Abstract
Purpose
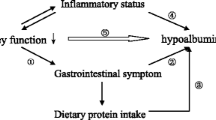
Serum albumin is one of the strongest mortality predictors in maintenance hemodialysis (MHD) patients. Yet, the degree to which serum albumin represents dietary protein intake or an inflammatory state, among others, is not clear. We hypothesize that these inadequate protein intake and inflammation contribute somewhat equally to hypoalbuminemia.
Methods
In a cross-sectional analysis, we examined correlates of low serum albumin, <3.8 g/dL, in 812 MHD patients in whom interleukin-6 (IL-6) and normalized protein nitrogen appearance (nPNA), also known as normalized protein catabolic rate (nPCR), were also measured. Logistic regression estimated odds ratios were employed, and spline models were plotted to examine the likelihood of relatively low serum albumin <3.8 g/dL.
Results
Mean age (±SD) of patients was 54 ± 15 years; 53 % of patients were men, 50 % Hispanic, 31 % African–American, and 55 % diabetic. The mean dialysis vintage was 31 ± 34 months (median: 19, inter-quartile range: 7–44 months). The baseline serum albumin, averaged over a 3-month period (mean ± SD), was 3.88 ± 0.38 g/mL. The unadjusted correlation coefficients of l IL-6 and nPNA with serum albumin were −0.36 and +0.20, respectively (p < 0.001 for each comparison). The likelihood for an albumin <3.8 gr/dL increased linearly with decreasing nPNA and rising serum IL-6. This trend was steeper with increasing serum IL-6 up to a concentration of 30 ng/mL.
Conclusions
Both low protein intakes and a high state of inflammation are associated with low serum albumin in MHD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many individuals with advanced chronic kidney disease (CKD) who receive chronic hemodialysis (HD) treatment suffer from various degrees of protein-energy wasting (PEW) [1–3]. The International Society of Renal Nutrition and Metabolism (ISRNM) Expert Panel recently proposed a set of criteria for the detection of PEW, among which low serum albumin is one of the key diagnostic criteria [2].
Since maintenance hemodialysis (MHD) patients have little or no glomerular filtration rate (GFR), the change in serum urea nitrogen between and during hemodialysis sessions becomes a reliable indicator of dietary nitrogen intake, provided the patient is in a steady state. Although indirect, this measure of protein intake is referred to as the urea kinetic-based protein nitrogen appearance (PNA) or protein catabolic rate (PCR), which is usually normalized (n) to a typical body weight, hence, the nPNA. It was previously showed that the nPNA is a strong and independent predictor of mortality in MHD patients [4].
Protein-energy wasting (PEW) and inflammation are closely associated in MHD patients [5, 6]. Chronic inflammation is common in uremia, in part because of factors related to the GFR and to the standard dialysis procedure itself [7, 8], but also because of a genetic predisposition [9, 10]. Measures of inflammation and PEW are important predictors of mortality in patients with end-stage renal failure [11, 12].
In maintenance dialysis patients, a low serum albumin level is an indicator of more severe comorbidity, worse general health status, and protein-energy wasting (PEW) [13]. Hypoalbuminemia is a strong predictor of both cardiovascular [14] and all-cause mortality [15] and post-transplant mortality [16] in patients with different stages of CKD including those undergoing chronic dialysis treatments [17]. Although the serum albumin is a strong and clinically important predictor of adverse outcomes, it is not entirely known, which clinical markers are associated with serum albumin.
Although previous studies have shown that both dietary protein intake and inflammatory markers [18–20] are associated with serum albumin in MHD patients, to our knowledge, no study in the last 10 years has examined the association between protein intake and inflammatory markers and serum albumin level in MHD patients. We hypothesized that low serum albumin is associated with higher level of inflammatory markers and lower protein intake.
Methods
Patient population
We studied MHD patients who were participating in the Nutritional and Inflammatory Evaluation in Dialysis (NIED) Study [21]. The original patient cohort was derived from a pool of over 3,000 MHD outpatients over 5 years in eight DaVita Inc. chronic dialysis facilities in the South Bay Los Angeles area [see NIED Study Web site at http://www.NIEDStudy.org for more details]. Inclusion criteria were outpatients who had been undergoing MHD for at least 8 weeks, were 18 years or older, and who signed the Institutional Review Board approved consent form. Patients with an anticipated life expectancy of less than 6 months (for example, due to a metastatic malignancy or advanced HIV/AIDS disease) were excluded. From October 1, 2001 through December 31, 2006, 893 MHD patients from eight DaVita dialysis facilities in the Los Angeles South Bay area signed the informed consent form and underwent the periodic evaluations of the NIED Study. For this study, data including baseline serum albumin were available in 812 MHD patients.
