Abstract
Background
Early diagnosis of kidney allograft dysfunction is crucial for the management and long-term survival of transplanted kidneys. We investigated whether neutrophil gelatinase-associated lipocalin (NGAL), interleukin 18 (IL-18), and liver-type fatty acid-binding protein (L-FABP) are capable of being used as novel biomarkers of acute kidney allograft dysfunction.
Methods
We measured serum and urine NGAL, urine IL-18, and urine L-FABP levels on the first 3 days after transplantation. To assess the diagnostic sensitivity of these biomarkers, a receiver-operating characteristic curve (ROC) was plotted, and the area under the curve (AUC) was calculated to quantify the accuracy of the parameter. Sections from paraffin-embedded biopsy specimens were examined by immunohistochemistry for NGAL expression.
Results
Twelve cases were clinically diagnosed as acute rejection (AR) by renal biopsy. Urine NGAL was the most sensitive of these markers for detection of acute kidney allograft dysfunction. The cutoff value of urine NGAL was 66.0 ng/ml, with an AUC of 0.79 (95 % CI 0.68–0.88). Sensitivity of serum NGAL was about the same as urine NGAL with an AUC of 0.75 (0.64–0.85). IL-18 and L-FABP were 0.584 (95 % CI 0.433–0.725) and 0.612 (95 % CI 0.460–0.749), respectively. NGAL was more useful than other biomarkers to detect AR of kidney allograft dysfunction. NGAL staining intensity was significantly increased in the proximal tubules of the transplants with AR than in transplants that were not acutely rejected.
Conclusion
Urine NGAL level was found to be the most sensitive biomarker of acute kidney allograft dysfunction after living-donor kidney transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Great improvements in clinical technologies have made kidney transplantation the most effective treatment for end-stage renal disease. However, early diagnosis of kidney allograft dysfunction is crucial to the management and long-term survival of the transplanted kidney [1]. Acute kidney injury (AKI) in allografts can result from several different etiologies. Early after transplantation, acute tubular necrosis manifested as delayed graft function (DGF) or slow graft function; acute rejection (AR) and drug toxicity are the leading causes of AKI [2]. Clinicians have been searching for noninvasive tools that would allow early and accurate diagnosis of acute kidney allograft dysfunction without performing a kidney biopsy [3].
Allograft function after kidney transplantation is commonly monitored by measuring serum creatinine (Cr) concentration. However, serum Cr concentrations are greatly affected by numerous nonrenal factors, including gender, age, body weight, muscle metabolism, and protein intake [4], and serum Cr values do not reflect the severity of renal injury until a steady state has been reached, which may take several days. The serum Cr levels of kidney transplant recipients are also significantly affected by the donor kidney’s function, acute tubular necrosis, and the accumulation of Cr in both serum and urine before surgery, making it very difficult to detect AKI in the early postoperative period. Early detection and treatment of subclinical rejection, before progression to renal dysfunction, improves outcomes [5, 6]; it is rather invasive and is not suitable for very early diagnosis of kidney allograft dysfunction.
The quest for a way to improve the early diagnosis of AKI is a target of intense research. Several biomarkers of AKI, including neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), and liver fatty acid-binding protein (L-FABP), have been assessed in human populations [7]. NGAL, which is also known as lipocalin 2, is currently the most promising novel biomarker of AKI, and it can predict the time to dialysis and graft recovery using serum and urinary IL-18 levels [8]. Very little is known about changes in serum urinary L-FABP levels after kidney transplantation [9]. We therefore investigated whether the three biomarkers, NGAL, IL-18, and L-FABP, are sensitive enough to detect early kidney allograft dysfunction within 2 weeks after living-donor kidney transplantation by following after operation associated with early kidney allograft dysfunction.
