Abstract
Purpose
To quantitatively assess the histological and ultrastructural changes resulting from aging in the human testis.
Methods
Age-related histological and ultrastructural changes were evaluated using light microscopy, transmission electron microscopy (TEM) and immunohistochemistry on 41 testicular samples obtained from elderly men and, respectively, assigned to group A (n = 20), 54–69 years old or group B (n = 21), 70–89 years old. Testicular samples derived from 17 young men were used for control.
Results
The numbers of Sertoli cells in the aged groups were significantly lower than that in the controls (p < 0.05). With the exception of the Sertoli cell ratios (germ cells/Sertoli cells) of spermatogonia and primary spermatocytes, results showed lower levels of the Sertoli cell ratios of round spermatids and elongated spermatids in the elderly men compared with the young men (p < 0.05). A similar degenerative pattern of the organelles was shown in germ cells and Sertoli cells in the aging testes under TEM. Immunohistochemistry revealed an increased apoptosis index (AI) (0.81 ± 0.13) accompanied by a decreased proliferation index (PI) (30.08 ± 4.86) in the group B (p < 0.05), while both AI and PI were similar between the group A (0.54 ± 0.06; 36.38 ± 7.38) and the controls (0.50 ± 0.15; 40.55 ± 7.92) (p > 0.05).
Conclusions
Aging has negative influence on testicular morphology and spermatogenesis, and the failure of spermatogenic cell development is evident from the spermatid level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a continuous process characterized by the gradual, morphological and functional degeneration of organs, among which the gonad represents a special case. Menopause is the clinical sign of ovarian aging as the ovary exerts normal physiological functions at a distinct period from menarche until menopause. By contrast, the aging process of the testis is quite relative as there is no precise clinical symptom for the decline in testicular function [1]. Although spermatogenesis usually continues uninterrupted throughout adult life, there are some decreases that occur in both quality and quantity in elderly men when compared with young men [1–3], and such alterations may be principally explained by age-related degenerations in the testis [4].
The morphological characteristics and functions of spermatogenic cells are largely based on the integration of the histological structure of the testis. Aging can decrease testicular size and numbers of germ cells, Sertoli cells and Leydig cells [5–7]. Our previous stereological study has also demonstrated a decline in peritubular cells in the testis during aging process [8]. Sperm characteristics as well as sperm fertilizing capacity are reported to exhibit a precipitous decline with aging [9, 10].
Spermatogenesis is closely associated with the morphological alterations of the testis [11]. Spermatogenesis is a highly sophisticated process involving the progressive differentiation of spermatogonia into mature spermatozoa. This process undergoes consecutive divisions including spermatogonia, primary spermatocytes and secondary spermatocytes, round spermatids, elongated spermatids and mature spermatozoa. Ultrastructural analyses of the aging human testes revealed that maturation of spermatogenesis arrested at spermatogonium and spermatocyte levels [12, 13]. However, most of these studies are focused on morphological alterations without further quantitative analysis, and the effects of aging on spermatogenesis merit further exploration.
The present study was conducted to further elucidate the influences of aging on testicular histological changes and, in particular, on stage-specific spermatogenesis by quantitative methods. This would deepen our insight into the understanding of the physiological and pathophysiological events taking place in the aging testis.
Materials and methods
Subjects and samples
All of the testicular tissue samples were obtained from the First Affiliated Hospital of Jinan University, PR China. The testes from 41 elderly men undergoing bilateral orchidectomy for advanced prostate cancer therapy were assigned to one of the two aged groups: Group A (n = 20), 54–69 years old, mean age 63.3 ± 4.6 years; Group B (n = 21), 70–89 years old, mean age 75.5 ± 4.2 years. None of these cases had previously received gonadotherapy which might affect spermatogenesis prior to orchidectomy, and all the patients had one or more children. The control group comprised 17 testicular tissue samples obtained by biopsy from patients who underwent assisted reproductive treatment and aged 24–40 years old (mean age 32.5 ± 3.4 years). All of the testicular tissue samples, including that in the aged groups and the control group, revealed normal appearance by histopathological examination. This study was performed in agreement with the local ethical committee, and informed consent was obtained from each subject.