The medical chart of each MHD patient was reviewed by a collaborating physician, and data pertaining to underlying kidney disease, cardiovascular history, and other comorbid conditions were extracted. A modified version of the Charlson Comorbidity Index, that is, without the age and kidney disease components, was used to assess the severity of comorbidities [22].
Anthropometric measures
Body weight was measured immediately after a hemodialysis treatment. Other anthropometric measurements were taken while patients were undergoing a hemodialysis treatment or within 5–20 min after termination of the treatment. Biceps skinfold and triceps skinfold thicknesses were measured with a conventional skinfold caliper using standard techniques as previously described [23, 24].
Near-infrared interactance
To estimate the percentage of body fat and fat-free body mass, near-infrared (NIR) interactance was measured at the same time as the anthropometric measurements [25]. A commercial near-infrared interactance sensor with a coefficient of variation of 0.5 % for total body fat measurement (portable Futrex 6100®, Gaithersburg, Maryland, http://www.futrex.com) was used. NIR measurements were taken by placing, for several seconds on the upper aspect of the arm without a vascular access, a Futrex® sensor, and entering the required data (date of birth, gender, weight, and height) of each patient. NIR measurements of body fat appear to correlate significantly with other nutritional measures in MHD patients.
Laboratory tests
Pre-dialysis blood samples and post-dialysis serum urea nitrogen were obtained on a mid-week day, which coincided chronologically with the drawing of quarterly blood tests in the DaVita facilities. The single-pool Kt/V was used to represent the weekly dialysis dose. All routine laboratory measurements were taken by DaVita® Laboratories (Deland, FL) using automated methods.
Serum high-sensitivity C-reactive protein (CRP) was measured by a turbidometric immunoassay in which a serum sample is mixed with latex beads coated with antihuman CRP antibodies forming an insoluble aggregate (manufacturer: WPCI, Osaka, Japan, unit: mg/L, normal range: <3.0 mg/L) [26, 27]. Interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) were measured by immunoassay kits based on a solid-phase sandwich ELISA using recombinant human IL-6 and TNF-α (manufacturer: R&D Systems, Minneapolis, MN; units: pg/mL; normal range: IL-6: <9.9 pg/mL, TNF-α: <4.7 pg/mL) [28, 29]. CRP and the cytokines were measured in the General Clinical Research Center Laboratories of Harbor-UCLA Medical Center. Serum transthyretin (prealbumin) was measured using immunoprecipitation analysis. Plasma total homocysteine concentrations were determined by high-performance liquid chromatography in the Harbor-UCLA Clinical Laboratories.
Statistical methods
Chi-square test and independent sample t test were employed to examine the differences between the two groups of patients with serum albumin <3.8 g/dL and ≥3.8 g/dL. Pearson′s correlation coefficient (r) was used for the analyses of linear associations. CRP and IL-6 both were log-transformed because of the severe skewness. Multivariate regression analyses and analysis of covariance were performed to obtain adjusted p values controlled for case-mix and other covariates. Multivariate logistic regression analysis was utilized to examine the strength of association between case-mix and other variables of nutrition and inflammation with the presence of hypoalbuminemia (i.e., serum albumin <3.8 g/dL).
To express and compare the ability of the two inflammatory markers of interest (i.e., IL-6 and CRP) and the two nutritional markers of interest (i.e., nPNA and serum creatinine) and their mutual combinations in predicting serum albumin level <3.8 g/dL, we constructed receiver operating characteristic (ROC) curves each of the aforementioned predictors and serum albumin <3.8 g/dL as the reference variable. Then, the differences between the areas under ROC curves were examined using “roccomp” command in Stata. In a ROC curve, sensitivity (y-axis) is plotted versus one minus specificity (x-axis) for each possible cutoff value of IL-6, CRP, nPNA, creatinine, or probability function obtained from the logistic regression models containing one marker of inflammation and one marker of nutrition and serum albumin <3.8 g/dL as dependent (reference) variable [30]. Values are expected to be between 0.5 (indicating no discriminative ability) and 1.0 (indicating highest detection accuracy).