Materials and methods
We performed a prospective, single-center, observational cohort study of living-donor kidney transplant patients to evaluate serum NGAL levels and urinary levels of NGAL, IL-18, and L-FABP as biomarkers for detection of early kidney allograft dysfunction within 2 weeks after transplantation. Consecutive adult patients scheduled for living-donor kidney transplantation at Tokyo Womens’ Medical University, Tokyo, Japan, between March 2010 and June 2011 were eligible for enrollment. The study protocol was approved by the Tokyo Women’s Medical University Hospital Ethics Committee and the Department of Urology. Written informed consent was obtained from the recipients before enrollment. All patients received a triple-drug immunosuppressive regimen that consisted of tacrolimus, mycophenolate mofetil, and methylprednisolone starting 1 week prior to transplantation. The anti-CD25 monoclonal antibody basiliximab was also given at a dose of 20 mg on postoperative day (POD) 0 and POD 4 as a desensitization protocol.
We collected urine and blood specimens first 6 h and daily for POD 3. All specimens were immediately centrifuged at 3,000 RPM for 10 min at 4 °C, and the supernatant was divided into tubes and frozen at −80 °C. No additives were used. Serum Cr was measured at baseline, just before the kidney transplantation, routinely measured at least daily in the POD 1–3 and at 1 and 3 months. Estimated glomerular filtration rate (eGFR) was calculated from the serum Cr values by using the MDRD equation for Japanese [10].
The serum and urine NGAL (U-NGAL) assays were performed by using a standardized clinical platform (INVERNESS analyzer for serum samples and ARCHITECTR analyzer for urine samples, Abbott Diagnostics, Abbott Park, Illinois, USA) as previously described [11]. The coefficient of variation for the NGAL was approximately 5 % in serum and 8 % in urine, respectively. The urine IL-18 and L-FABP assays were performed by using a commercial ELISA kit (MBL No7620, Nagoya, IBL No27777, Takasaki, Japan) according to the manufacturer’s recommendations. The measurements were performed in duplicate and blinded to sample sources and clinical outcome. AR was diagnosed on the basis of the protocol biopsy findings according to the Banff 2007 criteria [12], the ultrasonography findings, and physical findings in the early period after transplantation. Early allograft dysfunction was diagnosed when there was an increase in serum Cr level of approximately 20 % or an increase of more than 0.3 mg/dl from the baseline value.
The serial sections of the biopsy specimens were evaluated immunohistochemically stained for NGAL. Paraffin sections were deparaffinized through xylene and descending grades of ethanol, then processed in an autoclave for 5 min at 105 °C, and fixed with 0.3 % methanol containing H2O2 for 30 min at room temperature. The sections were then incubated with a rabbit polyclonal antibody against human NGAL (Santa Cruz Biotechnology, Tokyo, Japan) at a 1:100 dilution for 1 h at room temperature as previously described [13]. After washing three times with PBS, the Envision System (Dako Cytomation, Tokyo, Japan) was used as the secondary antibody, and the reaction was visualized with 0.02 % diaminobenzidine tetrahydrochloride. As a negative control, the primary antibody was excluded. Six renal specimens in which no morphological abnormalities were detected by light or immunofluorescent microscopy were used as controls.
The SAS version 9.1 software program (SAS Institute Inc, Cary, NC) was used to perform the statistical analyses. All data are reported as mean ± SD. The Mann–Whitney test was used to compare the data in the AR(+) group and AR(−) group, and the Fisher exact test was used to compare categorical variables. A p value <0.05 was considered statistically significant. We assessed the diagnostic test characteristics of the candidate biomarkers, by plotting conventional receiver-operating characteristic (ROC) curves and used a nonparametric approach to compare their discriminatory power [14]. We identified biomarker cutoff levels that maximized sensitivity and specificity based on the ROC analysis.
Results
Serum and urine samples from 71 patients who underwent living-donor kidney transplantation were analyzed. The baseline characteristics of the 71 recipients are shown in Table 1. Their mean age was 46.6 ± 14.1 years, mean duration to protocol renal biopsy after transplantation was 14.0 ± 2.7 months, and mean duration of pre-transplantation hemodialysis was 53.7 ± 53.2 months. Twelve patients had been diagnosed with cellular AR by POD 14, and the other causes of allograft dysfunction were urinary tract infection, recurrence of focal segmental glomerulosclerosis, thrombotic microangiopathy, calcineurin-inhibitor drug toxicity, and histopathology of acute tubular necrosis.