Histological examination
Testicular tissues were fixed in Bouin solution for 2 h and in 10 % neutral-buffered formalin for 2–4 h, and then subjected to paraffin embedding and cut into 5-μm sections. Then sections were stained with hematoxylin and eosin. The method used for quantitative analysis of spermatogenesis was according to the procedure described previously [14, 15]. In brief, the numbers of Sertoli cells, spermatogonia, primary spermatocytes, round spermatids, and elongated spermatids were counted within 20 randomly selected seminiferous tubules presenting an approximately circular cross section under light microscopy. The rate of germ cells was indicated by Sertoli cell ratio, as evaluated by the number of each type of germ cells to the number of Sertoli cells in the same tubule segment.
Transmission electron microscopy (TEM) procedure
TEM was performed on small testicular tissue samples (1 mm3). The specimens were fixed in 2.5 % glutaraldehyde at 4 °C for 2 h. After washing, the specimens were fixed in 1 % osmium tetroxide for 2 h, dehydrated and embedded in Epon-812. Thin sections were double-stained with uranyl acetate and lead citrate. Observations were performed under a JEM-100CXII TEM (NEC Corporation, Tokyo, Japan).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
An in situ cell apoptosis detection kit (Boster, Wuhan, China) was used for the evaluation of apoptosis in testicular tissues according to the manufacturer’s instructions. The formalin-fixed sections were deparaffinized, and pretreated with 3 % hydrogen peroxide to quench endogenous peroxidase. Subsequently, the sections were incubated in TdT/dUTP solution at 37 °C for 2 h. After rinsed, sections were incubated in biotinylated anti-digoxigenin antibody and strept avidin–biotin complex (SABC), respectively, for 30 min in a humidified chamber at 37 °C. The sections were rinsed again, revealed with 3,3′-diaminobenzidine (DAB, Boster, Wuhan, China) and counterstained with hematoxylin. Positive controls involved sections of mucosa of small intestine provided by the manufacturer. Negative controls were incubated in a TdT-free solution. For the evaluation of apoptosis, 20 randomly selected cross sections of the seminiferous tubules were chosen, and the apoptosis index (AI) was defined as the percentage of seminiferous tubules containing TUNEL-positive cells.
Proliferating cell nuclear antigen (PCNA) immunohistochemistry
All of the reagents were purchased from Boster Ltd., Wuhan, China. Paraffin-embedded sections were deparaffinized and incubated in 3 % hydrogen peroxide to quench endogenous peroxidase. Subsequently, the sections were heated in a microwave oven in 0.01 M citrate buffer solution. After incubated with normal goat serum for 20 min at room temperature, the sections were added with PCNA mouse mono-clonal PC10 antibody overnight at 4 °C (1:200 dilution). After washed, the sections were exposed to a solution containing a goat anti-mouse secondary antibody (1:200 dilution) for 20 min at room temperature. Then the sections were incubated with the SABC for 20 min at 37 °C. The PCNA antigen was finally detected by treating the sections with a DAB substrate kit. Positive controls involved sections of tonsillar lymphoid follicles. Negative controls without the primary antibody were performed. 20 randomly selected cross sections of the seminiferous tubules were chosen, and the percentage of PCNA positive-stained cells was used to indicate the proliferative index (PI).
Statistical analysis
Results were presented as mean ± standard deviation (SD). Data were analyzed by SPSS V.11.0 software (SPSS, Chicago, IL, USA). Differences among groups were determined by one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls (SNK) for multiple comparisons. Statistical significance was set up at p < 0.05.
Results
Histological characteristics
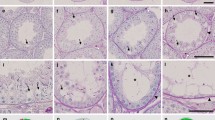
In contrast to the active spermatogenesis and ordered spermatogenic cells found in the young controls (Fig. 1a), progressively atrophic lesions were frequently seen in the aging testis, albeit these histological changes were to some extent individually variable. Seminiferous tubules in the aged cases showed reduced height of seminiferous epithelium. Degenerative spermatogenesis characterized by the disarrangement of spermatogenic cells and sloughing off of premature spermatids from the seminiferous epithelium into the tubular lumen were frequently noted (Fig. 1b). The basement membrane lying adjacent to Sertoli cells was substantially thickened and folded in the elderly cases. In the aged men, the lesion areas might vary from foci of hyaline degeneration in the seminiferous tubular wall and occasional disappearance of spermatogenic cells to completely sclerosed tubules accompanied by fibrosis and nodular hyperplasia in the peritubular tissues.
