A, Asamoto M, Ochiya T, Shirai T (2008) Glutathione S-transferase Pi mediates proliferation of androgen-independent prostate cancer cells. Carcinogenesis 29(6):1134–1138
Kim KH, Kwon HC, Oh SY, Kim SH, Lee S, Kwon KA, Jang JS, Kim MC, Kim SJ, Kim HJ (2011) Clinicopathologic significance of ERCC1, thymidylate synthase and glutathione S-transferase P1 expression for advanced gastric cancer patients receiving adjuvant 5-FU and cisplatin chemotherapy. Biomarkers 16(1):74–82
Noda E, Maeda K, Inoue T, Fukunaga S, Nagahara H, Shibutani M, Amano R, Nakata B, Tanaka H, Muguruma K, Yamada N, Yashiro M, Ohira M, Ishikawa T, Hirakawa K (2012) Predictive value of expression of ERCC 1 and GST-p for 5-fluorouracil/oxaliplatin chemotherapy in advanced colorectal cancer. Hepatogastroenterology 59(113):130–133
Li M, Ittmann MM, Rowley DR, Knowlton AA, Vaid AK, Epner DE (2003) Glutathione S-transferase pi is upregulated in the stromal compartment of hormone independent prostate cancer. Prostate 56(2):98–105
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391(6669):806–811
Quinn DI, Tangen CM, Hussain M, Lara PN Jr, Goldkorn A, Moinpour CM, Garzotto MG, Mack PC, Carducci MA, Monk JP, Twardowski PW, Van Veldhuizen PJ, Agarwal N, Higano CS, Vogelzang NJ, Thompson IM Jr (2013) Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol 14(9):893–900
Yang Y, Zhou Z, He S, Fan T, Jin Y, Zhu X, Chen C, Zhang ZR, Huang Y (2012) Treatment of prostate carcinoma with (galectin-3)-targeted HPMA copolymer-(G3-C12)-5-fluorouracil conjugates. Biomaterials 33(7):2260–2271
Iida K, Zheng R, Shen R, Nanus DM (2012) Adenoviral neutral endopeptidase gene delivery in combination with paclitaxel for the treatment of prostate cancer. Int J Oncol 41(4):1192–1198
Bostwick DG, Meiers I, Shanks JH (2007) Glutathione S-transferase: differential expression of alpha, mu, and pi isoenzymes in benign prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma. Hum Pathol 38(9):1394–1401
Antognelli C, Mearini L, Talesa VN, Giannantoni A, Mearini E (2005) Association of CYP17, GSTP1, and PON1 polymorphisms with the risk of prostate cancer. The Prostate 63(3):240–251
O’Brien ML, Vulevic B, Freer S, Boyd J, Shen H, Tew KD (1999) Glutathione peptidomimetic drug modulator of multidrug resistance-associated protein. J Pharmacol Exp Ther 291(3):1348–1355
Eilers T, Machtens S, Tezval H, Blaue C, Lichtinghagen R, Hagemann J, Jonas U, Serth J (2007) Prospective diagnostic efficiency of biopsy washing DNA GSTP1 island hypermethylation for detection of adenocarcinoma of the prostate. Prostate 67(7):757–763
Lu M, Xia L, Luo D, Waxman S, Jing Y (2004) Dual effects of glutathione-S-transferase-P1 on As203 action in prostate cancer cells: enhancement of growth inhibition and inhibition of apoptosis. Oncogene 23(22):3945–3952
Sowery RD, Hadaschik BA, So AI, Zoubeidi A, Fazli L, Hurtado-Coll A, Gleave ME (2008) Clusterin knockdown using the antisense oligonucleotide OGX-011 re-sensitizes docetaxel-refractory prostate cancer PC-3 cells to chemotherapy. BJU Int 102(3):389–397
Naiki T, Asamoto M, Toyoda-Hokaiwado N, Naiki-Ito A, Tozawa K, Kohri K, Takahashi S, Shirai T (2012) Organ specific Gst-pi expression of the metastatic androgen independent prostate cancer cells in nude mice. Prostate 72(5):533–541
Chung LW, Baseman A, Assikis V, Zhau HE (2005) Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol 173(1):10–20
Saleh EM, El-Awady RA, Anis N (2013) Predictive markers for the response to 5-fluorouracil therapy in cancer cells: constant-field gel electrophoresis as a tool for prediction of response to 5-fluorouracil-based chemotherapy. Oncol Lett 5(1):321–327
Conflicts of interest
The authors report no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, P., Xie, J., Zhu, X. et al. shRNA-mediated GSTP1 gene silencing enhances androgen-independent cell line DU145 chemosensitivity. Int Urol Nephrol 46, 1115–1121 (2014). https://doi.org/10.1007/s11255-013-0616-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-013-0616-7