Table 2 shows the mean serum and urine biomarker concentrations, serum Cr concentrations, and eGFR values of the AR(+) group and AR(−) group. There were significant differences between the AR(+) group and AR(−) group in mean serum NGAL and urine NGAL levels at all time points measured (Table 2, Fig. 1). The level of IL-18 shows a difference only at 6 h point. There were no statistically significant differences between the groups in mean L-FABP levels at any time point measured. Recovery of renal function in the AR(+) group was significantly delayed at 1 and 3 months after transplantation. The mean urine volume of the AR(+) group was similar to those of the AR(−) group. There was no significant difference in mean serum tacrolimus levels in the AR(+) group and AR(−) group.
We also investigated the relationship between serum Cr and eGFR values after transplantation. On POD 1 serum Cr levels were negatively correlated with eGFR values in both the AR(+) group (r = −0.6442, p = 0.0238) and the AR(−) group (r = −0.4585, p = 0.0003) on POD 1. There were also negative correlations between the serum Cr and eGFR values in the AR(+) group (r = −0.6270, p = 0.0291) and AR(−) group (r = −0.5549, p ≤ 0.0001) on POD 2. On POD 3, the serum Cr levels were significantly correlated with the eGFR values in the AR(−) group (r = −0.5975, p < 0.0001) but not in the AR(+) group (r = −0.4321, p = 0.0749).
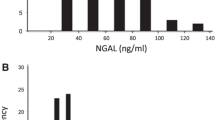
We performed an ROC analysis to determine whether the NGAL values were better predictors of AR than the values of the other biomarkers. Figure 2 and Table 3 show the area under the ROC curve (AUC) for each biomarker and their sensitivity, specificity, and positive and negative likelihood ratios. On POD 3 after transplantation, the AUC of the serum NGAL values did not significantly differ from the AUC of the L-FABP values (p = 0.20) or IL-18 values (p = 0.06), whereas the AUC of the urinary NGAL values was significantly larger than the AUC of the urinary L-FABP values (p = 0.02) or the urinary IL-18 values (p = 0.01).
Both the serum NGAL levels and the urine NGAL levels were more strongly associated with AR than the levels of the other biomarkers were. The IL-18 levels were not very sensitive, but their specificity was the same as that of NGAL on POD 3. Figure 3 shows scatter plots of each of the biomarker values on POD 3, and the optimal cutoff point for the serum NGAL levels and the urine NGAL levels was 161.9 and 66.0 ng/ml, respectively.
NGAL staining in the control kidney biopsy specimens was minimal to absent, whereas NGAL was easily detected in transplant kidneys, primarily in the proximal tubules, but also in the distal nephron segments in a cytoplastic distribution. The glomeruli were not stained. No neutrophils, macrophages, or inflammatory interstitial cells with significant NGAL staining were detectable. The tubular NGAL staining intensity was significantly increased in the kidneys with AR (Fig. 4b), whereas little staining was observed in the kidneys without AR (Fig. 4a).
Discussion
The results of this study clearly showed that NGAL in both serum and urine is a powerful biomarker of AR during the early postoperative period. Current diagnostic markers for detection of AR in the early postoperative periods, such as serum Cr, are neither sensitive nor specific. A sensitive biomarker of renal injury is needed to detect allograft rejection.
Kusaka et al. suggested that monitoring serum NGAL levels might allow prediction of graft recovery and the need for hemodialysis after kidney transplantation from donors after cardiac death [15]. Urinary NGAL could be an early predictor of DGF as reported by Parikh et al. [16]. They observed that in patients with DGF, peak postoperative serum Cr requiring dialysis typically occurred 2–4 days after transplantation, whereas urine NGAL and IL-18 values on day 0 were maximally elevated in the DGF group when compared to living-donor kidney and diseased donor kidney with prompt graft function.