a Seminiferous tubules in the young men showed normal height of seminiferous epithelium (H) and regular arrangement of spermatogenic cell layers (triangle). b Seminiferous tubules with reduced height of seminiferous epithelium (H) in a 78-year-old man. Spermatogenic cells arranged loosely (triangle) and the basement membrane was thickened (arrow). Bar = 40 μm
Quantitative analysis of the histological findings
The numbers of Sertoli cells and the Sertoli cell ratios of germ cells are shown in Table 1. The number of Sertoli cells per seminiferous tubule in group A (16.5 ± 5.6) and group B (15.3 ± 7.8) was, respectively, significantly lower than that in control group (21.7 ± 5.7) (p < 0.05), whereas it displayed a slight but not significant lower-level in group B than that in group A (p > 0.05). The Sertoli cell ratios of spermatogonia and primary spermatocytes did not differ among group A (1.33 ± 0.42; 1.46 ± 0.75), group B (1.32 ± 0.58; 1.43 ± 0.86) and control group (1.41 ± 0.38; 1.72 ± 0.72) (p > 0.05). The Sertoli cell ratio of round spermatids in group A (0.87 ± 0.74) as well as group B (0.94 ± 0.77) was remarkably lower than that in control group (1.63 ± 0.91) (p < 0.05), and this indicator was similar between the former two aged groups (p > 0.05). The Sertoli cell ratio of elongated spermatids in group A (0.88 ± 0.73) and group B (0.81 ± 0.79) did not differ from that in control group (1.43 ± 0.92) (p > 0.05), albeit a numerical decline was actually found in these two aged groups.
Ultrastructural observations
With TEM, typical nuclei, steady nuclear chromatin and intact organelles such as mitochondria, endoplasmic reticulum and Golgi apparatus were visible in the Sertoli cells of the young controls (Fig. 2a). Inter-Sertoli junctional complexes were abundant between adjacent Sertoli cell membranes in the young men. The spermatogonia, oval in shape, were noted to lie in the basal lamina, and their nuclei were compact, and the chromatin was dense. The spermatocytes with heterochromatin were closely associated with Sertoli cell cytoplasm. Round and elongated spermatids with condensed chromatin were embedded in the cytoplasm of Sertoli cells relatively near the lumen. Unlike the young men, the elderly men showed remarkably thick patterns in the basement membrane of seminiferous tubules. The Sertoli cells were highly vacuolated, and degenerative appearances were seen in the organelles, characterized by the presence of endoplasmic reticulum cisternae and lysosome paramorphia (Fig. 2b). Inter-Sertoli cell tight junctions were less frequent. Germ cell loss occurred frequently throughout the process of spermatogenesis, especially from the stage of primary spermatocytes. The remaining spermatids were seen to display poorly condensed chromatin, with conspicuous vacuoles in the caudal cytoplasm and mitochondria arranged in a disordered fashion.
a Representative electron micrograph of the testis from the young men showing the typical nucleus of Sertoli cell (N) and a developed whorl of endoplasmic reticulum (ER). b Representative electron micrograph of the testis from the elderly men showing malformed nuclei in Sertoli cells (N). Increased vacuoles (V) were noted to present in the bottom of Sertoli cells and the organelles in the cytoplasm were less present. Bar = 1 μm
AI and PI of germ cells
The TUNEL assay revealed that apoptotic signals were predominantly located in the nuclei of spermatogonia and primary spermatocytes (Figs. 3a, b). AI in group B (0.81 ± 0.13) was significantly increased in comparison with control group (0.50 ± 0.15) (p < 0.05). However, there was no significant change of AI in group A (0.54 ± 0.06) when compared with group B or control group, respectively (p > 0.05) (Table 2).