Case-mix and comorbidity covariates included gender, age, race, and ethnicity (Hispanics, Blacks, Asians, and others), diabetes mellitus, the modified Charlson Comorbidity Index, dialysis vintage (number of months on MHD treatment), and clinical and laboratory measures of MICS. Unadjusted and case mix-adjusted non-linear associations between IL-6 and CRP with the presence of serum albumin <3.8 g/dL were also examined using restricted cubic splines as an alternative to inappropriate linearity assumptions [31]. Fiducial limits are given as mean ± SD (standard deviation) or median and inter-quartile range; odds ratios include 95 % confidence interval (CI) levels. A p value <0.05 or a 95 % CI that did not span 1.0 was considered to be statistically significant. Descriptive and multivariate statistics were carried out with the statistical software “Stata version 10.0” (Stata Corporation, College Station, Texas).
Results
Baseline characteristics
Mean age (±SD) of patients was 54 ± 15 years; 53 % of patients (n = 430) were men, 50 % (n = 410) were Hispanic, 32 % (n = 258) were African–American, and 56 % (n = 455) were diabetic. The mean dialysis vintage was 31 ± 34 months (median: 19, inter-quartile range: 7–44 months). The average (mean ± SD) baseline serum albumin in the 812 MHD patients was 3.88 ± 0.38 ng/mL.
Table 1 shows baseline demographic, clinical, and laboratory variables according to the 6 a priori-selected groups of albumin in 812 maintenance hemodialysis patients. Hypoalbuminemic patients were older, more likely to be diabetic, and had more comorbidities. The inflammatory profile also shows that hypoalbuminemic patients had higher serum concentrations of CRP and IL-6, higher WBC levels, and a lower percentage of lymphocytes. Moreover, hypoalbuminemic patients had a lower nPNA and serum creatinine level. However, the differences between variables such as ethnicity, primary insurance, erythropoietin dose, serum ferritin, phosphorus, bicarbonate, and TNF-α were not significant in different albumin subgroups.
Table 2 shows the correlation coefficients of relevant clinical, nutritional, and inflammatory measures with serum albumin levels in 812 MHD patients. Serum albumin was negatively correlated with age, MIS score, IL-6, CRP, white blood cell count, and erythropoietin dose. Furthermore, serum albumin was positively correlated with blood hemoglobin, serum nPNA, prealbumin, creatinine, and TIBC.
Non-linear associations
Figure 1 illustrates the cubic spline curves depicting non-linear association between serum albumin concentrations and nPNA and serum creatinine. Figure 1a shows that the serum albumin concentration increases with increasing nPNA up to around 1.4 g/kg/day. Figure 1b shows a rather linear positive association between serum albumin concentrations and serum creatinine up to a serum creatinine concentration of about 14 mg/dL. Figure 2 also shows the non-linear associations between serum albumin and measures of inflammation. It shows that the declining concentrations of serum albumin with the severity of inflammatory measures is more prominent with regard to serum IL-6 than with CRP; however, decline in serum albumin with increasing serum CRP shows a more linear and consistent trend.
Table 3 shows the unadjusted and multivariate adjusted odds ratios (OR) for the occurrence of a serum albumin concentration <3.8 g/dL among the study population. It shows that women had more than twice the risk of being hypoalbuminemic even after adjustment for case-mix and other markers of PEW. Even though there was no unadjusted association between hypoalbuminemia and race/ethnicity, African/American patients had an OR of 2.36 of hypoalbuminemia after adjustment for other relevant confounders. Higher concentrations of serum prealbumin, creatinine, and TIBC were associated with a lower likelihood of hypoalbuminemia even after multivariate adjustments. Table 3 also shows that each 1-unit increment in the logarithm of serum IL-6 or CRP was associated, respectively, with a 24 and 16 % increase in the odds of having serum albumin <3.8 g/dL. Figure 3 indicates the unadjusted and case mix-adjusted associations of IL-6 and CRP with the probability of a serum albumin <3.8 g/dL. In general, the higher concentrations of IL-6 and CRP were associated with greater odds of a serum albumin <3.8 g/dL.