Human NGAL was originally identified as a 25-kD protein covalently bound to gelatinase in neutrophils. NGAL is normally expressed at very low levels in several human tissues, including kidney, lung, stomach, and colon tissue [17]. NGAL has been studied in various clinical conditions and proven predictive of diagnosis and prognosis [18, 19]. In patients after kidney transplantation, tissue NGAL staining in early protocol biopsies and urinary NGAL levels have been shown to predict delayed initial graft function and outcome [8, 16, 20]. The present study showed that NGAL staining intensity was significantly increased in the proximal tubular cells of living-related allografts with AR. Mishra et al. obtained protocol kidney biopsy specimens 1 h after anastomosis and found that the immunohistochemical staining intensity for NGAL was correlated with cold ischemia time, peak postoperative serum Cr levels, and dialysis requirement [21]. Four patients developed DGF requiring dialysis during the first week post-transplantation; all of these patients displayed the most intense NGAL staining in their first protocol biopsies. NGAL staining in early biopsies from patients receiving cadaveric kidney was strongly correlated with peak postoperative serum Cr, which occurred days later. Taken together, NGAL staining intensity in early protocol biopsy specimens provides a novel predictor of AKI following kidney transplantation.
AKI has been demonstrated to result in increased NGAL mRNA expression in distant organs, especially in the liver and spleen, and the over-expressed NGAL protein is most likely released into the circulation and contributes to the systemic pool [22]. NGAL is freely filtered by the glomerulus and completely reabsorbed by the proximal tubule. Moreover, any decrease in glomerular filtration rate resulting from AKI would be expected to decrease the clearance of NGAL and lead to further accumulation in the systemic pool [23]. Both serum and urine NGAL concentrations have been shown to be strongly correlated with serum Cr concentrations. Assessment of urinary NGAL concentrations at the time of Cr-based diagnosis of AKI may offer prognostic information that may improve monitoring and care in the identification of patients for clinical trials of new treatments for AKI.
A serum NGAL cutoff value of 161.9 ng/ml made it possible to discriminate patients with AR from patients with other causes of acute allograft function. More importantly, we demonstrated urine NGAL, at a cutoff value of 60 ng/ml, is a sensitive and specific predictor of AR as the underlying pathology in our cohort. The optimal cutoff values calculated in this study reflect in part the homogeneity of our cohort of allograft recipients on maintenance immunosuppression. This is in accordance with a significantly lower cutoff value reported in selected clinical settings than in cross-sectional observations in an emergency department or intensive care unit [18].
The results of the present study have several potential implications. First, we prospectively recruited a relatively homogeneous cohort of only living-related kidney transplant recipients. Second, the present study is the first to report how a standardized point-of-care platform can be useful for predicting AR based on measurements in serum and urine samples in living-related kidney transplant recipients. The majority of biomarkers of AKI described thus far, as well as NGAL, have been measured in urine, and simultaneous measurement of other serum and urine biomarkers as potential predictors of AKI may be informative [24]. Urinary diagnostic tests have several advantages over measurements of substances in blood, including the noninvasive nature of specimen collection, the smaller number of interfering proteins, and the potential for the development of self-testing kits.
The present study had several limitations. The first limitation was the small number of patients. Although our results were of clear clinical and statistical significance, they will need to be validated in a larger population. Second, the classification of patients with acute allograft dysfunction was based on an increase in serum Cr concentration as the diagnostic criterion for acute allograft dysfunction. However, AR was diagnosed on the basis of the protocol biopsy findings according to the Banff 2007 criteria. Third, the correlations between NGAL values and clinical conditions are descriptive, and the NGAL levels did not affect diagnostic or therapeutic interventions in our cohort.