Light micrographs of TUNEL-positive spermatogenic cells in seminiferous tubules from the young men (a) and the aged men (b). a A TUNEL-positive primary spermatocyte was presented in seminiferous tubule from the young men (arrow). b Arrow pointed to a TUNEL-positive spermatogonium in seminiferous tubule from the aged men. Bar = 20 μm
Compared with control group (Fig. 4a), the number of PCNA-positive cells reduced remarkably in the aged groups (Fig. 4b). PCNA-positive staining was shown in the nuclei of spermatogonia and primary spermatocytes, and the staining was more obvious in the latter than that in the former. Spermatids and Sertoli cells were devoid of PCNA staining (Fig. 4b). In group B, PI (30.08 ± 4.86) was dramatically decreased in comparison with control group (40.55 ± 7.92) (p < 0.05). Like AI, PI in group A did not differ from that in group B or control group (p > 0.05) (Table 2).
Light micrographs of PCNA-positive spermatogenic cells in seminiferous tubules from the young men (a) and the aged men (b). a Spermatogonia (arrows) and primary spermatocytes (dotted arrows) labeled with PCNA were noted in seminiferous tubules from the young men. b The number of PCNA-positive cells reduced markedly in the aged men. Arrows pointed to the PCNA-positive spermatogonia and dotted arrows pointed to the PCNA-positive primary spermatocytes. Bar = 20 μm
Discussion
Unlike its pronounced and age-specific effects on female reproduction, aging is thought to cause moderate damage to male fertility without a precise threshold. Therefore, the onset and severity of testicular changes caused by aging remain to be fully illustrated. In the present study, quantitative methods were applied to determine the histological changes in the aging testis. As revealed by the data obtained, a striking decline did emerge with aging concerning the numbers and morphological aspects of germ cells, Sertoli cells, and even testicular peritubular tissues. The age-related increase in apoptotic activity and decrease in proliferation in the testis may account for these degenerative changes.
The present findings endorsed previous studies describing a trend of regression in Sertoli cells during the process of aging [12, 16]. In addition to a decline in the cell number, ultrastructural evaluation further showed a complete loss of normal appearance in the organelles of Sertoli cells in the elderly men in contrast to that in the young men. An increased number of vacuoles involving the endocytosis of degenerative germ cells were seen in the cytoplasm. Moreover, odd-shaped nuclei, vesiculated endoplasmic reticulum and irregular lysosomes were more frequent in the elderly cases. These histological degradations of Sertoli cells may presumably reflect their diminished biological functions with aging.
Distinct from the widespread existence of typical Sertoli–Sertoli junctions in the young controls, inter-Sertoli junctional complexes were rarely seen in the aged cases. There is a strong correlation between the existence of inter-Sertoli tight junctions and the integrity of the blood-testis barrier (BTB) [17]. Thus, the degeneration in inter-Sertoli tight junctions may be taken to indicate the presence of a damaged BTB in the old men. Integrity of the BTB can assure the development of germ cells to take place in an immune privileged microenvironment in the apical compartment. Such age-related disruption in the BTB may serve as a contributor leading to the abnormal spermatogenesis because of the degeneration of immunological barrier [16]. Herein, the underlying mechanism implicated in the breakdown of inter-Sertoli tight junctions in the aging testis is worth further discussing. Cell junction restructuring in the testis is proposed to be under the control of steroid hormones [18, 19]. Therefore, it is rational to speculate that steroid hormone alterations steaming from senescence may appear as a crucial factor accounting for the age-related damages in the BTB.
Increased apoptosis accompanied by diminished proliferation in germ cells of the aging testis was revealed in the study, and this is established as an intrinsic characteristic during testicular aging [20]. TUNEL assay in a earlier study displayed a significant increase in the apoptotic activity of primary spermatocytes in elderly men [15]. Furthermore, aging is reported to have negative impacts on the proliferation in testicular germ cells [21]. Our present data was thought to further supplement these conclusions by additional quantitative histological analysis. A decrease trend in the numbers of germ cells was only found in the aging testes at the stages from round spermatids until elongated spermatids, while spermatogonium and primary spermatocytes were similar between the aged cases and the young controls. Thus, the arrested development of spermatogenic cells seems to commence from the spermatid level. There were previous reports suggesting that reduced spermatogenic potential occurred at the primary spermatocyte level and this had direct effects on decreased spermatid production [22, 23]. Other investigators argued that sperm maturation arrested at spermatogonium level, because both A and B spermatogonia were occasionally found in the aging testes [12, 13]. In the present study, although the difference in the numbers of spermatocytes did not reach a statistical significance, the elderly men did show a numerical decrease compared with the young men, with further considerable reduction occurring at the spermatid level. Highly individual viability and small sample sizes may contribute to the discrepancy. Although accelerated apoptosis may to some extent affect the process of sperm maturation, it is significant to note that germ cell loss during spermatogenesis occurs throughout the adult life [24], whereas sperm maturation arrest is only found in men with advancing age. Therefore, it is advisable that maturation failure does not simply refer to a diminution in germ cells but certain age-related mechanisms must be at work.