Table 4 shows the death hazard ratios according to the progressive integers of serum albumin concentrations in the 812 MHD patients. The association was linear, and lower serum albumin levels were associated with higher risks of death.
Figure 4 illustrates the combined (three-dimensional) association of serum IL-6 and nPNA with the odds of a serum albumin <3.8 g/dL. As shown in Fig. 4, the odds of a serum albumin <3.8 g/dL increases linearly with decreasing nPNA, and there is an increasing trend toward hypoalbuminemia with increment of serum IL-6. This trend is steeper with increasing serum IL-6 concentrations up to about 30 ng/mL.
Discussion
In this cross-sectional study in the twenty-first century, we showed that both low protein intake and high level of inflammatory markers are associated with low serum albumin concentrations in MHD patients. This finding has important clinical implications, since physicians commonly employ the serum albumin level to evaluate the nutritional status and overall health of a MHD patient. It is important to know that each 1-unit increment in the logarithm of serum IL-6 or CRP was associated, respectively, with a 24 and 16 % increase in the odds of the serum albumin being <3.8 g/dL.
Many previous epidemiological studies have shown a strong association between serum albumin and prospective mortality in chronic dialysis patients, including CV death, and post-transplant mortality in dialysis patients [13, 16, 32]. Evidence indicates that both malnutrition and inflammation are the major causes of severe hypoalbuminemia in MHD patients [33]. Nutritional support has been shown to improve hypoalbuminemia and different outcomes in patients with CKD [16, 32, 34, 35]. Indeed, several randomized and nonrandomized prospective trials in hypoalbuminemic MHD patients indicate that nutritional support may increase serum albumin [36], but it has not effect on clinical outcomes [37–39]. Increased dietary protein in hypoalbuminemic patients thus might confer a survival advantage compared to hypoalbuminemic patients with lower dietary protein intakes.
We found that each 1-unit increment in the logarithm of the serum IL-6 or CRP was associated, respectively, with a 24 and 16 % increase the odds of having a serum albumin <3.8 g/dL. Kaysen et al. [40] reported that in MHD patients, the serum albumin concentration changes with inflammation and poor nutritional intake due to their effects on albumin catabolism and synthesis, respectively. These authors showed that the markers of nutritional status primarily affected albumin synthesis, whereas inflammation caused hypoalbuminaemia by increasing the albumin fractional catabolic rate [40]. In another study, Kaysen et al. showed that increasing inflammation and higher dietary protein intake exerted competing effects on serum albumin in MHD patients [18]. In our study using a patients’ population from twenty-first century, we found similar results. However, all of these previous studies were performed before 2000 when the dialysis practice and pattern were different than nowadays.
Serum albumin levels decrease with inflammation due to several causes, including reduced synthesis, increased catabolism, and translocation of albumin to extravascular pools [41, 42]. TNF-α and IL-1 directly suppress appetite [43, 44]. Cytokines may also induce catabolism, leading to a wasting illness that is indistinguishable from prolonged starvation. In particular, IL-6 and TNF-α induce muscle breakdown in rats and IL-6 appears to mediate experimental cancer cachexia [45]. Administering IL-6 receptor antibodies prevented these effects in mice transgenic for IL-6 [46]. Therefore, the inflammatory response may induce protein-energy malnutrition by decreased intake, in addition to increased catabolism.
Our study should be qualified for several potential limitations. Like all observational studies, the present study cannot prove causality. Another limitation is that dietary intake was not measured. The nPNA is considered a valid surrogate for dietary protein intake under steady-state conditions. However, particularly in the face of inflammation, the nPNA may overestimate dietary protein intake because of an increase in endogenous nitrogen breakdown. Furthermore, whether PNA should be normalized to body weight is a matter of controversy, as it can be misleading in obese, malnourished, and edematous individuals [47]. It is also important to note that nPNA, according to the equations, underestimates dietary protein intake by roughly 6–8 g of protein per day and more accurately reflects “measured” net protein degradation as determined by the urea nitrogen appearance. Moreover, we do not have data from peritoneal dialysis patients; therefore, our findings cannot be extrapolated to patients undergoing chronic peritoneal dialysis.