In conclusion, the results of the present study demonstrate the ability of NGAL to guide diagnosis of AR in living-donor kidney transplant recipients. Whether NGAL has the potential to drive therapeutic interventions remains to be determined. In future studies it will be important to validate the sensitivity and specificity of these biomarker panels in clinical specimens from large kidney transplantation recipient cohorts.
References
Alachkar N, Rabb H, Jaar BG (2011) Urinary biomarkers in acute kidney transplant dysfunction. Nephron Clin Pract 118:c173–c181
Ojo AO, Wolfe RA, Held PJ et al (1997) Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 63:968–974
Halawa A (2011) The early diagnosis of acute renal graft dysfunction: a challenge we face. The role of novel biomarkers. Ann Transplant 16:90–98
Perrone RD, Madias NE, Levey AS (1992) Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 38:1933–1953
Kee TY, Chapman JR, O’Connell PJ et al (2006) Treatment of subclinical rejection diagnosed by protocol biopsy of kidney transplants. Transplantation 82:36–42
Kurtkoti J, Sakhuja V, Sud K et al (2008) The utility of 1- and 3-month protocol biopsies on renal allograft function: a randomized controlled study. Am J Tansplant 8:317–323
Coca SG, Yalavarthy R, Concato J et al (2008) Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int 73:1008–1016
Hall IE, Yarlagadda SG, Coca SG et al (2010) IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 21:189–197
Yamamoto T, Noiri E, Ono Y et al (2007) Renal L-type fatty acid-binding protein in acute ischemic injury. J Am Soc Nephrol 18:2894–2902
Imai E, Matsuo S, Makino H et al (2010) Chronic Kidney Disease Japan Cohort study: baseline characteristics and factors associated with causative diseases and renal function. Clin Exp Nephrol 14:558–570
Parikh CR, Devarajan P, Zappitelli M et al (2011) Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 22:1737–1747
Solez K, Colvin RB, Racusen LC et al (2008) Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 8:753–760
Nitta K, Horita S, Honda K et al (1999) Glomerular expression of cell-cycle-regulatory proteins in human crescentic glomerulonephritis. Virchows Arch 435:422–427
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristics curves: a nonparametric approach. Biometrics 44:837–845
Kusaka M, Kuroyanagi Y, Mori T et al (2008) Serum neutrophil gelatinase-associated lipocalin as a predictor of organ recovery from delayed graft function after kidney transplantation from donors after cardiac death. Cell Transplant 17:129–134
Parikh CR, Jani A, Mishra J et al (2006) Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 6:1639–1645
Xu S, Venge P (2000) Lipocalins as biochemical markers of disease. Biochim Biophys Acta 1482:298–307
Haase M, Bellomo R, Devarajan P et al (2009) Accuracy of neutrophil-gelatinase associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury. A systematic review and meta-analysis. Am J Kidney Dis 54:1012–1024
Soni SS, Cruz D, Bobek I et al (2010) NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol 42:141–150
Hollmen ME, Kyllonen LE, Inkinen KA et al (2011) Urine neutrophil gelatinase-associated lipocalin is a marker of graft recovery after kidney transplantation. Kidney Int 79:89–98
Mishra J, Ma Q, Kelly C et al (2006) Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol 21:856–863
Schmidt-Ott KM, Mori K, Kalandadze A et al (2007) Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18:407–413
Schmidt-Ott KM, Mori K, Kalandadze A et al (2006) Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens 15:442–449
Devarajan P (2007) Emerging biomarkers of acute kidney injury. Contrib Nephrol 156:203–212
Acknowledgments
We thank Naoki Kohei for his valuable suggestion and appreciate Mayuko Tsuboi, Ai Munekawa, and Atsuko Teraoka for their technical assistance.
Conflict of interest
None of the authors has any financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kohei, J., Ishida, H., Kazunari, T. et al. Neutrophil gelatinase-associated lipocalin is a sensitive biomarker for the early diagnosis of acute rejection after living-donor kidney transplantation. Int Urol Nephrol 45, 1159–1167 (2013). https://doi.org/10.1007/s11255-012-0321-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-012-0321-y