Sertoli cells can support spermatogenic cells during maturation in terms of physical attachment, nutrient provisions, and protection from immune attack. Therefore, interactions between germ cells and supporting somatic cells are crucial for normal spermatogenesis. In the aging testis, extracellular spaces of the Sertoli cells were seen to be dilated because of the detachment of germ cells from seminiferous epithelium [12]. In addition, Sertoli cells and spermatogenic cells from aging testes with regressed appearances showed a parallel selective loss to each other [25, 26]. Taken together, disruptions in the communication between Sertoli cells and germ cells are likely to be one of the mechanisms involved in the onset of age-associated sperm maturation arrest.
Basement membranes of the seminiferous epithelium were noted to become thickened and convoluted in the aging testis. Thickened basement membrane is a well-recognized morphological feature of the aging testis, albeit the causality remains to be established [4, 27]. Sertoli cells and testicular peritubular cells are reported to be two active participants required for the production and deposition of basement membrane in the testis [28, 29]. Although aging has no influence on the expression of the genes involved in the deposition of basement membrane [30], morphology of the peritubular tissues can be affected by steroid hormones [31], of which the levels are in close association with the aging process. Therefore, altered concentrations of steroid hormones in the elderly men may serve as an additional factor resulting in these structural anomalies.
However, there is a considering limitation in the present study that all the data was obtained by histological quantitative analysis, while the application of stereological methods, which could allow us to gather the data of three-dimensional objects through the interpretation of two-dimensional images, may permit more unbiased and reliable results of the size, shape and number of the aging male germ cells. And this should be further illustrated in our following studies.
In summary, abnormal histological structure and impaired spermatogenesis are present in the aging human testes. These atrophic changes do not appear to have a specific pathological etiology other than their relation to age, suggesting that a possible intrinsic degenerative mechanism may exist in the aging testis. More researches aiming to uncover the age-related alterations by means of quantitative methods are undoubtedly conducive to expanding the understanding of the regulatory mechanisms of physiological and pathological events in the aging testis.
References
Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D (2003) The association of age and semen quality in healthy men. Hum Reprod 18:447–454
Haidl G, Jung A, Schill WB (1996) Ageing and sperm function. Hum Reprod 11:558–560
Plas E, Berger P, Hermann M, Pfluger H (2000) Effects of aging on male fertility? Exp Gerontol 35:543–551
Pop OT, Cotoi CG, Plesea IE, Enache SD, Popescu FC, Enache MA, Plesea RM (2011) Correlations between intralobular interstitial morphological changes and epithelial changes in ageing testis. Rom J Morphol Embryol 52:339–347
Neaves WB, Johnson L, Porter JC, Parker CR Jr, Petty CS (1984) Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab 59:756–763
Paniagua R, Martin A, Nistal M, Amat P (1987) Testicular involution in elderly men: comparison of histologic quantitative studies with hormone patterns. Fertil Steril 47:671–679
Well D, Yang H, Houseni M, Iruvuri S, Alzeair S, Sansovini M, Wintering N, Alavi A, Torigian DA (2007) Age-related structural and metabolic changes in the pelvic reproductive end organs. Semin Nucl Med 37:173–184
Xia Y, Zhu WJ, Hao SF, Liang WB, Li J (2012) Stereological analysis of age-related changes of testicular peritubular cells in men. Arch Gerontol Geriatr 55:116–119
Chen QJ, Zhu WJ, Li J (2006) Effects of aging on spermatogenesis, sperm maturation and fertility in mice. J Reprod Contracept 17:9–14