The strengths of our study include the well-characterized cohort, the comprehensive clinical and laboratory evaluations including body composition measures, detailed evaluation of comorbid states by study physicians at baseline, and the measurements of pro-inflammatory cytokines and markers. In addition, the subjects were selected without having any prior knowledge of their inflammatory status.
Conclusions
We showed both measures of low protein intake and high levels of inflammatory markers are associated with low serum albumin in MHD patients.
References
Clinical practice guidelines for nutrition in chronic renal failure (2000) K/DOQI, National Kidney Foundation. Am J Kidney Dis 35:S1–S140
Fouque D, Kalantar-Zadeh K, Kopple J et al (2008) A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73:391–398
Ricks J, Molnar MZ, Kovesdy CP, Kopple JD, Norris KC, Mehrotra R, Nissenson AR, Arah OA, Greenland S, Kalantar-Zadeh K (2011) Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis 58(4):574–582
Shinaberger CS, Kilpatrick RD, Regidor DL et al (2006) Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis 48:37–49
Kalantar-Zadeh K, Kopple JD, Block G et al (2001) A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38:1251–1263
Kaizu Y, Ohkawa S, Odamaki M et al (2003) Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis 42:295–302
Raj DS, Dominic EA, Pai A et al (2005) Skeletal muscle, cytokines, and oxidative stress in end-stage renal disease. Kidney Int 68:2338–2344
Caglar K, Peng Y, Pupim LB et al (2002) Inflammatory signals associated with hemodialysis. Kidney Int 62:1408–1416
Maruyama Y, Nordfors L, Stenvinkel P et al (2005) Interleukin-1 gene cluster polymorphisms are associated with nutritional status and inflammation in patients with end-stage renal disease. Blood Purif 23:384–393
Balakrishnan VS, Guo D, Rao M et al (2004) Cytokine gene polymorphisms in hemodialysis patients: association with comorbidity, functionality, and serum albumin. Kidney Int 65:1449–1460
Rambod M, Bross R, Zitterkoph J et al (2009) Association of malnutrition-inflammation score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis 53:298–309
Molnar MZ, Czira ME, Rudas A et al (2011) Association of the malnutrition-inflammation score with clinical outcomes in kidney transplant recipients. Am J Kidney Dis 58:101–108
Kalantar-Zadeh K, Kilpatrick RD, Kuwae N et al (2005) Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 20:1880–1888
Stenvinkel P, Heimburger O, Paultre F et al (1999) Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55:1899–1911
Kovesdy CP, George SM, Anderson JE et al (2009) Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr 90:407–414
Molnar MZ, Kovesdy CP, Bunnapradist S et al (2011) Associations of pretransplant serum albumin with post-transplant outcomes in kidney transplant recipients. Am J Transplant 11:1006–1015
Kalantar-Zadeh K, Kopple JD (2001) Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis 38:1343–1350
Kaysen GA, Chertow GM, Adhikarla R et al (2001) Inflammation and dietary protein intake exert competing effects on serum albumin and creatinine in hemodialysis patients. Kidney Int 60:333–340
Kaysen GA, Rathore V, Shearer GC et al (1995) Mechanisms of hypoalbuminemia in hemodialysis patients. Kidney Int 48:510–516
Kaysen GA, Gambertoglio J, Jimenez I et al (1986) Effect of dietary protein intake on albumin homeostasis in nephrotic patients. Kidney Int 29:572–577
Colman S, Bross R, Benner D et al (2005) The nutritional and inflammatory evaluation in dialysis patients (NIED) study: overview of the NIED study and the role of dietitians. J Ren Nutr 15:231–243
Rattanasompattikul M, Feroze U, Molnar MZ, Dukkipati R, Kovesdy CP, Nissenson AR, Norris KC, Kopple JD, Kalantar-Zadeh K (2011) Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int Urol Nephrol. doi:10.1007/s11255-011-0085-9
Nelson EE, Hong CD, Pesce AL et al (1990) Anthropometric norms for the dialysis population. Am J Kidney Dis 16:32–37
Levin A, Bakris GL, Molitch M et al (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71:31–38
Kalantar-Zadeh K, Dunne E, Nixon K et al (1999) Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol Dial Transplant 14:169–175
Ridker PM, Rifai N, Rose L et al (2002) Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 347:1557–1565
Erbagci AB, Tarakcioglu M, Aksoy M et al (2002) Diagnostic value of CRP and Lp(a) in coronary heart disease. Acta Cardiol 57:197–204
Pecoits-Filho R, Barany P, Lindholm B et al (2002) Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 17:1684–1688
Beutler B, Cerami A (1989) The biology of cachectin/TNF—a primary mediator of host response. Ann Rev Immunol 7:625–655
Pepe MS, Janes H, Longton G et al (2004) Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol 159:882–890
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8:551–561
Mehrotra R, Duong U, Jiwakanon S et al (2011) Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am J Kidney Dis 58:418–428
Noori N, Kovesdy CP, Dukkipati R, Feroze U, Molnar MZ, Bross R, Nissenson AR, Kopple JD, Norris KC, Kalantar-Zadeh K (2011) Racial and ethnic differences inmortality of hemodialysis patients: role of dietary and nutritional status and inflammation. Am J Nephrol 33(2):157–167
Kalantar-Zadeh K, Cano NJ, Budde K et al (2011) Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat Rev Nephrol 7:369–384
Dezfuli A, Scholl D, Lindenfeld SM et al (2009) Severity of hypoalbuminemia predicts response to intradialytic parenteral nutrition in hemodialysis patients. J Ren Nutr 19:291–297
Cano N, Labastie-Coeyrehourq J, Lacombe P et al (1990) Perdialytic parenteral nutrition with lipids and amino acids in malnourished hemodialysis patients. Am J Clin Nutr 52:726–730
Cano NJ, Fouque D, Roth H et al (2007) Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: a 2-year multicenter, prospective, randomized study. J Am Soc Nephrol 18:2583–2591
Kopple JD, Cheung AK, Christiansen JS Djurhuus CB, El Nahas M, Feldt-Rasmussen B, Mitch WE, Wanner C, Göthberg M, Ikizler TA (2011) OPPORTUNITY&trade: a large-scale randomized clinical trial of growth hormone in hemodialysis patients. Nephrol Dial Transplant 26(12):4095–4103
Wolfson M, Jones MR, Kopple JD (1982) Amino acid losses during hemodialysis with infusion of amino acids and glucose. Kidney Int 21:500–506
Kaysen GA, Dubin JA, Muller HG et al (2002) Relationships among inflammation nutrition and physiologic mechanisms establishing albumin levels in hemodialysis patients. Kidney Int 61:2240–2249
Rothschild MA, Oratz M, Schreiber SS (1972) Albumin synthesis (second of two parts). N Engl J Med 286:816–821
Rothschild MA, Oratz M, Schreiber SS (1972) Albumin synthesis. 1. N Engl J Med 286(14):748–757
Brebner K, Hayley S, Zacharko R et al (2000) Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology 22:566–580
Goodman MN (1994) Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med 205:182–185
Strassmann G, Fong M, Kenney JS et al (1992) Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Investig 89:1681–1684
Tsujinaka T, Fujita J, Ebisui C et al (1996) Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Investig 97:244–249
Canaud B, Leblanc M, Garred LJ et al (1997) Protein catabolic rate over lean body mass ratio: a more rational approach to normalize the protein catabolic rate in dialysis patients. Am J Kidney Dis 30:672–679
Acknowledgments
The authors are thankful to Ms. Stephanie Griffith and Dr. Victor Goh, at Harbor-UCLA GCRC Core Laboratories for the management of blood samples and measuring inflammatory markers. This study was supported by a research grant from the National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Disease grant # DK61162 (for KKZ), a research grant from DaVita, Inc (KKZ), and a General Clinical Research Center (GCRC) grant # M01-RR00425 from the National Centers for Research Resources, National Institutes of Health. MZM received the grants from the National Developmental Agency (KTIA-OTKA-EU 7KP-HUMAN-MB08-A-81231) from the Research and Technological Innovation Fund, and is recipient of the Hungarian Eötvös Scholarship (MÖB/77-2/2012).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y., Molnar, M.Z., Rattanasompattikul, M. et al. Relative contributions of inflammation and inadequate protein intake to hypoalbuminemia in patients on maintenance hemodialysis. Int Urol Nephrol 45, 215–227 (2013). https://doi.org/10.1007/s11255-012-0170-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-012-0170-8