Hellstrom WJ, Overstreet JW, Sikka SC, Denne J, Ahuja S, Hoover AM, Sides GD, Cordell WH, Harrison LM, Whitaker JS (2006) Semen and sperm reference ranges for men 45 years of age and older. J Androl 27:421–428
Russell LD, Malone JP, Karpas SL (1981) Morphological pattern elicited by agents affecting spermatogenesis by stimulation. Tissue Cell 13:369–380
Paniagua R, Nistal M, Saez FJ, Fraile B (1991) Ultrastructure of the aging human testis. J Electron Microsc Tech 19:241–260
Paniagua R, Nistal M, Amat P, Rodriguez MC, Martin A (1987) Seminiferous tubule involution in elderly men. Biol Reprod 36:939–947
Hikim AP, Wang C, Lue Y, Johnson L, Wang XH, Swerdloff RS (1998) Spontaneous germ cell apoptosis in humans: evidence for ethnic differences in the susceptibility of germ cells to programmed cell death. J Clin Endocrinol Metab 83:152–156
Kimura M, Itoh N, Takagi S, Sasao T, Takahashi A, Masumori N, Tsukamoto T (2003) Balance of apoptosis and proliferation of germ cells related to spermatogenesis in aged men. J Androl 24:185–191
Levy S, Serre V, Hermo L, Robaire B (1999) The effects of aging on the seminiferous epithelium and the blood-testis barrier of the Brown Norway rat. J Androl 20:356–365
Yan HH, Cheng CY (2005) Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci USA 102:11722–11727
Lui WY, Lee WM (2009) Molecular mechanisms by which hormones and cytokines regulate cell junction dynamics in the testis. J Mol Endocrinol 43:43–51
Perheentupa A, Huhtaniemi I (2009) Aging of the human ovary and testis. Mol Cell Endocrinol 299:2–13
Pastor LM, Zuasti A, Ferrer C, Bernal-Manas CM, Morales E, Beltran-Frutos E, Seco-Rovira V (2011) Proliferation and apoptosis in aged and photoregressed mammalian seminiferous epithelium, with particular attention to rodents and humans. Reprod Domest Anim 46:155–164
Salama M, Tsuji M, Tamura M, Kagawa S (1998) Impact of aging and diabetes mellitus on the expression of the proliferating cell nuclear antigen in rat testicular tissue. Arch Androl 40:95–107
Johnson L, Nguyen HB, Petty CS, Neaves WB (1987) Quantification of human spermatogenesis: germ cell degeneration during spermatocytogenesis and meiosis in testes from younger and older adult men. Biol Reprod 37:739–747
Johnson L, Grumbles JS, Bagheri A, Petty CS (1990) Increased germ cell degeneration during postprophase of meiosis is related to increased serum follicle-stimulating hormone concentrations and reduced daily sperm production in aged men. Biol Reprod 42:281–287
Johnson L, Petty CS, Neaves WB (1983) Further quantification of human spermatogenesis: germ cell loss during postprophase of meiosis and its relationship to daily sperm production. Biol Reprod 29:207–215
Syed V, Hecht NB (2002) Disruption of germ cell-Sertoli cell interactions leads to spermatogenic defects. Mol Cell Endocrinol 186:155–157
Syed V, Hecht NB (2001) Selective loss of Sertoli cell and germ cell function leads to a disruption in sertoli cell-germ cell communication during aging in the Brown Norway rat. Biol Reprod 64:107–112
Murray MJ, Meacham RB (1993) The effect of age on male reproductive function. World J Urol 11:137–140
Skinner MK, Tung PS, Fritz IB (1985) Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol 100:1941–1947
Richardson LL, Kleinman HK, Dym M (1995) Basement membrane gene expression by Sertoli and peritubular myoid cells in vitro in the rat. Biol Reprod 52:320–330
Richardson LL, Kleinman HK, Dym M (1995) The effects of aging on basement membrane in the testis. J Androl 16:118–126
Kondarewicz A, Kolasa A, Zawislak B, Baranowska-Bosiacka I, Marchlewicz M, Wenda-Rozewicka L, Wiszniewska B (2011) Testis morphology in rats chronically treated with letrozole, an aromatase inhibitor. Folia Histochem Cytobiol 49:677–684
Acknowledgments
This work was financed by the National “Twelfth Five-Year” Plan for Science and Technology Support (2012BAI32B03).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jiang, H., Zhu, WJ., Li, J. et al. Quantitative histological analysis and ultrastructure of the aging human testis. Int Urol Nephrol 46, 879–885 (2014). https://doi.org/10.1007/s11255-013-0610-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-013-0610-0